Synchronous Versus Metachronous Multiple Malignant Tumors Involving the Digestive Tract: Predictors of Survival from a Single-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Patients and Definitions
2.3. Data Collection
2.4. Outcomes and Statistical Analysis
3. Results
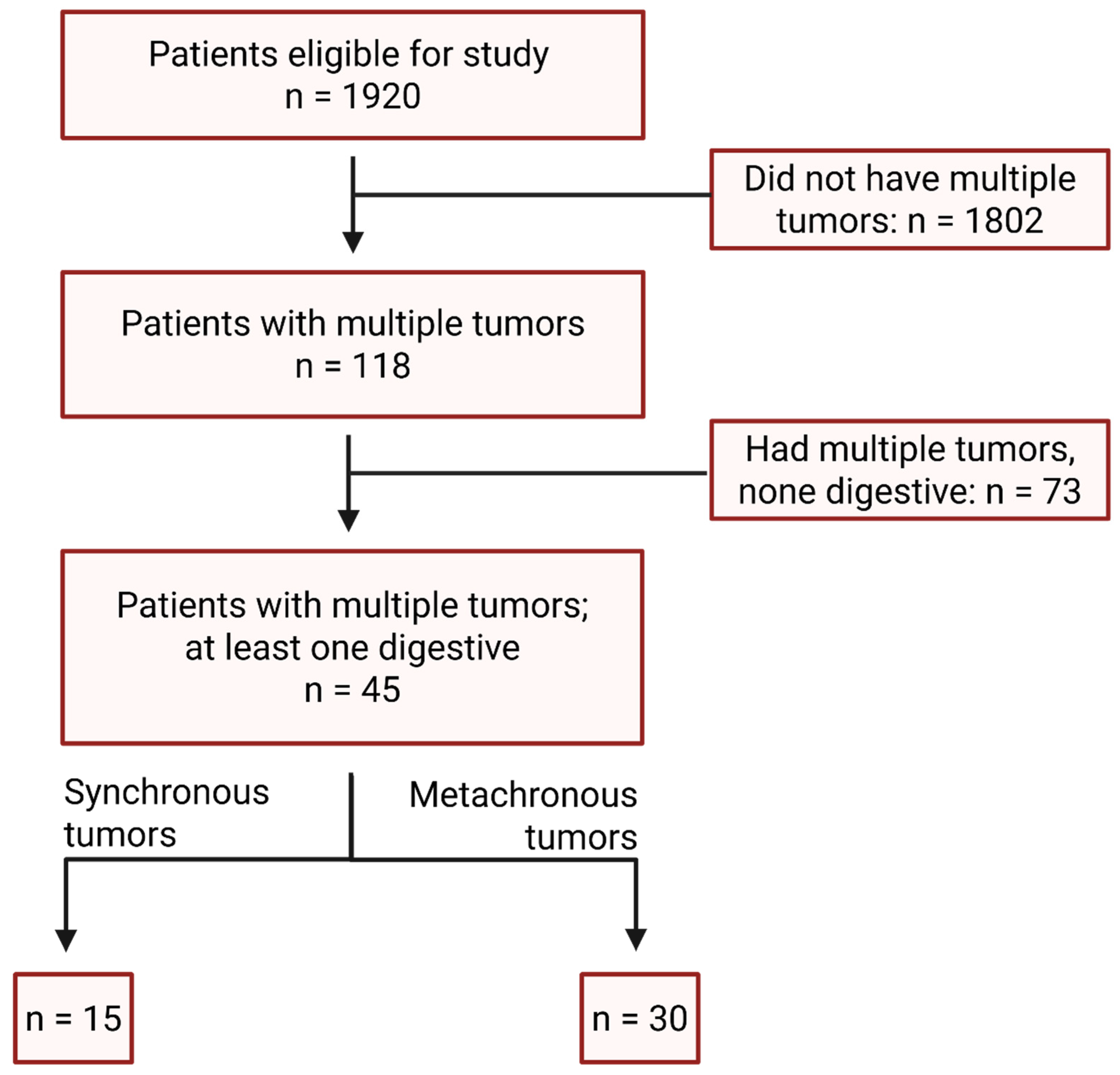
3.1. Cohort Assembly and Baseline Characteristics
3.2. Tumor Associations
3.3. Treatment Intent
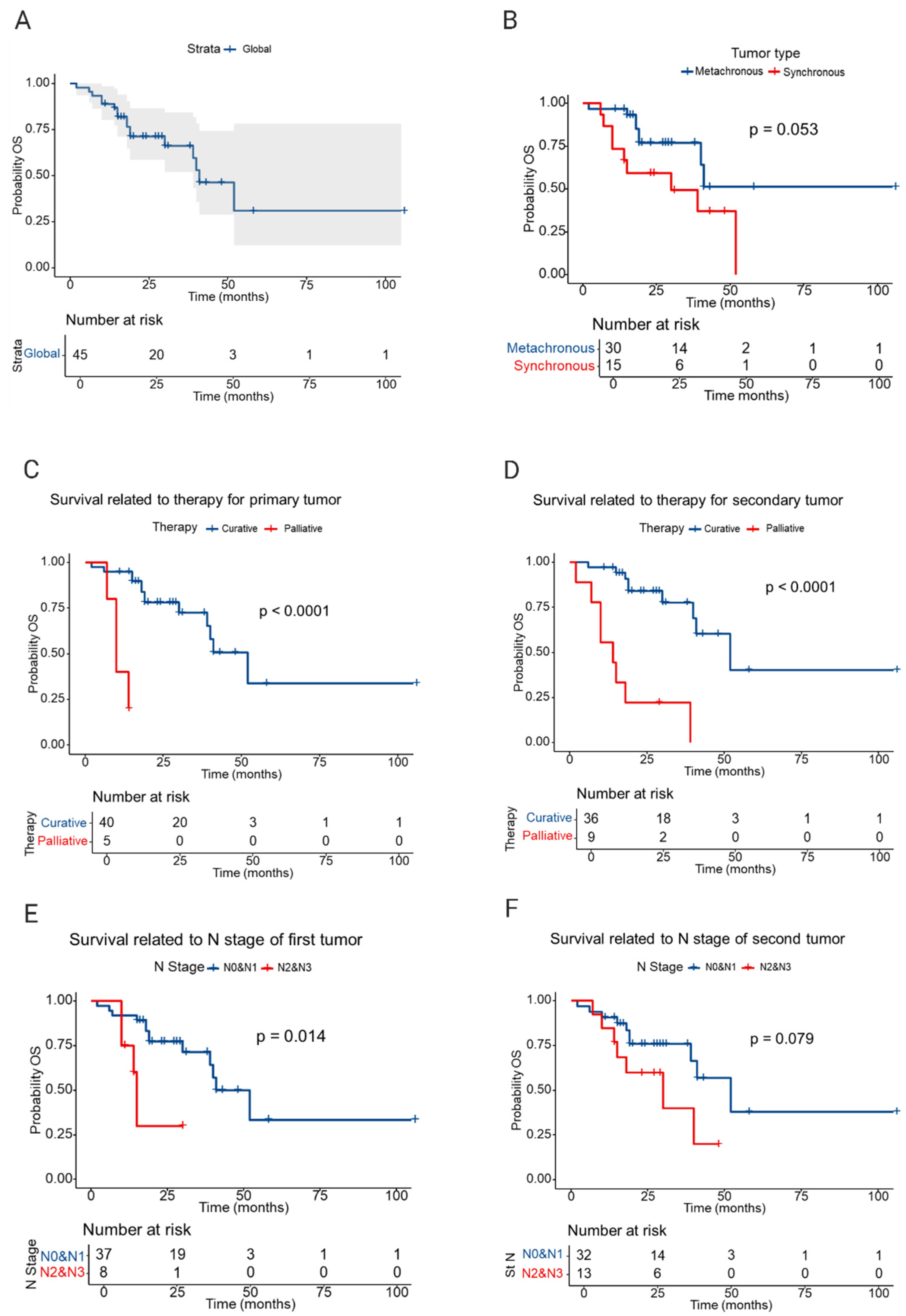
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Copur, M.S.; Manapuram, S. Multiple primary tumors over a lifetime. Oncology 2019, 33, 629384. [Google Scholar] [PubMed]
- Adamo, M.B.; Johnson, C.H.; Ruhl, J.L.; Dickie, L.A. SEER Program Coding and Staging Manual; National Cancer Institute: Bethesda, MD, USA, 2012; NIH publication no. 12-5581. [Google Scholar]
- International Agency for Research on Cancer. International Rules for Multiple Primary Cancers (ICD-O Third Edition); Internal Report no. 2004/02; IARC: Lyon, France, 2004. [Google Scholar]
- Vogt, A.; Schmid, S.; Heinimann, K.; Frick, H.; Herrmann, C.; Cerny, T.; Omlin, A. Multiple primary tumours: Challenges and approaches, a review. ESMO Open 2017, 2, e000172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, C.; Guo, W.; Li, S.; Bai, O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS ONE 2015, 10, e0125754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, L.; Zhou, C.; Cheng, X.; Li, Q.; Tang, Y.; Li, F.; Huang, T.; Tu, S. A pancancer analysis of the clinical and genomic characteristics of multiple primary cancers. Sci. Rep. 2024, 14, 2367. [Google Scholar] [CrossRef]
- Tanjak, P.; Suktitipat, B.; Vorasan, N.; Juengwiwattanakitti, P.; Thiengtrong, B.; Songjang, C.; Therasakvichya, S.; Laiteerapong, S.; Chinswangwatanakul, V. Risks and cancer associations of metachronous and synchronous multiple primary cancers: A 25-year retrospective study. BMC Cancer 2021, 21, 1045. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, X.; Shen, Y.; Wang, F.; Yang, J.; Wang, B.; Chen, Z.; Li, P.; Zhang, X.; Li, S.; et al. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine 2017, 96, e6799. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhang, L.H.; Xue, J.N.; Wang, Y.C.; Yang, X.; Zhang, N.; Liu, D.; Wang, Y.Y.; Xun, Z.Y.; Li, Y.R.; et al. High incidence combination of multiple primary malignant tumors of the digestive system. World J. Gastroenterol. 2022, 28, 5982–5992. [Google Scholar] [CrossRef]
