Exercise Modulation of the Myostatin–FOXO Pathway in Murine Models of Cancer Cachexia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Analysis
2.4. Quality Assessment
2.5. Data Synthesis
3. Results
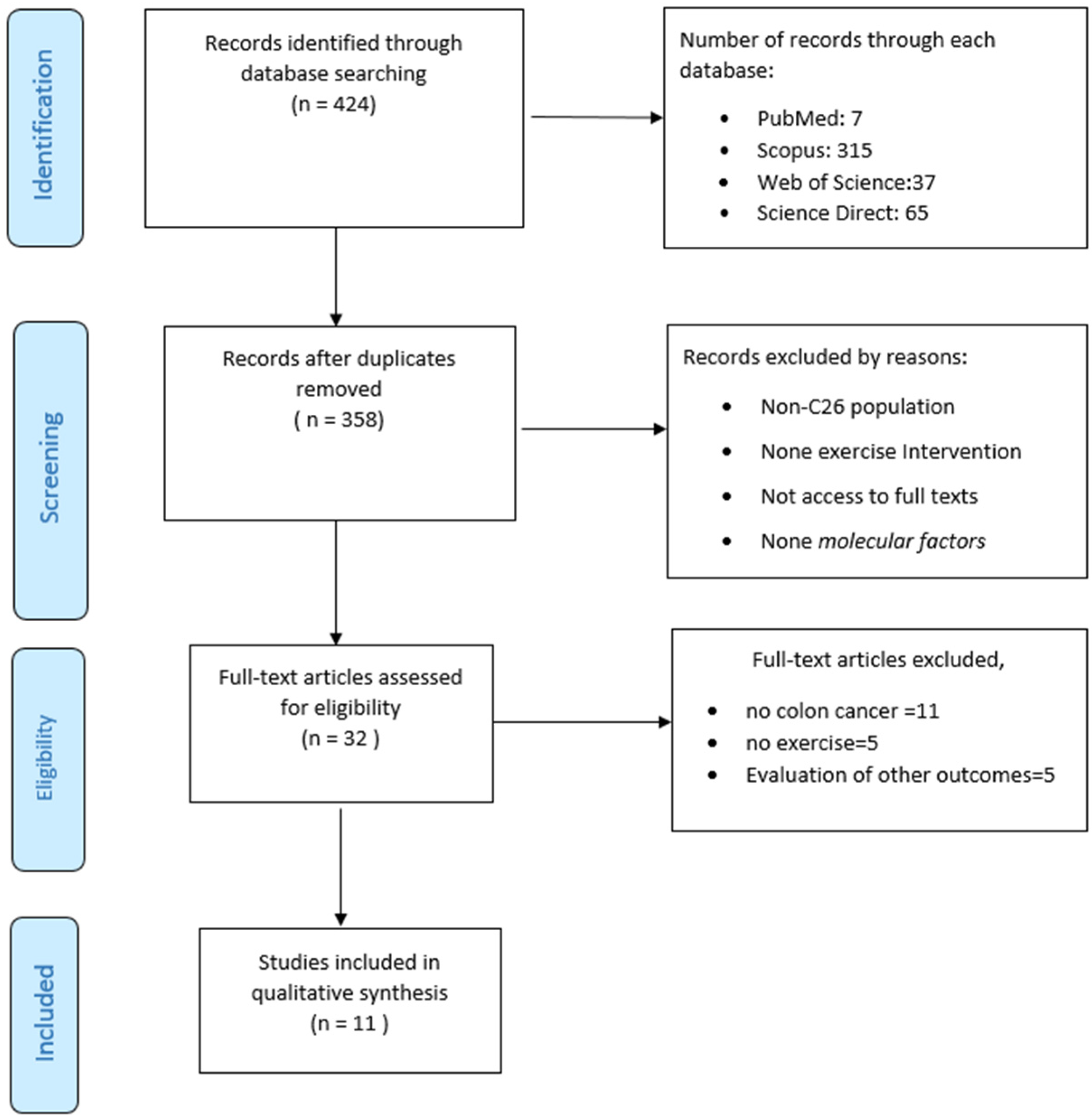
3.1. Study Selection and Characteristics
3.2. Characteristics of Exercise Programs
3.3. Methodological Quality and Risk of Bias
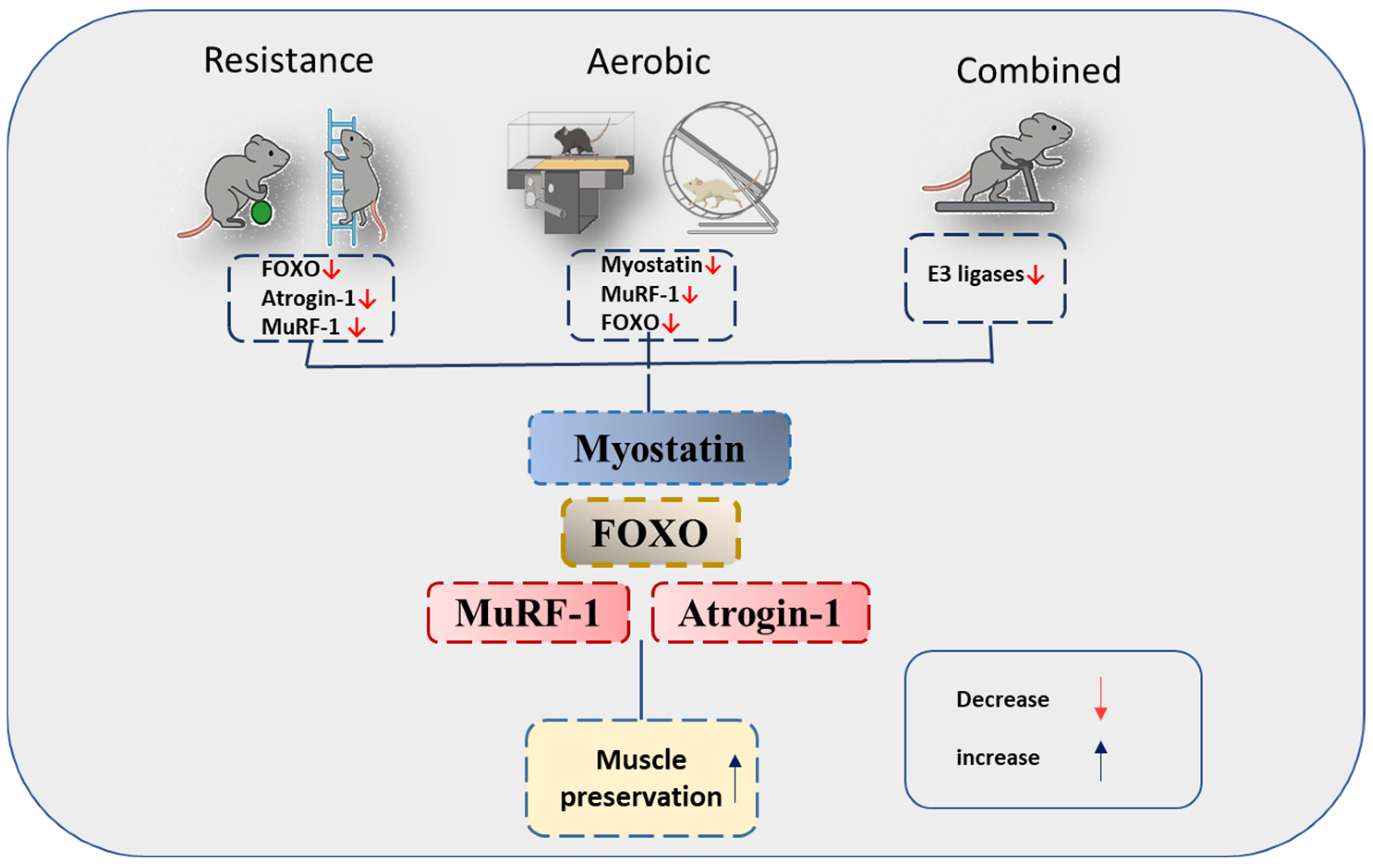
3.4. Outcomes
3.4.1. Myostatin
3.4.2. FOXO
3.4.3. MuRF-1
3.4.4. Atrogin-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tijerina, A.J. The Biochemical Basis of Metabolism in Cancer Cachexia. Dimens. Crit. Care Nurs. 2004, 23, 237–243. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020, 18, 646–654. [Google Scholar] [CrossRef]
- Tan, B.H.; Fearon, K.C. Cytokine gene polymorphisms and susceptibility to cachexia. Curr. Opin. Support. Palliat. Care 2010, 4, 243–248. [Google Scholar] [CrossRef]
- Coletti, D. Chemotherapy-induced muscle wasting: An update. Eur. J. Transl. Myol. 2018, 28, 7587. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.; den Braver, N.R.; Berkhof, J.; Langius, J.A.; Verheul, H.M. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef]
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-induced muscle wasting: Latest findings in prevention and treatment. Ther. Adv. Med. Oncol. 2017, 9, 369–382. [Google Scholar] [CrossRef]
- Han, H.Q.; Mitch, W.E. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr. Opin. Support. Palliat. Care 2011, 5, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Vernus, B.; Chelh, I.; Cassar-Malek, I.; Gabillard, J.-C.; Hadj Sassi, A.; Seiliez, I.; Picard, B.; Bonnieu, A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 2014, 71, 4361–4371. [Google Scholar] [CrossRef]
- Han, H.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347. [Google Scholar] [CrossRef]
- MacKenzie, M.G.; Hamilton, D.L.; Pepin, M.; Patton, A.; Baar, K. Inhibition of myostatin signaling through Notch activation following acute resistance exercise. PLoS ONE 2013, 8, e68743. [Google Scholar] [CrossRef]
- Cole, C.L.; Kleckner, I.R.; Jatoi, A.; Schwarz, E.M.; Dunne, R.F. The role of systemic inflammation in cancer-associated muscle wasting and rationale for exercise as a therapeutic intervention. JCSM Clin. Rep. 2018, 3, 1–19. [Google Scholar] [CrossRef]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef]
