Pediatric Early Warning System (PEWS) Association with ICU Mortality in Children with Acute Lymphoblastic Leukemia: A Cohort Study from Kazakhstan
Abstract
1. Introduction
2. Methods
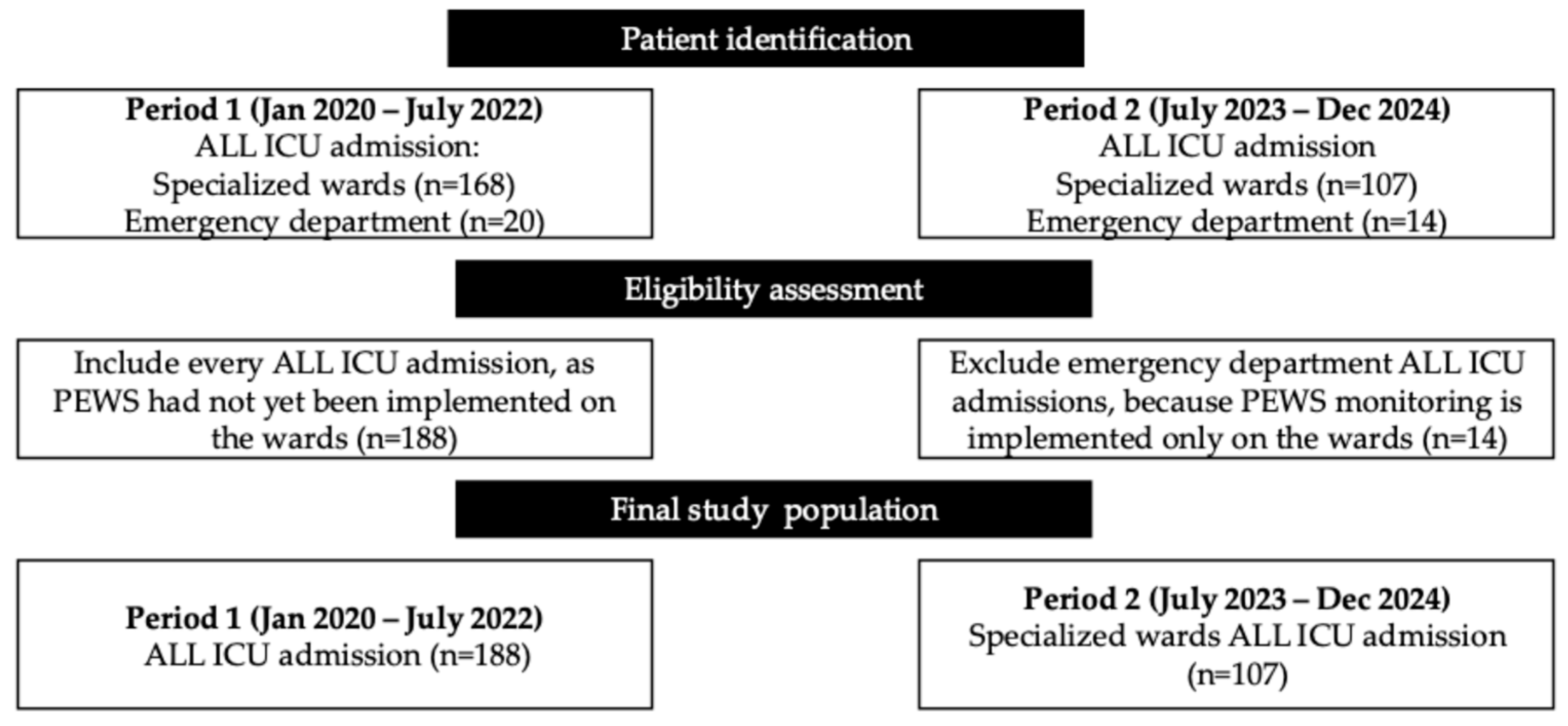
2.1. Research Design and Study Population
2.2. Sample Size and Power
2.3. Variables
2.3.1. Dependent Variable
2.3.2. Independent Variables
2.4. Statistical Analysis Plan
3. Results
3.1. General Characteristics
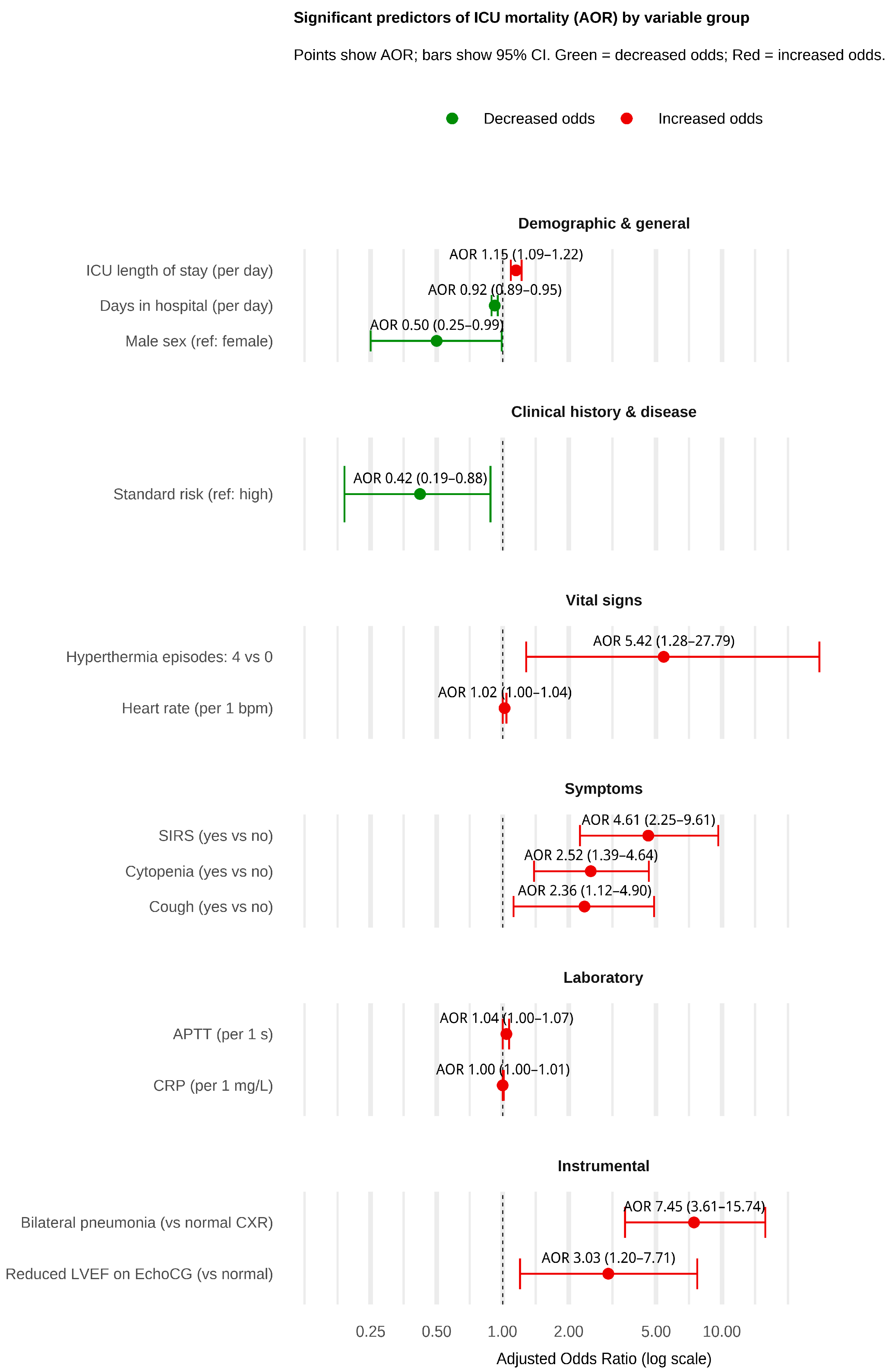
3.2. Factors Associated with ICU Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Yao, X.; Yang, L. Global, Regional, and National Burden of Children and Adolescents with Acute Lymphoblastic Leukemia from 1990 to 2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Front. Public Health 2025, 13, 1525751. [Google Scholar] [CrossRef]
- Ding, F.; Deng, L.; Xiong, J.; Cheng, Z.; Xu, J. Analysis of Global Trends in Acute Lymphoblastic Leukemia in Children Aged 0–5 Years from 1990 to 2021. Front. Pediatr. 2025, 13, 1542649. [Google Scholar] [CrossRef]
- Jaime-Pérez, J.C.; Jiménez-Castillo, R.A.; Herrera-Garza, J.L.; Gutiérrez-Aguirre, H.; Marfil-Rivera, L.J.; Gómez-Almaguer, D. Survival Rates of Adults With Acute Lymphoblastic Leukemia in a Low-Income Population: A Decade of Experience at a Single Institution in Mexico. Clin. Lymphoma Myeloma Leuk. 2017, 17, 60–68. [Google Scholar] [CrossRef]
- Jabeen, K.; Ashraf, M.S.; Iftikhar, S.; Belgaumi, A.F. The Impact of Socioeconomic Factors on the Outcome of Childhood Acute Lymphoblastic Leukemia (ALL) Treatment in a Low/Middle Income Country (LMIC). J. Pediatr. Hematol. Oncol. 2016, 38, 587–596. [Google Scholar] [CrossRef]
- Tai, E.W.; Ward, K.C.; Bonaventure, A.; Siegel, D.A.; Coleman, M.P. Survival among Children Diagnosed with Acute Lymphoblastic Leukemia in the United States, by Race and Age, 2001 to 2009: Findings from the CONCORD-2 Study. Cancer 2017, 123, 5178–5189. [Google Scholar] [CrossRef]
- Mussina, K.; Kuanova, B.; Syssoyev, D.; Gaipov, A.; Poddighe, D.; Shaikhyzada, K.; Aimyshev, T.; Galiyeva, D. Epidemiology of Pediatric Hematological Malignancies in Kazakhstan: Data from Unified National Electronic Healthcare System 2014–2021. Eur. J. Pediatr. 2024, 183, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the Republic of Kazakhstan. Clinical Protocol for the Diagnosis and Treatment of Acute Lymphoblastic Leukemia; Ministry of Health of the Republic of Kazakhstan: Astana, Kazakhstan, 2023.