- Ge, S.; Wang, B.; Wang, Z.; He, J.; Ma, X. Common Multiple Primary Cancers Associated With Breast and Gynecologic Cancers and Their Risk Factors, Pathogenesis, Treatment and Prognosis: A Review. Front. Oncol. 2022, 12, 840431. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Bai, L.; Zhao, Y.; Yang, Q. Epidemiological analysis of second primary malignant neoplasms in cancer survivors aged 85 years and older: A SEER data analysis (1975–2016). Sci. Rep. 2022, 12, 11688. [Google Scholar] [CrossRef]
- Kjaer, T.K.; Andersen, E.A.W.; Ursin, G.; Larsen, S.B.; Bidstrup, P.E.; Winther, J.F.; Borre, M.; Johansen, C.; Dalton, S.O. Cumulative incidence of second primary cancers in a large nationwide cohort of Danish cancer survivors: A population-based retrospective cohort study. Lancet Oncol. 2024, 25, 126–136. [Google Scholar] [CrossRef]
- Koivisto, H.A.M.; Nevala, A.O.; Miettinen, J.M.; Pitkäniemi, J.M.; Malila, N.K.; Heikkinen, S.M.M. Relative risk of second malignant neoplasms highest among young adult cancer patients—A population-based registry study in Finland. Acta Oncol. 2024, 63, 418–425. [Google Scholar] [CrossRef]
- Sung, H.; Hyun, N.; Leach, C.R.; Yabroff, K.R.; Jemal, A. Association of First Primary Cancer With Risk of Subsequent Primary Cancer Among Survivors of Adult-Onset Cancers in the United States. JAMA 2020, 324, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Lindor, N.M.; Petersen, G.M.; Hadley, D.W.; Kinney, A.Y.; Miesfeldt, S.; Lu, K.H.; Lynch, P.; Burke, W.; Press, N. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: A systematic review. JAMA 2006, 296, 1507–1517. [Google Scholar] [CrossRef]
- Fann, F.; Richardson, M.; Riegert-Johnson, D.; Young, C.C.; Sears, B.F. A novel insertion/deletion in APC promotor 1B is associated with both gastric and colon polyposis. Fam. Cancer 2025, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; McBride, A.; Yun, S.; Bhattacharjee, S.; Slack, M.; Martin, J.R.; Jeter, J.; Abraham, I. BRCA1 and BRCA2 Gene Mutations and Colorectal Cancer Risk: Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, S.S.; Gupta, S.; Weitzel, J.N.; Samadder, J. AGA Clinical Practice Update on Colorectal and Pancreatic Cancer Risk and Screening in BRCA1 and BRCA2 Carriers: Commentary. Gastroenterology 2020, 159, 760–764. [Google Scholar] [CrossRef]
