Clinical Outcomes of Full-Thickness Macular Holes with Epiretinal Proliferation Without Posterior Vitreous Detachment
Abstract
1. Introduction
1.1. Patients and Methods
1.2. Evaluations Using Swept-Source Optical Coherence Tomography
1.3. Surgical Procedures
1.4. Statistical Analyses
2. Results
2.1. Demographics
2.2. Clinical Characteristics and Clinical Course of FTMHs
2.3. Visual Acuity
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schimel, A.M.; Fisher, Y.L.; Flynn, H.W., Jr. Optical coherence tomography in the diagnosis and management of diabetic macular edema: Time-domain versus spectral-domain. Ophthalmic Surg. Lasers Imaging Retina 2011, 42, S41–S55. [Google Scholar] [CrossRef]
- Pang, C.E.; Spaide, R.F.; Freund, K.B. Epiretinal proliferation seen in association with lamellar macular holes: A distinct clinical entity. Retina 2014, 34, 1513–1523. [Google Scholar] [CrossRef]
- Itoh, Y.; Levison, A.L.; Kaiser, P.K.; Srivastava, S.K.; Singh, R.P.; Ehlers, J.P. Prevalence and characteristics of hyporeflective preretinal tissue in vitreomacular interface disorders. Br. J. Ophthalmol. 2016, 100, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Hubschman, J.P.; Govetto, A.; Spaide, R.F.; Schumann, R.; Steel, D.; Figueroa, M.S.; Sebag, J.; Gaudric, A.; Staurenghi, G.; Haritoglou, C.; et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br. J. Ophthalmol. 2020, 104, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.T.; Chen, S.N.; Yang, C.M. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: Clinical and surgical findings. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 629–638. [Google Scholar] [CrossRef]
- Lai, T.T.; Yang, C.M. Lamellar hole-associated epiretinal proliferation in lamellar macular hole and full-thickness macular hole in high myopia. Retina 2018, 38, 1316–1323. [Google Scholar] [CrossRef]