- Kasprzak, A. The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int. J. Mol. Sci. 2021, 22, 1565. [Google Scholar] [CrossRef]
- Directo, D.; Lee, S.R. Cancer Cachexia: Underlying Mechanisms and Potential Therapeutic Interventions. Metabolites 2023, 13, 1024. [Google Scholar] [CrossRef]
- Aulino, P.; Berardi, E.; Cardillo, V.M.; Rizzuto, E.; Perniconi, B.; Ramina, C.; Padula, F.; Spugnini, E.P.; Baldi, A.; Faiola, F.; et al. Molecular, cellular and physiological characterization of the cancer cachexia-inducing C26 colon carcinoma in mouse. BMC Cancer 2010, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Macleod, M.R.; O’Collins, T.; Howells, D.W.; Donnan, G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 2004, 35, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Tichy, L.; Allred, K.F.; Rezeli, E.T.; Coleman, M.F.; Allred, C.D.; Hursting, S.D.; Parry, T.L. Concurrent Physical Activity Protects Against C26 Adenocarcinoma Tumor-Mediated Cardiac and Skeletal Muscle Dysfunction and Wasting in Males. Cells 2025, 14, 924. [Google Scholar] [CrossRef] [PubMed]
- Tsitkanou, S.; Koopmans, P.; Peterson, C.; Cabrera, A.R.; Muhyudin, R.; Morena, F.; Khadgi, S.; Schrems, E.R.; Washington, T.A.; Murach, K.A.; et al. Myocellular adaptations to short-term weighted wheel-running exercise are largely conserved during C26-tumour induction in male and female mice. Exp. Physiol. 2025. [Google Scholar] [CrossRef]
- Dalle, S.; Hiroux, C.; Koppo, K. Endocannabinoid remodeling in murine cachexic muscle associates with catabolic and metabolic regulation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 167179. [Google Scholar] [CrossRef]
- Jee, H.; Park, E.; Hur, K.; Kang, M.; Kim, Y. High-intensity aerobic exercise suppresses cancer growth by regulating skeletal muscle-derived oncogenes and tumor suppressors. Front. Mol. Biosci. 2022, 9, 818470. [Google Scholar] [CrossRef] [PubMed]
- Fix, D.K.; Counts, B.R.; Smuder, A.J.; Sarzynski, M.A.; Koh, H.J.; Carson, J.A. Wheel running improves fasting-induced AMPK signaling in skeletal muscle from tumor-bearing mice. Physiol. Rep. 2021, 9, e14924. [Google Scholar] [CrossRef]
- Ranjbar, K.; Ballarò, R.; Bover, Q.; Pin, F.; Beltrà, M.; Penna, F.; Costelli, P. Combined exercise training positively affects muscle wasting in tumor-bearing mice. Med. Sci. Sports Exerc. 2019, 51, 1387–1395. [Google Scholar] [CrossRef]