- Jin, F.; Vajdic, C.M.; Poynten, I.M.; McGee-Avila, J.K.; Castle, P.E.; Grulich, A.E. Cancer risk in people living with HIV and solid organ transplant recipients: A systematic review and meta-analysis. Lancet Oncol. 2024, 25, 933–944. [Google Scholar] [CrossRef]
- Haas, C.B.; Shiels, M.S.; Pfeiffer, R.M.; D’aRcy, M.; Luo, Q.; Yu, K.; A Austin, A.; Cohen, C.; Miller, P.; Morawski, B.M.; et al. Cancers with epidemiologic signatures of viral oncogenicity among immunocompromised populations in the United States. J. Natl. Cancer Inst. 2024, 116, 1983–1991. [Google Scholar] [CrossRef]
- Saleem, N.; Wang, J.; Rejuso, A.; Teixeira-Pinto, A.; Stephens, J.H.; Wilson, A.; Kieu, A.; Gately, R.P.; Boroumand, F.; Chung, E.; et al. Outcomes of Solid Organ Transplant Recipients With Advanced Cancers Receiving Immune Checkpoint Inhibitors: A Systematic Review and Individual Participant Data Meta-Analysis. JAMA Oncol. 2025, 11, 1150–1159. [Google Scholar] [CrossRef]
- Mahmood, S.; Vu, K.; Tai, P.; Joseph, K.; Koul, R.; Dubey, A.; Yu, E. Radiation-induced second malignancies. Anticancer Res. 2015, 35, 2431–2434. [Google Scholar]
- Allodji, R.S.; Tucker, M.A.; Hawkins, M.M.; Le Deley, M.; Veres, C.; Weathers, R.; Howell, R.; Winter, D.; Haddy, N.; Rubino, C.; et al. Role of radiotherapy and chemotherapy in the risk of leukemia after childhood cancer: An international pooled analysis. Int. J. Cancer 2021, 148, 2079–2089. [Google Scholar] [CrossRef]
- Tringale, K.R.; Patel, K.H.; Hilal, L.; Wu, A.J.; Boe, L.; Zhang, Z.; Kim, N.; Navilio, J.; Reyngold, M.; Cuaron, J.; et al. Development of Second Malignancies Following Chemotherapy With or Without Radiation Therapy for the Treatment of Locally Advanced Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2025; in press. [Google Scholar] [CrossRef]
- Wood, M.E.; Vogel, V.; Ng, A.; Foxhall, L.; Goodwin, P.; Travis, L.B. Second malignant neoplasms: Assessment and strategies for risk reduction. J. Clin. Oncol. 2012, 30, 3734–3745. [Google Scholar] [CrossRef]
- Dos Anjos, G.R.; Machado, G.F.; de Barros, C.P.; de Andrade, V.P.; Maciel, R.M.B.; Cunha, L.L. Cancer survivorship in low- and middle-income countries: Challenges, needs, and emerging support strategies. Front. Public Health 2025, 13, 1601483. [Google Scholar] [CrossRef]