- Takahashi, H.; Inoue, M.; Itoh, Y.; Koto, T.; Hirota, K.; Kita, Y.; Hirakata, A. Macular dehiscence-associated epiretinal proliferation in eyes with full-thickness macular hole. Retina 2020, 40, 273–281. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Hsieh, Y.T.; Yang, C.M. Epiretinal membrane-induced full-thickness macular holes: The clinical features and surgical outcomes. Retina 2016, 36, 1679–1687. [Google Scholar] [CrossRef]
- Duker, J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D. Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am. J. Ophthalmol. 1995, 119, 752–759. [Google Scholar] [CrossRef]
- Govetto, A.; Dacquay, Y.; Farajzadeh, M.; Platner, E.; Hirabayashi, K.; Hosseini, H.; Schwartz, S.D.; Hubschman, J.P. Lamellar macular hole: Two distinct clinical entities? Am. J. Ophthalmol. 2016, 164, 99–109. [Google Scholar] [CrossRef]
- Compera, D.; Entchev, E.; Haritoglou, C.; Scheler, R.; Mayer, W.J.; Wolf, A.; Kampik, A.; Schumann, R.G. Lamellar hole-associated epiretinal proliferation in comparison to epiretinal membranes of macular pseudoholes. Am. J. Ophthalmol. 2015, 160, 373–384.e1. [Google Scholar] [CrossRef]
- Asaad, S.Z. Full-thickness macular hole progressing from lamellar macular hole with epiretinal proliferation. Case Rep. Ophthalmol. 2021, 12, 134–141. [Google Scholar] [CrossRef]
- Frisina, R.; Gius, I.; Palmieri, M.; Finzi, A.; Tozzi, L.; Parolini, B. Myopic traction maculopathy: Diagnostic and management strategies. Clin. Ophthalmol. 2020, 14, 3699–3708. [Google Scholar] [CrossRef]
- Hayashi, K.; Manabe, S.I.; Hirata, A.; Yoshimura, K. Posterior vitreous detachment in highly myopic patients. Investig. Ophthalmol. Vis. Sci. 2020, 61, 33. [Google Scholar] [CrossRef]