- Tatebayashi, D.; Himori, K.; Yamada, R.; Ashida, Y.; Miyazaki, M.; Yamada, T. High-intensity eccentric training ameliorates muscle wasting in colon 26 tumor-bearing mice. PLoS ONE 2018, 13, e0199050. [Google Scholar] [CrossRef]
- Hardee, J.P.; Counts, B.R.; Gao, S.; VanderVeen, B.N.; Fix, D.K.; Koh, H.J.; Carson, J.A. Inflammatory signalling regulates eccentric contraction-induced protein synthesis in cachectic skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 369–383. [Google Scholar] [CrossRef]
- Khamoui, A.V.; Park, B.S.; Kim, D.H.; Yeh, M.C.; Oh, S.L.; Elam, M.L.; Jo, E.; Arjmandi, B.H.; Salazar, G.; Grant, S.C.; et al. Aerobic and resistance training dependent skeletal muscle plasticity in the colon-26 murine model of cancer cachexia. Metab. Clin. Exp. 2016, 65, 685–698. [Google Scholar] [CrossRef]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M. Aerobic exercise and pharmacological treatments counteract cachexia by modulating autophagy in colon cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef]
- Pin, F.; Busquets, S.; Toledo, M.; Camperi, A.; Lopez-Soriano, F.J.; Costelli, P.; Argilés, J.M.; Penna, F. Combination of exercise training and erythropoietin prevents cancer-induced muscle alterations. Oncotarget 2015, 6, 43202. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Zhao, Y.-P.; Zhao, Y.; Deng, S.-L.; Yu, K. Regulation of myostatin on the growth and development of skeletal muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.L.; Unterman, T.G. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am. J. Physiol. Cell Physiol. 2007, 292, C188–C199. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, L.; Dieterlen, M.T.; Klaeske, K.; Haunschild, J.; Saeed, D.; Eifert, S.; Borger, M.A.; Jawad, K. Myostatin/AKT/FOXO Signaling Is Altered in Human Non-Ischemic Dilated Cardiomyopathy. Life 2022, 12, 1418. [Google Scholar] [CrossRef]
- Oyabu, M.; Takigawa, K.; Mizutani, S.; Hatazawa, Y.; Fujita, M.; Ohira, Y.; Sugimoto, T.; Suzuki, O.; Tsuchiya, K.; Suganami, T. FOXO1 cooperates with C/EBPδ and ATF4 to regulate skeletal muscle atrophy transcriptional program during fasting. FASEB J. 2022, 36, e22152. [Google Scholar] [CrossRef]
- Du, S.; Zheng, H. Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases. Cell Biosci. 2021, 11, 188. [Google Scholar] [CrossRef]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef]
