The Change in Healthcare-Associated Infections in Intensive Care Units Associated with the Coronavirus Disease 2019 in Taiwan
Abstract
1. Introduction
2. Materials and Methods
Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAIs | Healthcare-associated infections |
| DAHAIs | Device-associated HAIs |
| ICU | Intensive care unit |
| OR | Odds ratio |
| CRKP | Carbapenem-resistant K. pneumoniae |
| VREfm | Vancomycin-resistant Enterococcus faecium |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| MCs | Medical centers |
| COVID-19 | Coronavirus disease 2019 |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| AMR | Antimicrobial resistance |
| RHs | Regional hospitals |
| BSIs | Bloodstream infections |
| UTIs | Urinary traction infections |
| HAP | Healthcare-acquired pneumonia |
| CLABSIs | Central line-associated BSIs |
| CAUTIs | Catheter-associated UTIs |
| VAPs | Ventilation-associated pneumonia |
| CRAB | Carbapenem-resistant Acinetobacter baumannii |
| RR | Relative risk |
| HFNC | High-flow nasal cannula |
| DDD | Defined daily dose |
| ESBL | Extended-spectrum beta-lactamase |
| CHG | Chlorhexidine gluconate |
References
- Haque, M.; McKimm, J.; Sartelli, M.; Dhingra, S.; Labricciosa, F.M.; Islam, S.; Jahan, D.; Nusrat, T.; Chowdhury, T.S.; Coccolini, F.; et al. Strategies to prevent healthcare-associated infections: A narrative overview. Risk Manag. Heal. Policy 2020, 13, 1765–1780. [Google Scholar] [CrossRef]
- Liu, X.; Shrestha, R.; Koju, P.; Maharjan, B.; Shah, P.; Thapa, P.; Li, H. The direct medical economic burden of healthcare-associated infections and antimicrobial resistance: A preliminary study in a teaching hospital of Nepal. J. Glob. Antimicrob. Resist. 2022, 29, 299–303. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Shih, S.-M.; Chen, Y.-T.; Hsiung, C.A.; Kuo, S.-C. Clinical and economic impact of intensive care unit-acquired bloodstream infections in Taiwan: A nationwide population-based retrospective cohort study. BMJ Open 2020, 10, e037484. [Google Scholar] [CrossRef]
- MacEwan, S.R.; Beal, E.W.; Gaughan, A.A.; Sieck, C.; McAlearney, A.S. Perspectives of hospital leaders and staff on patient education for the prevention of healthcare-associated infections. Infect. Control Hosp. Epidemiol. 2022, 43, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Jin, Z.; Memish, Z.A.; Daboor, M.A.; Al-Ruzzieh, M.A.; Hussien, N.H.; Guclu, E.; Olmez-Gazioglu, E.; Ogutlu, A.; Agha, H.M.; et al. Risk factors for mortality in ICU patients in 10 middle eastern countries: The role of healthcare-associated infections. J. Crit. Care 2022, 72, 154149. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-R.; Lin, Y.-Y.; Yu, C.-P.; Yang, Y.-S.; Cheng, C.-G.; Cheng, C.-A. In Increased Involvement of Klebsiellapneumoniae and Enterococcusfaecium in Healthcare-Associated Infections of Intensive Care Units in Taiwan. Healthcare 2021, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Chen, Y.; Lin, S.; Chung, K.; Sheng, W.; Ko, W.; Hsueh, P.R. Changing aetiology of healthcare-associated bloodstream infections at three medical centres in Taiwan, 2000–2011. Epidemiol. Infect. 2014, 142, 2180–2185. [Google Scholar] [CrossRef]
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. 2021, 27, 1772–1776. [Google Scholar] [CrossRef]
- Huang, R.-C.; Chiu, C.-H.; Chiang, T.-T.; Tsai, C.-C.; Wang, Y.-C.; Chang, F.-Y.; Yang, Y.S.; Wang, C.H. Hospital-acquired infections in patients hospitalized with COVID-19: First report from Taiwan. J. Chin. Med. Assoc. 2022, 85, 922–927. [Google Scholar] [CrossRef]
- Ershova, K.; Savin, I.; Kurdyumova, N.; Wong, D.; Danilov, G.; Shifrin, M.; Alexandrova, I.; Sokolova, E.; Fursova, N.; Zelman, V.; et al. Implementing an infection control and prevention program decreases the incidence of healthcare-associated infections and antibiotic resistance in a Russian neuro-ICU. Antimicrob. Resist. Infect. Control. 2018, 7, 94. [Google Scholar] [CrossRef]
- Gozel, M.G.; Hekimoglu, C.H.; Gozel, E.Y.; Batir, E.; McLaws, M.L.; Mese, E.A. National Infection Control Program in Turkey: The healthcare associated infection rate experiences over 10 years. Am. J. Infect. Control. 2021, 49, 885–892. [Google Scholar] [CrossRef]
- Huang, C. The COVID-19 pandemic and the incidence of the non-COVID-19 pneumonia in adults. Front. Med. 2021, 8, 737999. [Google Scholar] [CrossRef]
- Gao, W.; Sanna, M.; Huang, G.; Hefler, M.; Tsai, M.-K.; Wen, C.-P. Examining population health during the COVID-19 pandemic: All-cause, pneumonia and influenza, and road traffic deaths in Taiwan. Ann. Intern. Med. 2021, 174, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers of Disease Control. The Healthcare-Associated of Intensive Care Units in Taiwan. 2022. Available online: https://www.cdc.gov.tw/En/Category/Page/J63NmsvevBg2u3I2qYBenw (accessed on 1 April 2023).