- Pang, C.E.; Spaide, R.F.; Freund, K.B. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina 2015, 35, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Haritoglou, C.; Gass, C.T.; Schaumberger, M.; Ulbig, M.W.; Kampik, A. Macular hole size as a prognostic factor in macular hole surgery. Br. J. Ophthalmol. 2002, 86, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Kato, T.; Hayashi, A. Epiretinal proliferation embedding combined with internal limiting membrane flap inversion for secondary macular hole: Two case reports. Am. J. Ophthalmol. Case Rep. 2022, 29, 101774. [Google Scholar] [CrossRef] [PubMed]
- Shiono, A.; Kogo, J.; Sasaki, H.; Yomoda, R.; Jujo, T.; Tokuda, N.; Kitaoka, Y.; Takagi, H. Hemi-temporal internal limiting membrane peeling is as effective and safe as conventional full peeling for macular hole surgery. Retina 2019, 39, 1779–1785. [Google Scholar] [CrossRef]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 2010, 117, 2018–2025. [Google Scholar] [CrossRef]
- Takahashi, K.; Morizane, Y.; Kimura, S.; Shiode, Y.; Doi, S.; Okanouchi, T.; Takasu, I.; Inoue, Y.; Shiraga, F. Results of lamellar macular hole-associated epiretinal proliferation embedding technique for the treatment of degenerative lamellar macular hole. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2147–2154. [Google Scholar] [CrossRef] [PubMed]

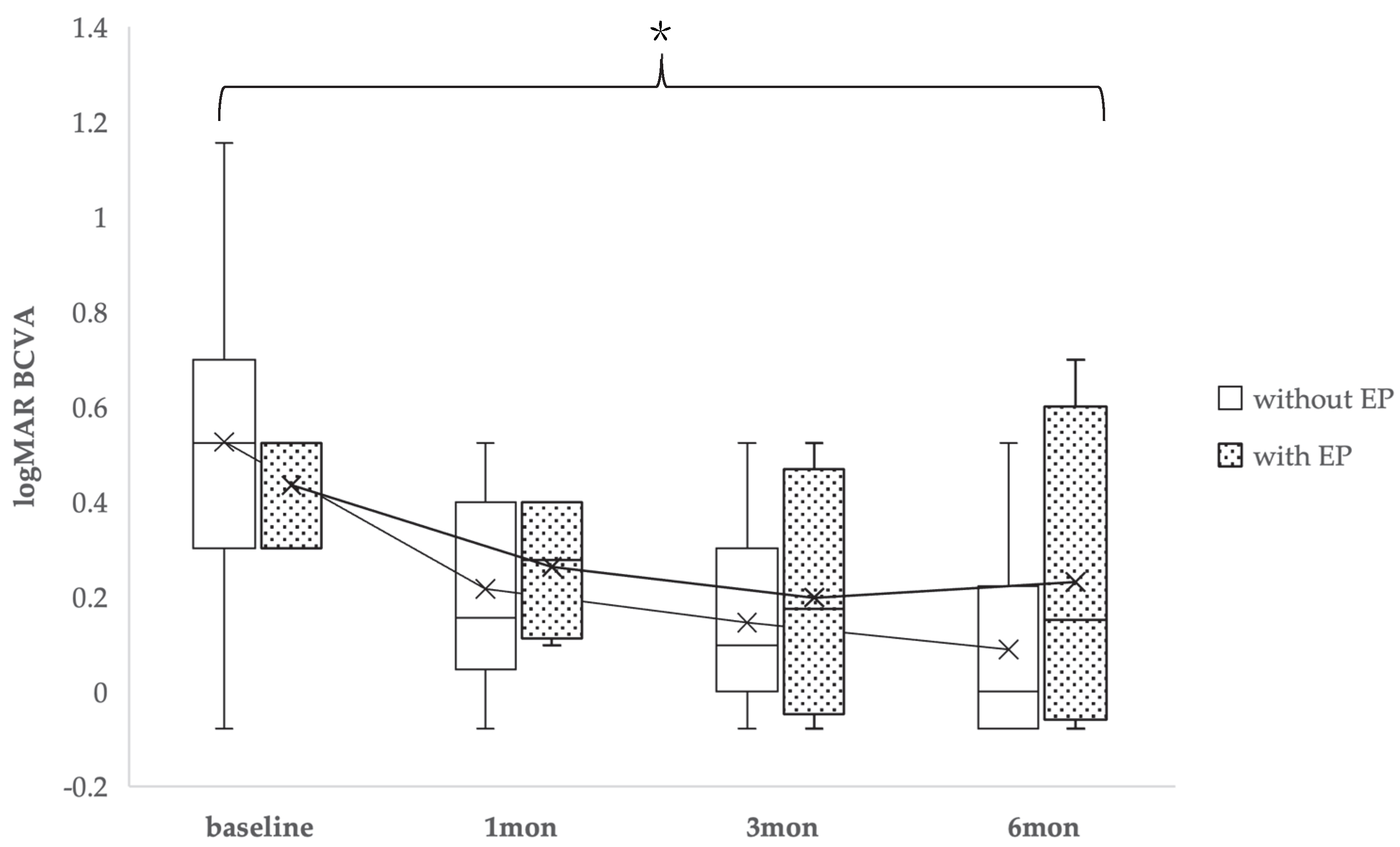

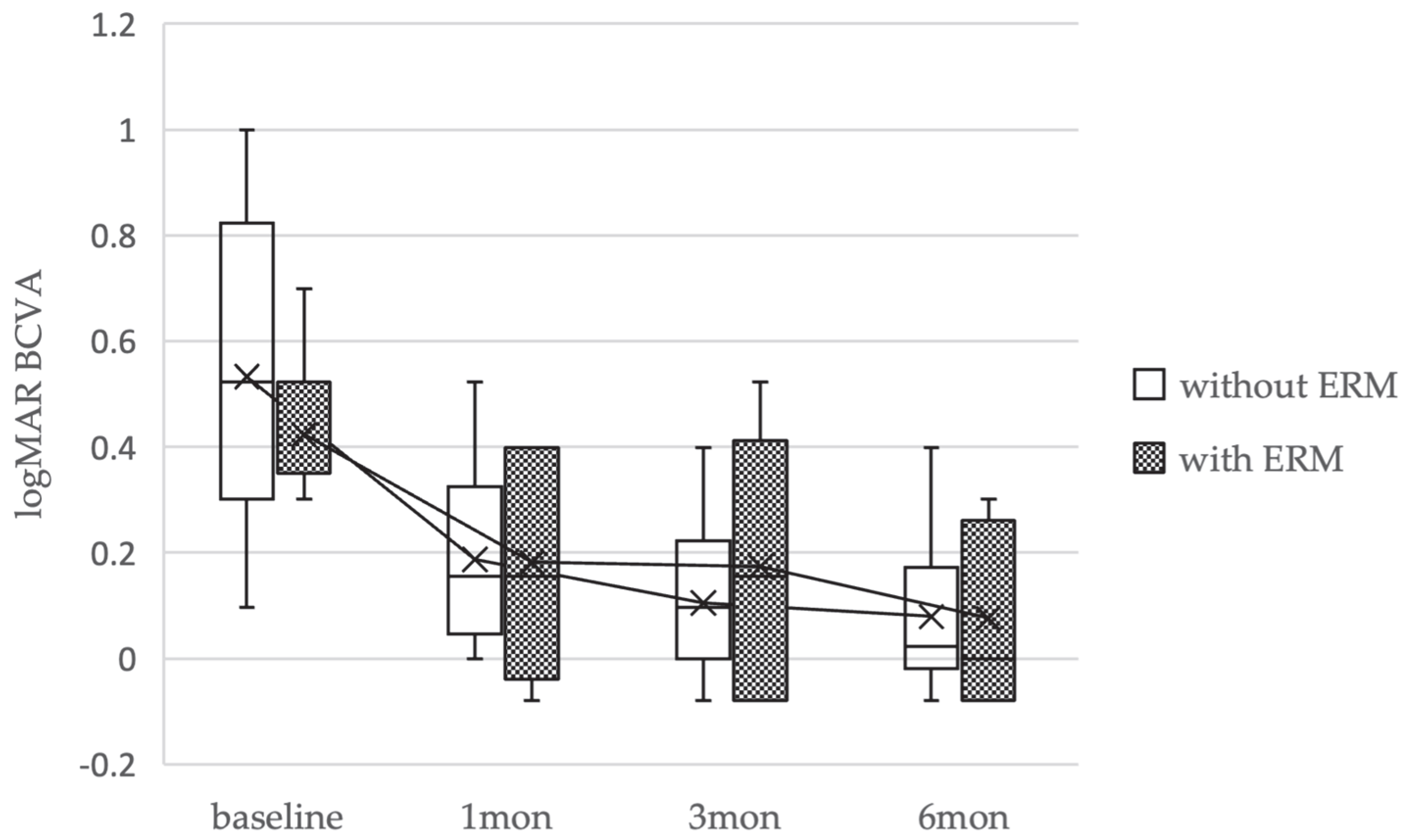
| Baseline Characteristics | FTMHs Without EP and Without PVD (Non-EP Group) (32 Eyes, 32 Patients) | FTMHs with EP Without PVD (EP Group) (5 Eyes, 5 Patients) | p Value |
|---|---|---|---|
| Age: mean ± SD (years) | 66.6 ± 9.0 | 63.4 ± 8.1 | 0.25 |
| Sex: n (%) | |||
| Male | 13 (41) | 2 (40) | |
| Female | 19 (59) | 3 (60) | |
| BCVA: logMAR, mean ± SD | 0.46 ± 0.20 | 0.32 ± 0.17 | 0.04 |
| Axial length (mm, mean ± SD) | 24.8 ± 1.9 | 24.3 ± 1.7 | 0.2 |
| Combined cataract surgery: n (%) | 27 (84%) | 5 (100%) | |
| Without combined cataract surgery: n (%) | 5 (16%) | 0 (0%) | |
| Pseudophakia | 5 | 0 | |
| Minimum MH size ± SD (μm) | 261.3 ± 145.6 | 285.6 ± 147.4 | 0.35 |
| Basal MH size ± SD (μm) | 546.5 ± 309.1 | 702.6 ± 397.4 | 0.16 |
| ERM: n (%) | 9 (28) | 5 (100) | 0.004 |