- Yuan, L.; Han, J.; Meng, Q.; Xi, Q.; Zhuang, Q.; Jiang, Y.; Han, Y.; Zhang, B.; Fang, J.; Wu, G. Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 2261–2268. [Google Scholar] [CrossRef]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) | Animal Model | Intervention | Comparison | Myostatin Pathway | Key Findings |
|---|---|---|---|---|---|
| Tichy et al. (2025) [23] | Male BALB/c mice, N = 10/group, 40 total (4 groups) | 4 weeks VWR | Sedentary non- tumor-bearing, tumor-bearing | MuRF-1, Atrogin-1, GDF8 (Western blot) | Tumor-bearing: ↑ MuRF-1, Atrogin-1, GDF8/11, muscle/cardiac wasting. VWR: ↓ tumor burden, ↓ MuRF-1, Atrogin-1, GDF8/11, GDF15 VWR reduced myostatin signaling and preserved muscle mass. |
| Tsitkanou et al. (2025) [24] | BALB/c mice, N = 8–11/group, 72 total (4 groups) | 4 weeks weighted VWR progressive loading (up to 4 g/day) | Healthy mice, tumor-bearing sedentary | MuRF-1, Atrogin-1 (RT-PCR) | C26: ↑ Atrogin-1/MuRF-1 VWR: ↓ Atrogin-1/MuRF-1, ↑ PGC1α Weighted running maintained fiber type and reduced catabolic genes. |
| Dalle et al. (2024) [25] | Male BALB/c mice, N = 5–6/group, 21 total (4 groups) | 16 days of VWR | Healthy controls, sedentary C26 mice | MuRF-1, Atrogin-1, FOXO (Western blot) | VWR: ↓ p-FOXO, ↔ MuRF-1/Atrogin-1, Short-term VWR attenuated FOXO activation without significant changes in E3 ligases, suggesting an anti-catabolic effect via FOXO pathway |
| Jee et al. (2022) [26] | Male CDF1 mice, N = 10/group, 40 total (4 groups) | 18 days high-intensity aerobic (90% max heart rate) | Healthy mice, tumor-bearing sedentary | MuRF-1 (RNA-seq) | Exercise: ↓ tumor growth, ↓ MuRF-1, ↓ CT26 proliferation (~20%) Short-term high-intensity aerobic exercise suppressed MuRF-1 expression and tumor growth, enhancing anabolic balance and overall survival in CRC-induced cachexia. |
| Fix et al. (2021) [27] | Male C57BL/6, ApcMin/+ mice, N = 6–10/group, 54 total (2 groups) | 4 weeks VWR, 12 h fast | Sedentary MIN, B6 mice | FOXO, MuRF-1, Atrogin-1 (Western blot) | VWR: ↓ AMPK, MuRF-1, autophagy, Atrogin-1, FOXO, ↑ mitochondrial quality VWR suppressed proteolytic genes and improved mitochondrial quality. |
| Ranjbar et al. (2019) [28] | Male BALB/c mice N = 6/group, 24 total (4 groups) | 6 weeks total (4 weeks pre- tumor + 11 days post-implantation); resistance: ladder climbing (20–50% BW); aerobic: motorized wheel (25 min/day, 4 days/week) | Healthy controls, tumor-bearing sedentary | MuRF-1, Atrogin-1 (RT-PCR) | C26 tumor: ↑ Atrogin-1, MuRF-1, ↓ muscle mass/strength. Combined exercise: ↔ Atrogin-1/MuRF-1 Combined (resistance + aerobic) training partially prevented muscle wasting and strength loss in C26- bearing mice by modulating autophagy and preserving mitochondrial function |