- Taiwan Centers of Disease Control. The Antimicrobial Consumptions of Taiwan in 2023. 2023. Available online: https://www.cdc.gov.tw/ (accessed on 10 October 2025).
- Taiwan Ministry of Health and Welfare. Survey on Service Volume of Medical Institutions in 2022. Available online: https://www.gender.ey.gov.tw/gecdb/Stat_Statistics_Category.aspx?fs=fTQP3HmkUvd1PbnmtSP3rw%40%40&cs1=aPi33EfnEATKPKjm9jJFBA%40%40&cs2=FuWFCi3De1SSQR5qlMyr0g%40%40) (accessed on 1 August 2024).
- Rong, R.; Lin, L.; Yang, Y.; Zhao, S.; Guo, R.; Ye, J.; Zhu, X.; Wen, Q.; Liu, D. Trending prevalence of healthcare-associated infections in a tertiary hospital in China during the COVID-19 pandemic. BMC Infect. Dis. 2023, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Tsou, H.H.; Kuo, S.C.; Lin, Y.H.; Hsiung, C.A.; Chiou, H.Y.; Chen, W.J.; Wu, S.I.; Sytwu, H.K.; Chen, P.C.; Wu, M.H.; et al. A comprehensive evaluation of COVID-19 policies and outcomes in 50 countries and territories. Sci. Rep. 2022, 12, 8802. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Infectious Disease Thematic Database. Isolation Policies in Taiwan. Available online: https://tidtd.nhri.edu.tw/quarantine-policy-timeline-2/ (accessed on 10 October 2025).
- Li, Y.; Li, C.; Chang, W.; Liu, L. High-flow nasal cannula reduces intubation rate in patients with COVID-19 with acute respiratory failure: A meta-analysis and systematic review. BMJ Open 2023, 13, e067879. [Google Scholar] [CrossRef]
- Liu, C.W.; Cheng, S.L. Application of High-Flow Nasal Cannula in COVID-19: A Narrative Review. Life 2022, 12, 1419. [Google Scholar] [CrossRef]
- Lee, Y.L.; Liu, C.E.; Tang, H.J.; Huang, Y.T.; Chen, Y.S.; Hsueh, P.R.; SMART Taiwan Group. Epidemiology and antimicrobial susceptibility profiles of Enterobacterales causing bloodstream infections before and during COVID-19 pandemic: Results of the Study for Monitoring Antimicrobial Resistance Trends (SMART) in Taiwan, 2018–2021. J. Microbiol. Immunol. Infect. 2024, 57, 446–456. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Chen, L.-Y.; Lin, S.-Y.; Chou, P.; Liao, S.-Y.; Wang, F.-D. Surveillance on secular trends of incidence and mortality for device–associated infection in the intensive care unit setting at a tertiary medical center in Taiwan, 2000–2008: A retrospective observational study. BMC Infect Dis. 2012, 12, 209. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, S.-Y.; Ko, W.-C.; Hsueh, P.-R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Taiwan Centers of Disease Control. Antimicrobial Resistance Surveillance Annual Report, Taiwan in 2021–2022. 2022. Available online: https://www.cdc.gov.tw/Category/MPage/4G8HuDdUN1k4xaBJhbPzKQ (accessed on 1 April 2023).