- Ranta, S.; Broman, L.M.; Abrahamsson, J.; Berner, J.; Fläring, U.; Myrberg, I.H.; Kalzén, H.; Karlsson, L.; Mellgren, K.; Nilsson, A.; et al. ICU Admission in Children with Acute Lymphoblastic Leukemia in Sweden: Prevalence, Outcome, and Risk Factors. Pediatr. Crit. Care Med. 2021, 22, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Kraguljac, A.P.; Croucher, D.; Christian, M.; Ibrahimova, N.; Kumar, V.; Jacob, G.; Kiss, A.; Minden, M.D.; Mehta, S. Outcomes and Predictors of Mortality for Patients with Acute Leukemia Admitted to the Intensive Care Unit. Can. Respir. J. 2016, 2016, 3027656. [Google Scholar] [CrossRef]
- Kembhavi, S.A.; Somvanshi, S.; Banavali, S.; Kurkure, P.; Arora, B. Pictorial Essay: Acute Neurological Complications in Children with Acute Lymphoblastic Leukemia. Indian J. Radiol. Imaging 2012, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, A.; Genova, S.; Maringhini, S.; Coffaro, G.; Ziino, O.; D’Angelo, P. Acute Respiratory Distress Syndrome Associated with Tumor Lysis Syndrome in a Child with Acute Lymphoblastic Leukemia. Pediatr. Rep. 2015, 7, 5760. [Google Scholar] [CrossRef]
- Lambert, V.; Matthews, A.; MacDonell, R.; Fitzsimons, J. Paediatric Early Warning Systems for Detecting and Responding to Clinical Deterioration in Children: A Systematic Review. BMJ Open 2017, 7, e014497. [Google Scholar] [CrossRef]
- Kemps, N.; Holband, N.; Boeddha, N.P.; Faal, A.; Juliana, A.E.; Kavishe, G.A.; Keitel, K.; van ‘t Kruys, K.H.; Ledger, E.V.; Moll, H.A.; et al. Validation of the Emergency Department-Paediatric Early Warning Score (ED-PEWS) for Use in Low- and Middle-Income Countries: A Multicentre Observational Study. PLOS Global Public Health 2024, 4, e0002716. [Google Scholar] [CrossRef]
- Alzaher, R.A.; Jamil, S.; Murabi, I.; Ahmari, E. Implementation of the Bedside Paediatric Early Warning System, Its Sustainability in Clinical Practice and Patient Outcomes: A Quality Improvement Initiative. BMJ Open Qual. 2025, 14, 2454. [Google Scholar] [CrossRef]
- Chong, S.L.; Goh, M.S.L.; Ong, G.Y.K.; Acworth, J.; Sultana, R.; Yao, S.H.W.; Ng, K.C.; Scholefield, B.; Aickin, R.; Maconochie, I.; et al. Do Paediatric Early Warning Systems Reduce Mortality and Critical Deterioration Events among Children? A Systematic Review and Meta-Analysis. Resusc. Plus 2022, 11, 100262. [Google Scholar] [CrossRef]