| Tatebayashi et al. (2018) [29] | Male CD2F1 mice, N = 5–7/group, 23 total (4 groups) | Eccentric contractions (acute, 14 sessions) | Tumor-bearing sedentary, healthy controls | Atrogin-1, MuRF-1, FOXO1 (RT-PCR) | ↑ mTORC1 signaling (p-p70S6K, p-rpS6), ↓ MuRF-1, ↔ Atrogin-1/FOXO1/REDD1, preserved gastrocnemius mass ECC-ES enhanced protein synthesis through mTORC1 activation and partially reduced FOXO1-driven proteolysis. |
| Hardee et al. (2018) [30] | C57BL/6, ApcMin/+ male mice, N = 42 total | Single session eccentric contractions | Healthy controls | MuRF-1, Atrogin-1 (Western blot) | MuRF-1 ↔, Atrogin-1 ↔ Acute ECC activated AKT/ERK but not E3 ligases. |
| Khamoui et al. (2016) [31] | BALB/c mice, N = 16–17/group, 49 total (3 groups) | 11 weeks (8 pre + 3 post tumor); treadmill 5–7 m/min; 5 days/week ladder 50–100% BW 3 days/week | Control, C26 | Atrogin-1, MuRF-1, myostatin (RT-PCR) | RT + C26 and AT + C26: ↔ Atrogin-1/MuRF-1/ myostatin, ↑ IGF-1Ea (RT), ↑ p-mTOR (AT), ↑ CSA/strength/muscle mass Aerobic enhanced mTOR activation; resistance promoted regeneration with limited anti-cachectic effect. |
| Pigna et al. (2016) [32] | Male BALB/c mice N = 7–8/group | 5 or 19 days VWR + AICAR or rapamycin | Tumor-bearing sedentary, Control | Atrogin-1, MuRF-1 (RT-PCR) | VWR: ↓ Atrogin-1, MuRF-1, counteracted muscle atrophy Short-term VWR suppressed proteolytic E3 ligases and mitigated tumor-induced muscle loss, independent of AMPK/mTOR modulation. |
| Pin et al. (2015) [33] | BALB/c, C57BL/6, male/female N = 6/group, ~24 total | 2 weeks low-intensity endurance (acute) or 8 weeks (6 pre-tumor, 2 post-tumor) + EPO | Control, EX-only, EPO-only, tumor-bearing | Atrogin-1, MuRF-1 (qPCR) | C26: ↑ Atrogin-1, ↑ MuRF-1 in sedentary TB; EX-only ↔ Atrogin-1/MuRF-1 (no protection); EX + EPO ↓ atrophy, ↑ PGC-1α, restored oxidative fibers Low-intensity EX alone failed or worsened atrophy, but EX + EPO activated PGC-1α and improved muscle oxidative phenotype, partially suppressing catabolic genes. |
| Molecular Target | Exercise Type(s) | Number of Studies | Direction of Change | Summary of Evidence |
|---|---|---|---|---|