- Lee, M.C.; Chang, H.; Sun, F.J.; Wu, A.Y.; Lu, C.H.; Lee, C.M. Association between Antimicrobial Consumption and the Prevalence of Nosocomial Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae in a Tertiary Hospital in Northern Taiwan. Am. J. Trop. Med. Hyg. 2022, 107, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Chu, C.-C.; Cheng, A.; Huang, Y.-T.; Hsueh, P.-R. Correlation between antimicrobial consumption and incidence of health-care-associated infections due to methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci at a university hospital in Taiwan from 2000 to 2010. J. Microbiol. Immunol. Infect. 2015, 48, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Hii, M.; Chang, H.-L.; Lin, L.-C.; Lee, Y.-L.; Liu, Y.-M.; Liu, C.-E.; Chen, C.H.; Cheng, Y.R.; Chang, C.Y. Changing epidemiology of candidemia in a medical center in middle Taiwan. J. Microbiol. Immunol. Infect. 2015, 48, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Yang Lauderdale, T.-L.; Huang, Y.-C. Evolution and population structures of prevalent methicillin-resistant Staphylococcus aureus in Taiwan. Front. Microbiol. 2021, 12, 725340. [Google Scholar] [CrossRef]
- Jean, S.-S.; Hsueh, P.-R.; Lee, W.-S.; Chang, H.-T.; Chou, M.-Y.; Chen, I.-S.; Wang, J.H.; Lin, C.F.; Shyr, J.M.; Ko, W.C.; et al. Nationwide surveillance of antimicrobial resistance among Enterobacteriaceae in intensive care units in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 215–220. [Google Scholar] [CrossRef]
- Tseng, Y.W.; Huang, C.W.; Chen, C.C.; Er, T.K. Assessment of antibiotic resistance patterns in Central Taiwan during the COVID-19 pandemic: A retrospective study. J. Infect. Public Health 2024, 17, 229–235. [Google Scholar] [CrossRef]
- Chang, H.C.; Chang, C.H.; Tien, K.L.; Tai, C.H.; Lin, L.M.; Lee, T.F.; Ku, S.C.; Fang, C.T.; Chen, Y.C.; Sheng, W.H. Impact of coronavirus disease 2019 (COVID-19) on antimicrobial resistance among major pathogens causing healthcare-associated infection. J. Formos. Med. Assoc. 2024, 123, 123–132. [Google Scholar] [CrossRef]
- Zhu, W.-M.; Yuan, Z.; Zhou, H.-Y. Risk factors for carbapenem-resistant Klebsiella pneumoniae infection relative to two types of control patients: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 23. [Google Scholar] [CrossRef]
- Taiwan Centers of Disease Control. Antimicrobial Agents Usage in Taiwan During 2021. 2022. Available online: https://www.cdc.gov.tw/File/Get?q=t9WnCInvvVMS9kUboNEwG4WgpE7_jCv77LnPRq-ahA8qJsp16pQaURPNoq-YXOk8rOPxRYaU05iElAmaD7nRv1JU1ssScWXEfG6JYcOdavjeKlsgjBb32PCAIM_hzeWQnzxC76d15dplS75hosZOMQ (accessed on 1 August 2024).
- Reis, M.A.O.; de Almeida, M.C.S.; Escudero, D.; Medeiros, E.A. Chlorhexidine gluconate bathing of adult patients in intensive care units in São Paulo, Brazil: Impact on the incidence of healthcare-associated infection. Braz. J. Infect. Dis. 2022, 26, 101666. [Google Scholar] [CrossRef]
- Izadi, N.; Eshrati, B.; Mehrabi, Y.; Etemad, K.; Hashemi-Nazari, S.S. The national rate of intensive care units-acquired infections, one-year retrospective study in Iran. BMC Public Health 2021, 21, 609. [Google Scholar] [CrossRef]
- Taiwan Centers of Disease Control. The Antimicrobial Resistant of Taiwan During COVID-19. 2022. Available online: https://www.cdc.gov.tw/File/Get?q=t9WnCInvvVMS9kUboNEwG6kcWFLUQ2PuJpCrSHkBMhj77SNvH3TjC1xNyquh63r8npxSKk3K4tbIn3Cw-FyuUev53IhkKNHrO1LvG7esSWGKc7ZVNdeH95zVA1tWv2Zw_naR6r8NwtjXEozg6NH2VdxBBEAXzykEpFfv4S4X-VNznSjKYjkotxRwk43i6jhWJ_BEZ-DOxtkM824nT03-_Q (accessed on 10 October 2025).

| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | p | |
|---|---|---|---|---|---|---|---|---|---|
| All HAIs | 5.7 | 5.29 | 4.93 | 5.19 | 5.41 | 5.2 | 5.26 | 5.17 | <0.001 * |
| BSIs | 2.13 | 2.11 | 1.93 | 1.99 | 2.11 | 2.17 | 2.83 | 2.41 | <0.001 * |
| UTIs | 2.08 | 1.92 | 1.84 | 1.87 | 1.92 | 1.95 | 2.05 | 1.87 | 0.222 |
| HAPs | 0.82 | 0.65 | 0.59 | 0.61 | 0.61 | 0.56 | 0.53 | 0.44 | <0.001 * |
| SSIs | 0.26 | 0.26 | 0.24 | 0.26 | 0.25 | 0.39 | 0.43 | 0.23 | <0.001 * |
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | p | |
|---|---|---|---|---|---|---|---|---|---|
| Medical ICUs | 6.12 | 5.52 | 5.44 | 5.44 | 5.94 | 5.92 | 6.29 | 6.12 | <0.001 * |
| Surgical ICUs | 6.94 | 6.49 | 5.93 | 6.68 | 6.51 | 6.26 | 6.21 | 6 | <0.001 * |
| Cardiac ICUs | 5.07 | 5.05 | 4.55 | 5.23 | 5.24 | 6.08 | 6.5 | 5.87 | <0.001 * |
| Pediatric ICUs | 2.61 | 2.35 | 2.16 | 2.08 | 2.09 | 1.96 | 2.21 | 1.97 | <0.001 * |
| General ICUs | 5.64 | 5.36 | 4.88 | 4.89 | 5.51 | 4.94 | 4.62 | 4.84 | <0.001 * |
| Overall | 5.7 | 5.29 | 4.93 | 5.19 | 5.41 | 5.2 | 5.26 | 5.17 | <0.001 * |
| Relative Risk | p | |
|---|---|---|
| Healthcare associated infections related to hospital-acquired pneumonias | 1.316 (95% CI: 1.225–1.414) | <0.001 * |
