1. Introduction
Pancreatic cancer (PC), predominantly pancreatic ductal adenocarcinoma (PDAC), remains one of the most lethal malignancies worldwide, with a 5-year survival rate of approximately 10% [
1]. According to the GLOBOCAN 2022 report, 510,992 new cases were diagnosed globally, ranking PC 12th among all cancers [
2]. Recent global epidemiologic updates continue to show a rising incidence and mortality of pancreatic cancer despite advances in systemic therapies. The poor prognosis of this malignancy is primarily due to its early systemic dissemination, high metastatic potential, and resistance to conventional chemotherapy [
3,
4,
5].
The aggressiveness and lethality of PDAC are largely determined by its highly desmoplastic and immunosuppressive tumor microenvironment, characterized by dense stroma, abundant inflammatory infiltrates, and immunosuppressive signaling that collectively promote tumor progression and therapeutic resistance [
4]. This inflammatory milieu not only supports tumor growth but also impairs host antitumor immunity, emphasizing the central role of systemic inflammation in pancreatic cancer biology.
Systemic inflammation can be quantified using circulating cytokines, acute-phase proteins, and hematologic cell ratios, which have been shown to correlate with disease progression and survival in several malignancies [
6]. Composite indices derived from complete blood counts, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and prognostic nutritional index (PNI), reflect the balance between tumor-induced inflammation and host immune competence. Among these, the Systemic Inflammation Response Index (SIRI)—calculated as neutrophil × monocyte/lymphocyte—integrates both pro-tumor and antitumor immune elements, providing a broader representation of the inflammatory and immunologic state [
7,
8,
9].
Previous studies have investigated the prognostic value of SIRI in resectable and locally advanced PC [
10,
11]. However, few have focused specifically on patients with metastatic pancreatic cancer, in whom systemic inflammation and immune dysfunction are most pronounced. Moreover, prior work primarily evaluated SIRI after chemotherapy, whereas its prognostic significance at the time of metastasis and before treatment initiation remains insufficiently characterized.
Although Qi et al. (2016) first described the prognostic value of SIRI in pancreatic cancer after chemotherapy [
11], our study provides novel insights by evaluating SIRI at the time of metastasis in a real-world, contemporary cohort treated between 2010 and 2024. Unlike earlier studies, we focused exclusively on metastatic patients receiving first-line and subsequent lines of chemotherapy, analyzed detailed clinical and biochemical parameters, and determined the optimal SIRI cutoff using receiver operating characteristic (ROC) analysis. Therefore, this study expands existing evidence by confirming the independent prognostic value of SIRI in advanced-stage pancreatic cancer and by proposing its integration into routine clinical decision-making.
2. Materials and Methods
2.1. Clinical Characteristics of the Cohort
The retrospective study included 147 patients diagnosed with pancreatic adenocarcinoma who were followed up at the Medical Oncology Department of Manisa Celal Bayar University Hospital between 2010 and 2024. Diagnosis was validated histopathologically. Data were obtained from the hospital system and patients’ oncological records. All collected data were anonymized; therefore, informed consent was not required. This study was conducted in accordance with the Declaration of Helsinki.
The patients were staged using the TNM Classification of Malignant Tumours, 8th edition. In the diagnosis and follow-up of the patients, positron emission tomography, contrast-enhanced computed tomography, contrast-enhanced magnetic resonance imaging, and magnetic resonance cholangiopancreatography examinations were used as standard radiological examinations.
Data on age, gender, performance score, comorbidities, smoking and alcohol use status, symptoms at the time of diagnosis, tumor localization, stage at the time of diagnosis, and chemotherapy history were obtained from the patient files. Carcinoembryonic antigen (CEA), CA-19-9, albumin, globulin, C-reactive protein (CRP), total bilirubin, ALP, neutrophil, lymphocyte, and monocyte values were obtained from the laboratory records of the hospital automation system.
The cutoff value for age was determined as 65, for Eastern Cooperative Oncology Group (ECOG) performance score as 2, and for body mass index (BMI) classification as 25.