- Kurakbayev, Y.; Turdaliyeva, B.; Manzhuova, L.; Shchukin, V.; Aimakhanova, A.; Aitbekov, K. Acute Lymphoblastic Leukemia in Children Hospitalized in the Intensive Care Unit: A Retrospective Analysis. Oncol. Radiol. Kazakhstan 2024, 2, 26–36. [Google Scholar] [CrossRef]
- Messova, A.M.; Zhunusov, Y.; Pivina, L.; Yolcu, S. Triage System: Literature Review, Problems and Solutions in Kazakhstan. Наука и здравоохранение 2018, 5, 23–30. [Google Scholar]
- Karibayeva, I.; Moiynbayeva, S.; Akhmetov, V.; Yerkenova, S.; Shaikova, K.; Moshkalova, G.; Mussayeva, D.; Tarakova, B.; Semenova, Y.; Ivankov, A.; et al. Interrupted Time Series Analysis of the Impact of the COVID-19 Pandemic and Compulsory Social Health Insurance System on Fertility Rates: A Study of Live Births in Kazakhstan, 2019–2023. Front. Public Health 2024, 12, 1454420–1454430. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.; Lane, S.; Siner, S.; Jones, D.; Lambert, C.; Mehta, F.; Eyton-Chong, C.K.; Davis, P.; Fitzsimons, J.; Lim, E.; et al. Assessing the Performance of Paediatric Early Warning Scores to Predict Critical Deterioration Events in Hospitalised Children (the DETECT Study): A Retrospective Matched Case-Control Study. BMC Pediatr. 2025, 25, 520. [Google Scholar] [CrossRef] [PubMed]
- Kurakbayev, Y.; Turdaliyeva, B.; Manzhuova, L.; Omarova, K.; Abdilova, G.; Kusainov, A.; Saparbayev, S.; Schukin, V. International Experience in Applying the System of Pediatric Early Warning Signs of Critical Conditions in Oncological Children: A Literature Review. Oncol. Radiol. Kazakhstan 2023, 68, 69–75. [Google Scholar] [CrossRef]
- Wu, L.; Jin, M.; Wang, R.; Yang, L.; Lai, X.; Yu, L.; Lin, D.; Huang, L.; Zhang, Y.; Zhang, J.; et al. Prognostic Factors of Sepsis in Children with Acute Leukemia Admitted to the Pediatric Intensive Care Unit. Pediatr. Blood Cancer 2023, 70, e30382. [Google Scholar] [CrossRef]
- Agulnik, A.; Muniz-Talavera, H.; Pham, L.T.D.; Chen, Y.; Carrillo, A.K.; Cárdenas-Aguirre, A.; Gonzalez Ruiz, A.; Garza, M.; Conde Morelos Zaragoza, T.M.; Soberanis Vasquez, D.J.; et al. Effect of Paediatric Early Warning Systems (PEWS) Implementation on Clinical Deterioration Event Mortality among Children with Cancer in Resource-Limited Hospitals in Latin America: A Prospective, Multicentre Cohort Study. Lancet Oncol. 2023, 24, 978–988. [Google Scholar] [CrossRef]