| Bloodstream infections related to central line associated bloodstream infections | 1.241 (95% CI: 1.178–1.32) | <0.001 * |
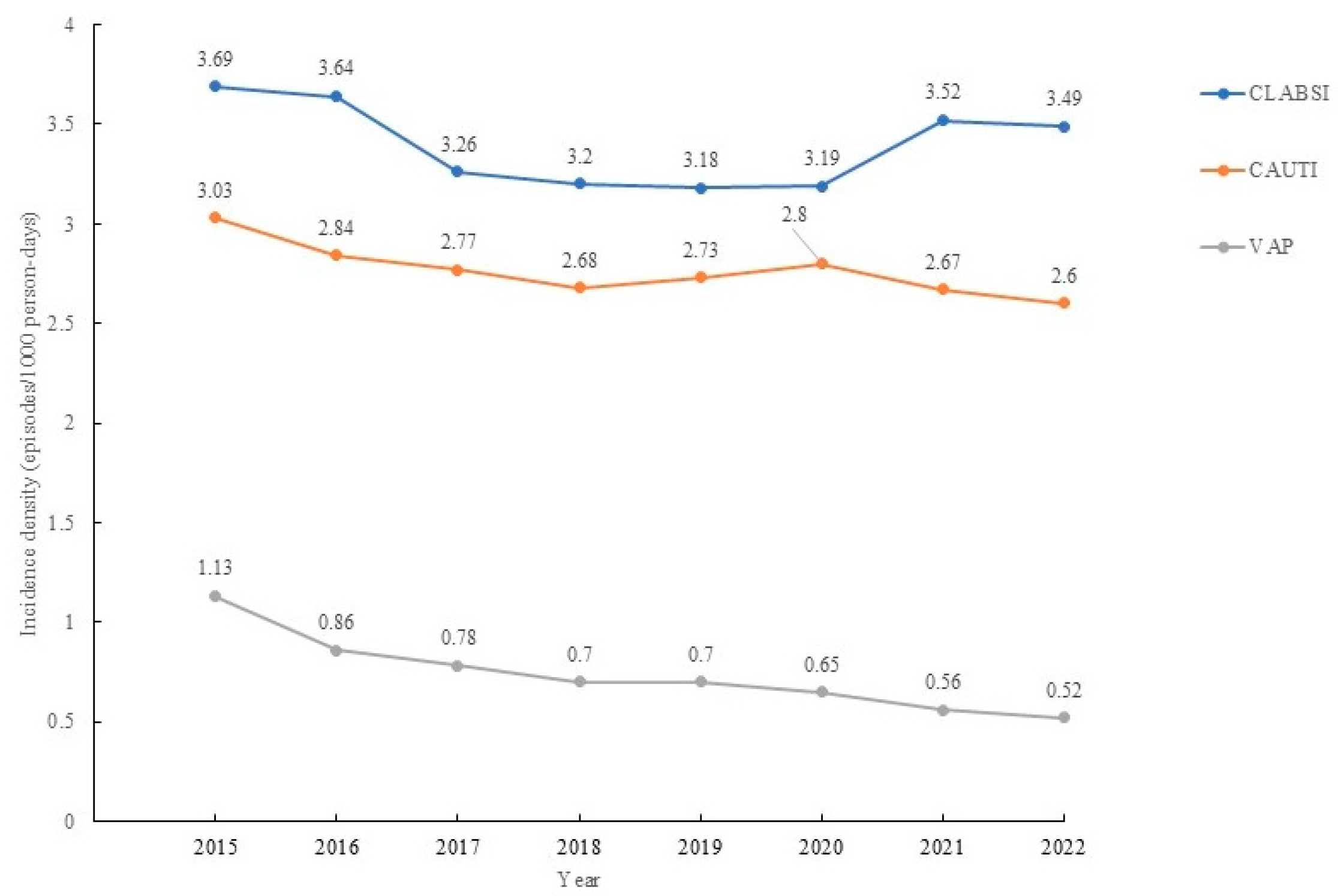
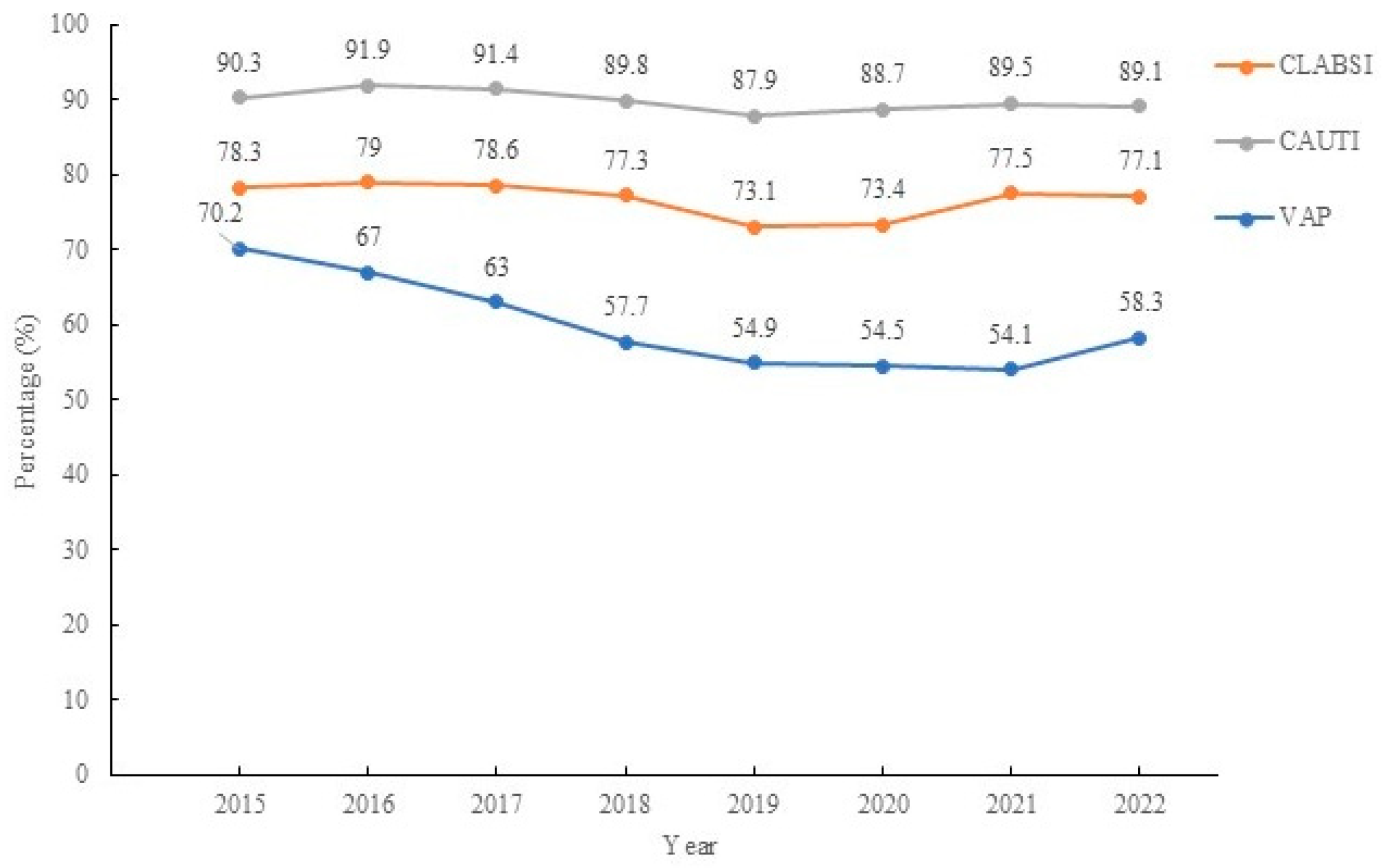
| Hospital acquired pneumonias related to ventilator-associated pneumonias | 2.324 (95% CI: 2.075–2.602) | <0.001 * |
| ATC code | BSIs | K. pneumoniae | E. faecium | E. coli | A. baumannii | P. aeruginosa | S. aureus |
|---|---|---|---|---|---|---|---|
| J01 | 0.679 | 0.795 | 0.986 | −0.948 | −0.981 | −0.995 | −0.925 |
| p | 0.321 | 0.205 | 0.014 * | 0.052 | 0.019 * | 0.005 * | 0.075 |
| J01C | −0.66 | −0.947 | −0.925 | 0.798 | 0.974 | 0.973 | 0.958 |
| p | 0.34 | 0.053 | 0.075 | 0.202 | 0.026 * | 0.027 * | 0.042 * |
| J01D | 0.704 | 0.791 | 0.979 | −0.946 | −0.976 | −0.992 | −0.914 |
| p | 0.321 | 0.209 | 0.021 * | 0.054 | 0.024 * | 0.008 * | 0.086 |
| ATC code | CRKP | MRSA | VREfm | CRAB | CRPA | CR E. coli |
|---|---|---|---|---|---|---|
| J01 | 0.97 | −0.992 | 0.746 | 0.879 | 0.83 | 0.895 |
| p | 0.03 * | 0.008 * | 0.254 | 0.121 | 0.17 | 0.105 |
| J01C | −0.98 | 0.945 | −0.919 | −0.722 | −0.937 | −0.768 |
| p | 0.02 * | 0.055 | 0.081 | 0.278 | 0.063 | 0.232 |