Patients with acute infections, recent surgical procedures (within 4 weeks), corticosteroid or granulocyte-colony stimulating factor (G-CSF) use, or any active inflammatory or autoimmune disorders were excluded. For patients presenting with biliary obstruction or cholangitis, baseline laboratory tests were obtained only after biliary drainage and normalization of infection markers (white blood cell count and C-reactive protein).
2.2. Biochemical Analysis and SIRI Calculation
SIRI was calculated using the following formula: SIRI = N × M/L, where N, M, and L represent peripheral neutrophil, monocyte, and lymphocyte counts at the time of metastasis, respectively. The optimal SIRI cutoff values for progression after chemotherapy in the cohort were determined using an ROC analysis. The results of the ROC analysis revealed that the optimal cutoff value for SIRI in this cohort was 1.86.
Progression-free survival (PFS) was calculated as the time interval between the initiation of treatment or best supportive care and the date of the first follow-up imaging showing disease progression. Progression was defined as an increase of more than 20% in the sum of the longest diameters of target lesions or the appearance of any new lesion. Overall survival (OS) was determined as the time from the date of diagnosis of metastasis to the date of death or, for patients who were still alive, the date of the last clinical follow-up.
Baseline hematologic parameters were obtained from blood samples drawn at the time of metastatic disease diagnosis, before initiation of any systemic chemotherapy or best supportive care.
2.3. Statistical Analysis
For statistical analysis, Statistical Package for the Social Sciences (SPSS) 15.0 (SPSS Inc., Chicago, IL, USA) was used. Descriptive statistics were presented as frequencies and percentages for categorical variables, and as mean, standard deviation, minimum, and maximum values for numerical variables. Survival rates were calculated using Kaplan–Meier analysis, while risk factors were assessed using Cox regression analysis. Because SIRI distribution was markedly right-skewed, it was log-transformed prior to inclusion in multivariate Cox regression models. This normalization reduced collinearity and resulted in a higher hazard ratio when adjusted for covariates. A statistical significance level of p < 0.05 was considered. Non-normally distributed variables, including SIRI and tumor markers, were presented as median (interquartile range). SIRI values were log-transformed for multivariate analyses to reduce skewness.
2.4. Statement on the Use of Artificial Intelligence
Artificial intelligence tools were used in a limited manner during the preparation of this manuscript. A large language model (ChatGPT, OpenAI, San Francisco, CA, USA; GPT-5) was employed to improve the linguistic clarity of the text, enhance coherence, and standardize English technical terminology. AI assistance was also utilized during the reference management process to check the accuracy of citations, match DOI numbers, and ensure consistency of reference formatting. No AI tools were used for data analysis, interpretation of results, or generation of scientific content. All AI-assisted outputs were carefully reviewed, verified, and edited by the authors, who take full responsibility for the integrity and accuracy of the final manuscript.
3. Results
In this study, the clinical and laboratory characteristics of 147 patients diagnosed with metastatic PC and treated with first-line chemotherapy at our center were retrospectively evaluated for their association with survival outcomes.
The median age at diagnosis was 63.2 years. Among the patients, 108 (73.5%) had an ECOG performance status of 0 or 1. A total of 57 patients (38.8%) had a BMI of 25 or greater. At baseline, 64 patients (43.5%) were active smokers, and 17 (11.6%) reported alcohol use. Regarding comorbidities, diabetes mellitus was present in 51 patients (34.7%) and coronary artery disease in 15 patients (10.2%). At diagnosis, abdominal pain was the most common symptom, observed in 138 patients (93.9%), followed by weight loss in 87 (59.2%) and jaundice in 32 (21.8%). Most patients (n = 117, 79.6%) presented with stage 4 disease. The primary tumor was located in the pancreatic head in 100 cases (68.0%). Surgical resection had not been performed in 102 patients (71.8%). The mean follow-up duration was 20.3 months. Review of outcomes showed that 135 patients (91.8%) had died during the follow-up period (
Table 1). 11 patients had incomplete treatment-related records (missing data on chemotherapy administration or response) and were therefore excluded from treatment-specific analyses.