- Melku, M.; Best, O.G.; Winter, J.M.; Thurgood, L.A.; Ahmed, M.; Kichenadasse, G.; Wassie, M.M.; Symonds, E.L. Risk Factors of Multiple Primary Cancers Among Colorectal Cancer Survivors. Cancers 2025, 17, 2145. [Google Scholar] [CrossRef]
- Issaka, R.B.; Chan, A.T.; Gupta, S. AGA Clinical Practice Update on Risk Stratification for Colorectal Cancer Screening and Post-Polypectomy Surveillance: Expert Review. Gastroenterology 2023, 165, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Yoshida, N.; Kinoshita, K.; Iwatsuki, M.; Yamashita, Y.I.; Chikamoto, A.; Watanabe, M.; Baba, H. Clinical and Prognostic Features of Patients With Esophageal Cancer and Multiple Primary Cancers: A Retrospective Single-institution Study. Ann. Surg. 2018, 267, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Yao, L.J.; Zhou, J.; Hu, H.; Wang, L.W. Clinical features of multiple primary malignancies: A retrospective analysis of 72 Chinese patients. Asian Pac. J. Cancer Prev. 2014, 15, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wen, R.; Zhou, L.; Gao, X.; Lou, Z.; Hao, L.; Meng, R.; Gong, H.; Yu, G.; Zhang, W. Clinicopathological features and prognosis of synchronous and metachronous colorectal cancer: A retrospective cohort study. Int. J. Surg. 2023, 109, 4073–4090. [Google Scholar] [CrossRef]
- Kollias, J.; Ellis, I.O.; Elston, C.W.; Blamey, R.W. Prognostic significance of synchronous and metachronous bilateral breast cancer. World J. Surg. 2001, 25, 1117–1124. [Google Scholar] [CrossRef]
- Bugter, O.; van Iwaarden, D.L.P.; Dronkers, E.A.C.; de Herdt, M.J.; Wieringa, M.H.; Verduijn, G.M.; Mureau, M.A.; Hove, I.T.; van Meerten, E.; Hardillo, J.A.; et al. Survival of patients with head and neck cancer with metachronous multiple primary tumors is surprisingly favorable. Head Neck. 2019, 41, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, J.; Shi, X.; Shen, J.; Liang, W.; Yang, C.; He, J. Prognosis of synchronous and metachronous multiple primary lung cancers: Systematic review and meta-analysis. Lung Cancer 2015, 87, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Izumi, N.; Tsukioka, T.; Inoue, H.; Hara, K.; Miyamoto, H.; Nishiyama, N. Prognosis associated with synchronous