| Myostatin (GDF-8) | Aerobic, Combined | 2 | ↓ or ↔ | two studies assessed myostatin; aerobic and combined exercise tended to lower or maintain basal levels. |
| FOXO1/3 | Aerobic, Resistance | 3 | ↓ | Most exercise modalities suppressed FOXO activation, reducing downstream proteolytic signaling. |
| MuRF-1 (Trim63) | Aerobic, Resistance, Combined | 11 | ↓ | Consistently downregulated across protocols; indicates inhibition of ubiquitin–proteasome activity. |
| Atrogin-1 (Fbxo32) | Aerobic, Resistance, Combined | 10 | ↓ | Closely follows FOXO suppression pattern; reduced expression observed in most models. |
| Author (Year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tichy et al. (2025) [23] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Tsitkanou et al. (2025) [24] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Dalle et al.(2024) [25] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Jee et al.(2022) [26] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Fix et al. (2021) [27] | Y | Y | Y | N | N | Y | Y | N | N | Y | 6 |
| Ranjbar et al. (2019) [28] | Y | N | Y | N | N | Y | Y | N | Y | Y | 6 |
| Tatebayashi et al. (2018) [29] | Y | Y | Y | N | N | Y | N | N | Y | Y | 6 |
| Hardee et al.(2018) [30] | Y | Y | Y | N | Y | Y | Y | N | Y | Y | 8 |
| Khamoui et al. (2016) [31] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Pigna et al. (2016) [32] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Pin et al.(2015) [33] | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare, Z.; Al Kitani, M.; Shahrbanian, S. Exercise Modulation of the Myostatin–FOXO Pathway in Murine Models of Cancer Cachexia: A Systematic Review. Medicina 2025, 61, 2022. https://doi.org/10.3390/medicina61112022
Zare Z, Al Kitani M, Shahrbanian S. Exercise Modulation of the Myostatin–FOXO Pathway in Murine Models of Cancer Cachexia: A Systematic Review. Medicina. 2025; 61(11):2022. https://doi.org/10.3390/medicina61112022
Chicago/Turabian StyleZare, Zahra, Mahfoodha Al Kitani, and Shahnaz Shahrbanian. 2025. "Exercise Modulation of the Myostatin–FOXO Pathway in Murine Models of Cancer Cachexia: A Systematic Review" Medicina 61, no. 11: 2022. https://doi.org/10.3390/medicina61112022
APA StyleZare, Z., Al Kitani, M., & Shahrbanian, S. (2025). Exercise Modulation of the Myostatin–FOXO Pathway in Murine Models of Cancer Cachexia: A Systematic Review. Medicina, 61(11), 2022. https://doi.org/10.3390/medicina61112022