Baseline laboratory parameters are summarized in
Table 2. The median (IQR) SIRI value was 1.94 (1.02–3.61), reflecting a right-skewed distribution consistent with chronic systemic inflammation. Mean CEA and CA 19-9 levels were elevated across the cohort, supporting the presence of high tumor burden and inflammatory activity.
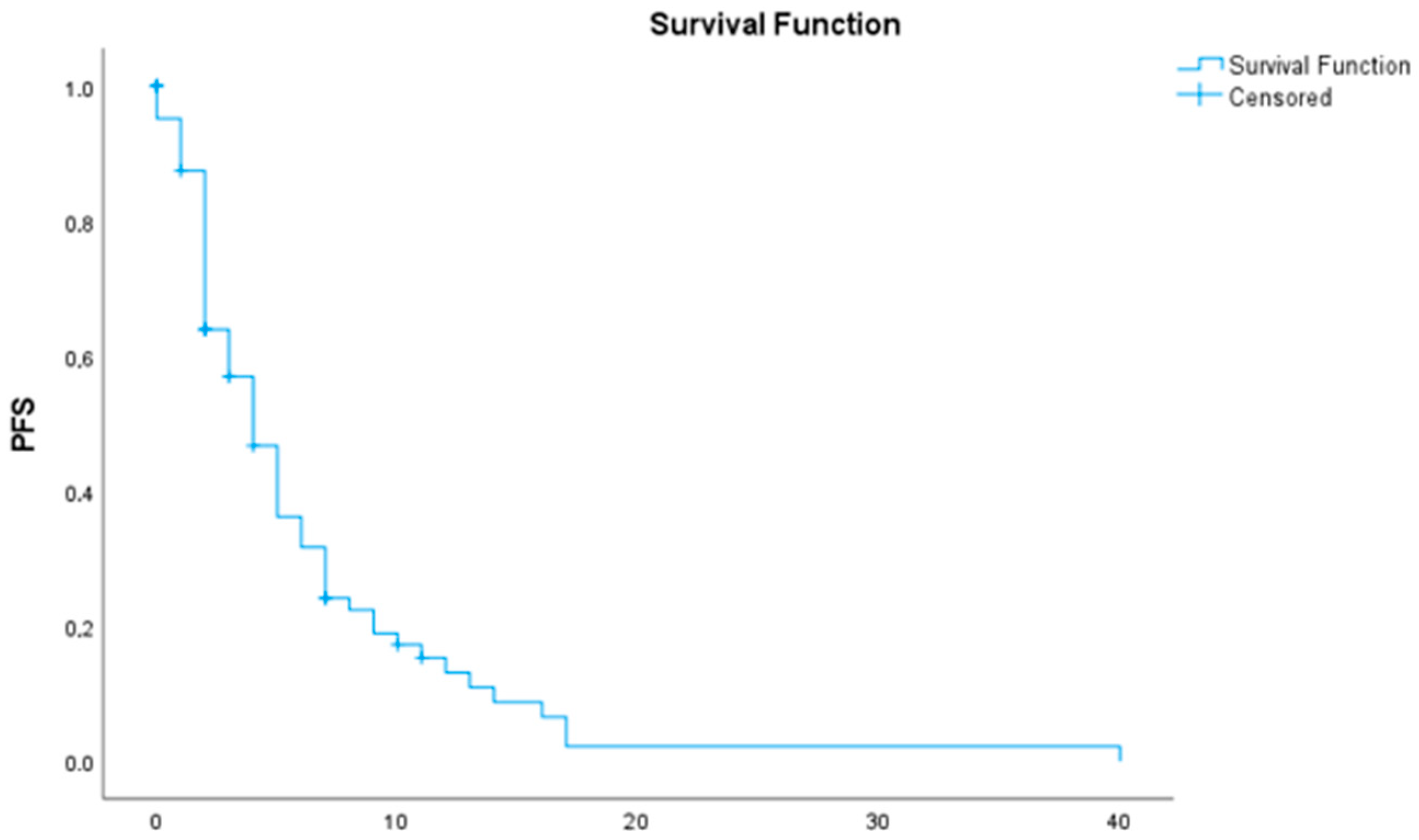
The median overall survival (OS) was 7 months (95% CI: 5.43–8.57) (
Table 3 and
Figure 1). The median progression-free survival (PFS) following first-line chemotherapy was 4 months (95% CI: 2.90–5.10) (
Table 4 and
Figure 2).