| J01D | 0.964 | −0.995 | 0.742 | 0.867 | 0.818 | 0.908 |
| p | 0.036 * | 0.005 * | 0.258 | 0.133 | 0.182 | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Y.; Chen, Y.-H.; Hsiao, C.-C.; Cheng, C.-G.; Cheng, C.-A. The Change in Healthcare-Associated Infections in Intensive Care Units Associated with the Coronavirus Disease 2019 in Taiwan. Medicina 2025, 61, 1971. https://doi.org/10.3390/medicina61111971
Wang C-Y, Chen Y-H, Hsiao C-C, Cheng C-G, Cheng C-A. The Change in Healthcare-Associated Infections in Intensive Care Units Associated with the Coronavirus Disease 2019 in Taiwan. Medicina. 2025; 61(11):1971. https://doi.org/10.3390/medicina61111971
Chicago/Turabian StyleWang, Chien-Ying, Yu-Hsuan Chen, Chih-Chun Hsiao, Chun-Gu Cheng, and Chun-An Cheng. 2025. "The Change in Healthcare-Associated Infections in Intensive Care Units Associated with the Coronavirus Disease 2019 in Taiwan" Medicina 61, no. 11: 1971. https://doi.org/10.3390/medicina61111971
APA StyleWang, C.-Y., Chen, Y.-H., Hsiao, C.-C., Cheng, C.-G., & Cheng, C.-A. (2025). The Change in Healthcare-Associated Infections in Intensive Care Units Associated with the Coronavirus Disease 2019 in Taiwan. Medicina, 61(11), 1971. https://doi.org/10.3390/medicina61111971