- Maccarana, T.; Pillon, M.; Bertozzi, V.; Carraro, E.; Cavallaro, E.; Bonardi, C.M.; Marchetto, L.; Reggiani, G.; Tondo, A.; Rosa, C.; et al. Oncological Pediatric Early Warning Score: A Dedicated Tool to Predict Patient’s Clinical Deterioration and Need for Pediatric Intensive Care Treatment. Pediatr. Hematol. Oncol. 2024, 41, 422–431. [Google Scholar] [CrossRef]
- Churpek, M.M.; Yuen, T.C.; Edelson, D.P. Risk Stratification of Hospitalized Patients on the Wards. Chest 2013, 143, 1758–1765. [Google Scholar] [CrossRef]
- Vaughn, J.L.; Kline, D.; Denlinger, N.M.; Andritsos, L.A.; Exline, M.C.; Walker, A.R. Predictive Performance of Early Warning Scores in Acute Leukemia Patients Receiving Induction Chemotherapy. Leuk. Lymphoma 2017, 59, 1498. [Google Scholar] [CrossRef] [PubMed]
- Simbaña-Rivera, K.; Torres-Roman, J.S.; Julca-Marin, D.; Guerrero, J.; Quispe-Vicuña, C.; Guerrero González, J.A.; Poterico, J.A.; Araujo, J.M. Mortality by Childhood Acute Lymphoblastic Leukemia: A Regional Analysis in Peru and Ecuador. Asian Pac. J. Cancer Prev. 2025, 26, 2879–2887. [Google Scholar] [CrossRef]
- Berkman, A.M.; Andersen, C.R.; Puthenpura, V.; Short, N.J.; Merriman, K.; Swaminathan, M.; Cuglievan, B.; McCall, D.; Dinardo, C.; Nunez, C.; et al. Risk of Early Death after Acute Leukemia Diagnosis among Adolescents and Young Adults. JNCI Cancer Spectr. 2025, 9, pkaf065. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, X.; Gao, C. Global Trends in Childhood Acute Lymphoblastic Leukemia Burden and Quality of Care Inequalities Across Regions, 1990 to 2021: A Systematic Analysis Using Global Burden of Disease Study 2021 Data. Am. J. Clin. Oncol. 2025, 48, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Zini, G.; Bassan, R. Diagnosis and Subclassification of Acute Lymphoblastic Leukemia. Mediterr. J. Hematol. Infect. Dis. 2014, 6, e2014073. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Shah, M.; Mehta, D.; Arora, S.; Patel, N.; Liu, D. Increased Mortality Among Patients with Acute Leukemia Admitted on Weekends Compared to Weekdays. Clin. Lymphoma Myeloma Leuk. 2017, 17, e33–e43. [Google Scholar] [CrossRef]