In the univariate Cox regression analysis, several clinical and laboratory factors were significantly associated with overall survival (OS). Patients with an ECOG performance status ≥ 2 exhibited a significantly increased risk of mortality compared to those with ECOG 0–1 (HR: 2.645, 95% CI: 1.782–3.925, p < 0.001). The presence of liver metastasis (HR: 2.113, 95% CI: 1.402–3.186, p < 0.001) and metastatic disease at diagnosis (HR: 2.414, 95% CI: 1.559–3.738, p < 0.001) were also associated with a poorer prognosis. Among inflammatory markers, each unit increase in SIRI was correlated with a statistically significant increase in mortality (HR: 1.001, 95% CI: 1.000–1.001, p < 0.001). Higher CEA levels (HR: 1.002, 95% CI: 1.000–1.003, p = 0.006), abdominal pain (HR: 2.525, 95% CI: 1.103–5.779, p = 0.028), and jaundice (HR: 1.685, 95% CI: 1.119–2.536, p = 0.012) were associated with worse OS. Conversely, patients who underwent prior surgery had a significantly lower risk of mortality (HR: 0.370, 95% CI: 0.243–0.565, p < 0.001). Nutritional and hepatic composite markers, such as the Albumin/ALP ratio (p = 0.005), were protective factors.
In the multivariate analysis, only a subset of these factors remained statistically significant. Multivariate Cox regression analysis revealed that patients with ECOG performance status ≥ 2 had a 2.09-fold higher risk of mortality compared to those with ECOG 0–1 (
p = 0.019). The presence of liver metastasis was associated with a 2.03-fold increased risk of death (
p = 0.027). Additionally, each unit increase in the Systemic Inflammation Response Index (SIRI) was associated with a 1.156-fold increase in mortality risk (
p < 0.001). Patients with a SIRI value > 1.86 had significantly shorter survival than those with SIRI ≤ 1.86 (median OS: 4 vs. 9 months,