or metachronous multiple primary malignancies in patients with completely resected non-small cell lung cancer. Surg. Today 2019, 49, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Sarver, M.M.; Rames, J.D.; Beasley, G.M.; Gao, J.; Jung, S.H.; Chen, S.C. Survival and tumor characteristics of patients presenting with single primary versus second primary melanoma lesions. J. Am. Acad. Dermatol. 2023, 88, 1033–1039. [Google Scholar] [CrossRef]
- Karapetyan, L.; Yang, X.; Knight, A.D.; Huang, Z.; Wang, H.; Sander, C.A.; Minnier, C.P.; Wilson, M.; Li, A.; Karunamurthy, A.; et al. Poorer survival outcomes in patients with multiple versus single primary melanoma. Cancer 2022, 128, 2098–2106. [Google Scholar] [CrossRef]
| Clinical Variable | Synchronous Malignant Tumors | Metachronous Malignant Tumors |
|---|---|---|
| Average age at diagnosis (years) | ||
| First malignant tumor | 66.28 | 61.9 |
| Second malignant tumor | 68.8 | |
| Sex | ||
| Male | 9 | 16 |
| Female | 5 | 15 |
| Tumor grade | ||
| G1 | 6 (18.75%) | 18 (29.5%) |
| G2 | 21 (65.62%) | 35 (57.37%) |
| G3 | 5 (15.62%) | 8 (13.11%) |
| TNM stage | ||
| I | 2 (7.4%) | 10 (15.1%) |
| II | 4 (14.8%) | 21 (31.8%) |
| III | 16 (55.5%) | 27 (40.9%) |
| IV | 5 (18.51%) | 8 (12.1%) |
| Treatment intent | ||
| Curative | ||
| First malignant tumor | 10 (32.25%) | 30 (47.61%) |
| Second malignant tumor | 10 (32.25%) | 26 (41.26%) |
| Third malignant tumor | 1 (3.22%) | 2 (3.17%) |
| Palliative | ||
| First malignant tumor | 5 (16.12%) | 0 (0.00%) |
| Second malignant tumor | 5 (16.12%) | 4 (6.34%) |
| Third malignant tumor | 0 (0.00%) | 1 (1.58%) |
| Patients diagnosed with a second malignant tumor during follow-up after treatment of the first malignant tumor | 2 | 16 |
| Clinical Variable | Deaths N (%) | RMST (Months) | Median Survival |
|---|---|---|---|
| The entire lot | 17/45 (37.77) | 54.00 | 41.00 |
| Tumor type | |||
| Metachronous | 8/30 (26.66) | 68.30 | N/A (40 at N/A) |
| Synchronous | 9/15 (60) | 31.30 | 30 (14 at N/A) |
| Treatment T1 | |||
| Curative | 13/40 (32.50) | 58.20 | 52.00 |
| Palliative | 4/5 (80.00) | 29.40 | 10.00 |
| Treatment T2 | |||
| Curative | 9/36 | 64.60 | 52.00 |
| Palliative | 8/9 (88.88) | 17.10 | 14.00 |
| Nodal stage T1 | |||
| 0–I | 13/37 (35.13) | 57.30 | 41.00 |
| II–III | 4/8 (50.00) | 40.90 | 14.00 |