| Category | Variables |
|---|---|
| Demographic and general | Age (years), sex (male/female), ethnicity (Kazakh/Russian/Other), BMI category (underweight/normal/overweight), number of ICU readmissions during the same hospitalization, number of ICU readmissions across different hospitalizations, total days in hospital, LoS before ICU admission, LoS in ICU and PEWS use based on the period. |
| Clinical history and disease characteristics | Blood group (O[I]/A[II]/B[III]/AB[IV]); Rh factor (positive/negative); ALL subtype (B-lineage/T-lineage/Other); FAB classification (L1/L2/L3); risk group (standard/intermediate/high); CNS leukemia (Yes/No); relapse (None/Once/Twice/Thrice); chemotherapy protocol (AIEOP 2009; ALL-REZ AIEOP 2009; INTERFANT; MLL-Baby AIEOP 2009-006; NHL-BFM 2004); days on chemotherapy. |
| Transfusion history | Total numbers of erythrocyte, platelet, plasma, and albumin transfusions. |
| Vital signs on ICU admission | Temperature, number of hyperthermia episodes within 24 h of ICU admission, heart rate, systolic and diastolic blood pressure, respiratory rate, saturation. |
| Clinical symptoms on ICU admission | SIRS, cough, hemorrhagic syndrome, and cytopenia (each Yes/No). |
| Laboratory parameters on ICU admission | Hemoglobin, erythrocyte count, white-cell count, absolute neutrophil count, platelet count, ESR, ALT, AST, total bilirubin, direct bilirubin, glucose, creatinine, urea, total protein, albumin, CRP, sodium, potassium, calcium, chloride, APTT, PTI, PI, INR, fibrinogen, urine specific gravity, urine pH, proteinuria. |
| Instrumental test results | Ultrasound: splenomegaly, hepatomegaly, lymphadenopathy, pancreatic enlargement, pleural effusion, ascitic fluid (each Yes/No). ECG: tachycardia, bradycardia, arrhythmia, normal. EchoCG: normal, pericarditis, reduced ejection fraction, ventricular hypertrophy. Chest X-ray: normal, bilateral pneumonia, right-sided pneumonia, left-sided pneumonia, lung abscess, pneumothorax with pneumonia. |
| PEWS Use (No) Mean ± SD/Frequency (%) | PEWS Use (Yes) Mean ± SD/Frequency (%) | p-Value | |
|---|---|---|---|
| Demographic and General Characteristics | |||
| Age | 7.54 ± 5.07 | 7.71 ± 5.23 | 0.79 |
| Female gender | 91 (48.4) | 37 (34.58) | 0.03 |
| Ethnicity | 0.85 | ||