p = 0.019) (
Table 5 and
Figure 3).
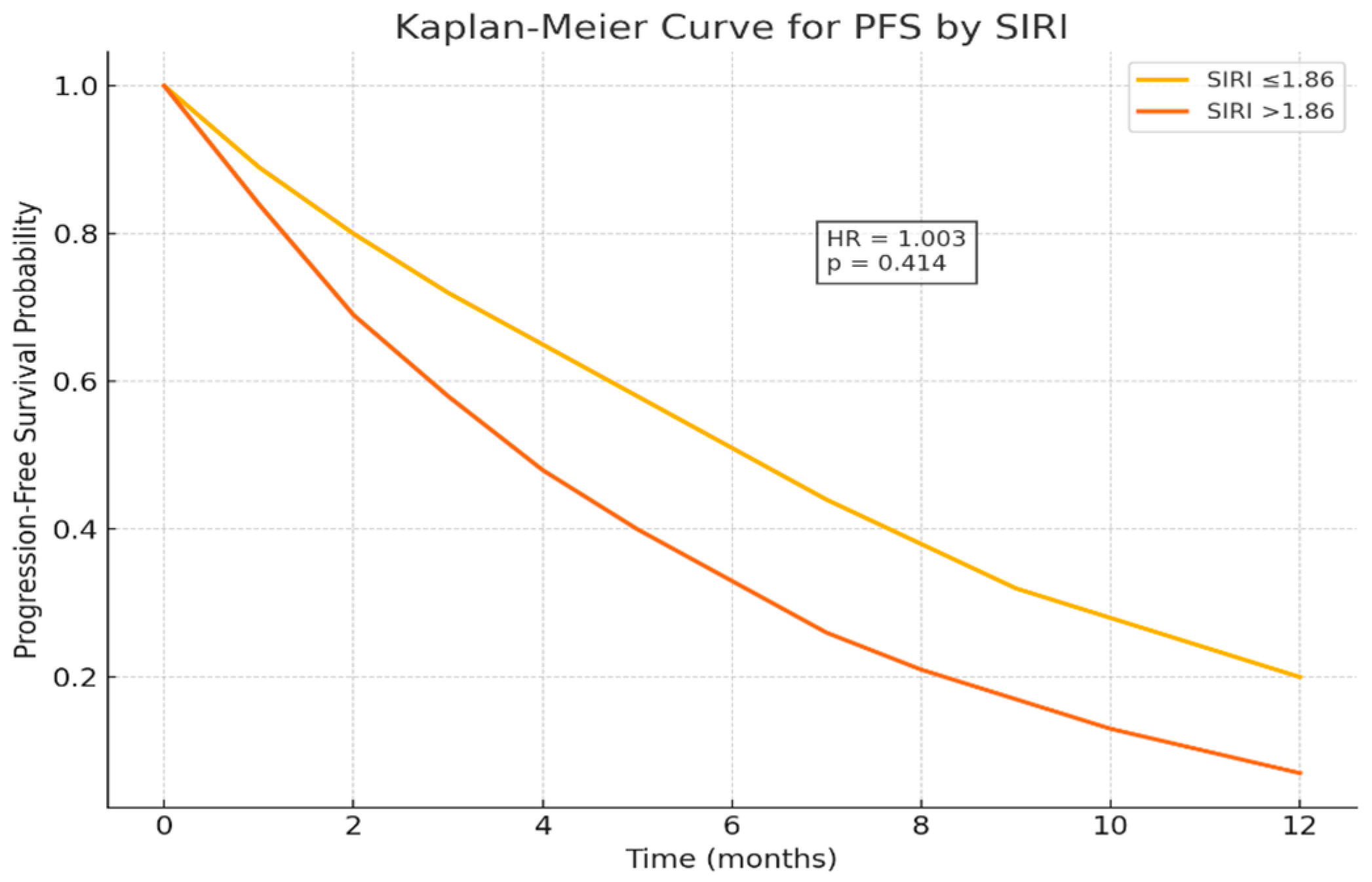
In the univariate Cox regression analysis for progression-free survival (PFS), an increase in carcinoembryonic antigen (CEA) levels was significantly associated with a higher risk of disease progression (HR: 1.003, 95% CI: 1.001–1.004, p = 0.003), highlighting the adverse prognostic impact of tumor burden. Peritoneal metastasis showed a non-significant trend toward shorter PFS (HR: 1.559, 95% CI: 0.926–2.626, p = 0.095). Other clinical parameters, including ECOG performance status ≥ 2 (HR: 1.709, 95% CI: 0.872–3.350, p = 0.118) and jaundice (HR: 1.463, 95% CI: 0.791–2.707, p = 0.225), did not reach statistical significance in the univariate analysis.
In the multivariate model, CEA remained an independent prognostic factor for PFS (HR: 1.003, 95% CI: 1.001–1.005,
p = 0.007), confirming its role as a marker of tumor aggressiveness. ECOG ≥ 2 also retained borderline prognostic relevance, although not statistically significant (HR: 1.802, 95% CI: 0.851–3.816,
p = 0.124). Peritoneal metastasis did not maintain independent significance in the multivariate analysis (
p > 0.05), suggesting that their univariate effects may be influenced by other clinical variables (
Table 6 and
Figure 4).
When analyzed according to SIRI and the novel composite score reflecting tumor-related biological burden, patients with SIRI ≤ 1.86 had a median OS of 9 months and a 1-year OS rate of 38.5%. In contrast, those with SIRI > 1.86 had a median OS of only 4 months and a 1-year OS rate of 24.3%, suggesting a strong association between elevated inflammatory burden and poor prognosis. The 1-year OS rates were Kaplan–Meier estimates at 12 months with 95% confidence intervals (
Table 7).