| Baseline Characteristics | FTMHs Without ERM and Without PVD (23 Eyes, 23 Patients) | FTMHs with ERM and Without PVD (9 Eyes, 9 Patients) | p Value |
|---|---|---|---|
| Age: mean ± SD (years) | 66.7 ± 7.6 | 66.2 ± 12.3 | 0.46 |
| Sex: n (%) | |||
| Male | 10 (43) | 3 (33) | |
| Female | 13(57) | 6 (66) | |
| BCVA: logMAR, mean ± SD | 0.49 ± 0.20 | 0.37 ± 0.15 | 0.09 |
| Axial length (mm, mean ± SD) | 24.7 ± 1.8 | 24.8 ± 1.9 | 0.42 |
| Combined cataract surgery: n (%) | 19 (83%) | 8 (88%) | |
| Without combined cataract surgery: n (%) | 4 (17%) | 1 (11%) | |
| Pseudophakia | 4 | 1 | |
| Minimum MH size ± SD (μm) | 257 ± 96.1 | 272 ± 237.7 | 0.14 |
| Basal MH size ± SD (μm) | 516 ± 316.1 | 623.4 ± 293.4 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakehashi, K.; Sekine, R.; Jujo, T.; Uchiyama, N.; Endo, A.; Tokuda, N.; Kitaoka, Y. Clinical Outcomes of Full-Thickness Macular Holes with Epiretinal Proliferation Without Posterior Vitreous Detachment. Medicina 2025, 61, 1975. https://doi.org/10.3390/medicina61111975
Kakehashi K, Sekine R, Jujo T, Uchiyama N, Endo A, Tokuda N, Kitaoka Y. Clinical Outcomes of Full-Thickness Macular Holes with Epiretinal Proliferation Without Posterior Vitreous Detachment. Medicina. 2025; 61(11):1975. https://doi.org/10.3390/medicina61111975
Chicago/Turabian StyleKakehashi, Kota, Reio Sekine, Tatsuya Jujo, Naoto Uchiyama, Akiko Endo, Naoto Tokuda, and Yasushi Kitaoka. 2025. "Clinical Outcomes of Full-Thickness Macular Holes with Epiretinal Proliferation Without Posterior Vitreous Detachment" Medicina 61, no. 11: 1975. https://doi.org/10.3390/medicina61111975
APA StyleKakehashi, K., Sekine, R., Jujo, T., Uchiyama, N., Endo, A., Tokuda, N., & Kitaoka, Y. (2025). Clinical Outcomes of Full-Thickness Macular Holes with Epiretinal Proliferation Without Posterior Vitreous Detachment. Medicina, 61(11), 1975. https://doi.org/10.3390/medicina61111975