| Kazakh | 135 (78.95) | 82 (76.64) | |
| Russian | 10 (5.85) | 8 (7.48) | |
| Other | 26 (15.2) | 17 (15.89) | |
| BMI | 0.04 | ||
| Underweight | 69 (40.35) | 28 (26.17) | |
| Normal weight | 84 (49.12) | 68 (63.55) | |
| Overweight | 18 (10.53) | 11 (10.28) | |
| ICU readmission (same hospitalization) | 0.29 | ||
| 1 | 165 (87.77) | 88 (82.24) | |
| 2 | 20 (10.64) | 15 (14.02) | |
| 3 | 3 (1.60) | 4 (3.74) | |
| ICU readmission (different hospitalizations) | <0.001 | ||
| 0 | 133 (79.64) | 102 (95.33) | |
| 1 | 27 (16.17) | 5 (4.67) | |
| 2 | 5 (2.99) | - | |
| 3 | 2 (1.2) | - | |
| Days in hospital | 38.66 ± 27.41 | 51.81 ± 25.12 | <0.001 |
| LoS before ICU | 15.02 ± 16.37 | 17.39 ± 18.05 | 0.26 |
| LoS in ICU | 6.02 ± 7.62 | 6.81 ± 8 | 0.41 |
| Mortality | 0.05 | ||
| No | 138 (73.40) | 90 (84.11) | |
| Yes | 50 (26.60) | 17 (15.89) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurakbayev, Y.; Kussainov, A.; Umbetov, K.; Zikiriya, Y.; Sarsekbayev, Y.; Turdaliyeva, B.; Nurgozhayeva, N.; Tolemisova, A. Pediatric Early Warning System (PEWS) Association with ICU Mortality in Children with Acute Lymphoblastic Leukemia: A Cohort Study from Kazakhstan. Medicina 2025, 61, 2054. https://doi.org/10.3390/medicina61112054
Kurakbayev Y, Kussainov A, Umbetov K, Zikiriya Y, Sarsekbayev Y, Turdaliyeva B, Nurgozhayeva N, Tolemisova A. Pediatric Early Warning System (PEWS) Association with ICU Mortality in Children with Acute Lymphoblastic Leukemia: A Cohort Study from Kazakhstan. Medicina. 2025; 61(11):2054. https://doi.org/10.3390/medicina61112054
Chicago/Turabian StyleKurakbayev, Yedil, Abay Kussainov, Kuanysh Umbetov, Yernur Zikiriya, Yergali Sarsekbayev, Botagoz Turdaliyeva, Nazira Nurgozhayeva, and Arai Tolemisova. 2025. "Pediatric Early Warning System (PEWS) Association with ICU Mortality in Children with Acute Lymphoblastic Leukemia: A Cohort Study from Kazakhstan" Medicina 61, no. 11: 2054. https://doi.org/10.3390/medicina61112054
APA StyleKurakbayev, Y., Kussainov, A., Umbetov, K., Zikiriya, Y., Sarsekbayev, Y., Turdaliyeva, B., Nurgozhayeva, N., & Tolemisova, A. (2025). Pediatric Early Warning System (PEWS) Association with ICU Mortality in Children with Acute Lymphoblastic Leukemia: A Cohort Study from Kazakhstan. Medicina, 61(11), 2054. https://doi.org/10.3390/medicina61112054