4. Discussion
This study demonstrates that the Systemic Inflammation Response Index (SIRI) serves as an independent prognostic biomarker in patients with metastatic pancreatic cancer receiving chemotherapy. Elevated SIRI levels (>1.86) were significantly associated with shorter overall survival (OS), confirming that systemic inflammation and immune suppression play pivotal roles in disease progression and treatment resistance. Although SIRI significantly predicted overall survival, it did not correlate with progression-free survival (p = 0.414), suggesting that it reflects long-term systemic inflammatory burden rather than short-term chemotherapy response.
The prognostic significance of SIRI aligns conceptually with other inflammation- and nutrition-based biomarkers, such as the Glasgow Prognostic Score (GPS), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and prognostic nutritional index (PNI) [
6,
12,
13]. GPS, which incorporates C-reactive protein (CRP) and albumin, has long been validated as a robust predictor of survival in gastrointestinal cancers. However, while GPS captures chronic inflammation and nutritional depletion, SIRI offers a more dynamic reflection of the immune–inflammatory equilibrium by integrating neutrophils, monocytes, and lymphocytes. Monocytes, in particular, contribute to tumor-associated macrophage formation and immunosuppression, thereby linking SIRI directly to tumor–immune interactions [
14]. Thus, SIRI provides a more comprehensive view of host–tumor crosstalk and may complement traditional indices like GPS for refined prognostic stratification.
Interestingly, SIRI significantly predicted overall survival but not progression-free survival (p = 0.414). This finding suggests that SIRI primarily reflects the chronic systemic inflammatory status of the host rather than immediate chemotherapy responsiveness. Similar patterns have been reported in other gastrointestinal malignancies, where elevated inflammation indices were linked to post-progression rather than treatment-phase survival.
The mechanistic basis underlying the prognostic impact of SIRI lies in the dual role of inflammation and immunity within the PDAC microenvironment. Neutrophils and monocytes facilitate tumor proliferation, angiogenesis, and invasion by secreting cytokines, chemokines, and matrix-degrading enzymes, while lymphocytes mediate anti-tumor cytotoxicity [
6,
14]. Consequently, a high SIRI reflects both an enhanced pro-tumor inflammatory response and a diminished adaptive immune capacity. This imbalance is particularly relevant in pancreatic cancer, characterized by desmoplasia, hypoxia, and immune exclusion. The integration of SIRI with emerging immunologic biomarkers—such as PD-L1 expression, tumor-infiltrating lymphocytes, and cytokine profiles—could further elucidate the interplay between systemic inflammation and immune suppression in PDAC. The prognostic significance of SIRI for OS but not PFS may reflect its role as a marker of long-term systemic inflammation and immune exhaustion rather than short-term chemotherapy sensitivity. Chronic inflammatory status influences survival through sustained tumor-promoting cytokine release and immune suppression even after disease progression.
Pancreatic ductal adenocarcinoma develops within a dense desmoplastic stroma characterized by activated fibroblasts and tumor-associated macrophages that secrete pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). These cytokines sustain systemic inflammation and immune suppression. Peripheral leukocyte-based indices such as SIRI serve as inexpensive surrogates for this cytokine-driven biology, providing a clinically accessible link between tumor microenvironment and host response.
From a clinical standpoint, SIRI represents an inexpensive, easily obtainable, and reproducible biomarker that can be derived from routine blood counts without additional cost. Its incorporation into baseline assessment and longitudinal monitoring could enhance risk stratification, early identification of aggressive disease, and prediction of treatment response. Patients with persistently elevated SIRI values may exhibit biologically aggressive tumors and might benefit from intensified therapeutic strategies or early inclusion in clinical trials targeting the inflammatory microenvironment.
Furthermore, our findings indicate that poor performance status (ECOG ≥ 2) and liver metastasis were also independent predictors of worse OS, consistent with established prognostic determinants in metastatic PC [
4,
5]. Elevated carcinoembryonic antigen (CEA) levels were additionally associated with shorter progression-free survival (PFS), reflecting higher tumor burden and more aggressive disease biology [
15].