| Nodal stage T2 | |||
| 0–I | 10/32 (31.25) | 60.90 | 52 |
| II–III | 7/13 (53.84) | 40.30 | 30 |
| Predictor | N | Deaths N | HR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | ||||
| T1 | 45 | 17 | ||
| T2 | 45 | 17 | 1.03 (0.97 to 1.10) | 0.382 |
| Sex | ||||
| Female | 20 | 8 | ||
| Male | 25 | 9 | 0.87 (0.33 to 2.27) | 0.768 |
| Tumor type | ||||
| Metachronous | 30 | 8 | - | |
| Synchronous | 15 | 9 | 2.49 (0.95 to 6.50) | 0.062 |
| Nodal stage T1 | ||||
| 0 | 37 | 13 | - | |
| II–III | 8 | 4 | 4.37 (1.24 to 15.3) | 0.021 |
| Nodal stage T2 | ||||
| 0 | ||||
| II–III | 13 | 7 | - | - |
| Grade T1 | ||||
| G1 | 17 | 7 | - | - |
| G2 | 25 | 9 | 1.03 (0.38 to 2.79) | 0.951 |
| G3 | 3 | 1 | 1.17 (0.14 to 9.70) | 0.887 |
| Grade T2 | ||||
| G1 | 8 | 3 | - | - |
| G2 | 27 | 10 | 0.80 (0.22 to 2.97) | 0.741 |
| G3 | 10 | 4 | 0.94 (0.21 to 4.30) | 0.936 |
| Treatment intent T1 | ||||
| Curative | 40 | 13 | - | - |
| Palliative | 5 | 4 | 20.5 (3.68 to 114) | <0.001 |
| Treatment intent T2 | ||||
| Curative | 36 | 9 | ||
| Palliative | 9 | 8 | 10.3 (3.051 to 30.5) | <0.001 |
| Predictor | N | Deaths | HR (95% CI) | p-Value | VIF |
|---|---|---|---|---|---|
| Tumor type | 1.0 | ||||
| Metachronous | 30 | 8 | - | ||
| Synchronous | 15 | 9 | 2.22 (0.84 to 5.86) | 0.1 | |
| Nodal stage T1 | |||||
| 0–I | 37 | 13 | - | ||
| II–III | 8 | 4 | 3.86 (1.04 to 14.3) | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprita, A.V.; Nitipir, C.; Achim, E.; Grama, F.A. Synchronous Versus Metachronous Multiple Malignant Tumors Involving the Digestive Tract: Predictors of Survival from a Single-Center Retrospective Study. Medicina 2025, 61, 1962. https://doi.org/10.3390/medicina61111962
Oprita AV, Nitipir C, Achim E, Grama FA. Synchronous Versus Metachronous Multiple Malignant Tumors Involving the Digestive Tract: Predictors of Survival from a Single-Center Retrospective Study. Medicina. 2025; 61(11):1962. https://doi.org/10.3390/medicina61111962
Chicago/Turabian StyleOprita, Alexandru Vlad, Cornelia Nitipir, Eduard Achim, and Florin Andrei Grama. 2025. "Synchronous Versus Metachronous Multiple Malignant Tumors Involving the Digestive Tract: Predictors of Survival from a Single-Center Retrospective Study" Medicina 61, no. 11: 1962. https://doi.org/10.3390/medicina61111962
APA StyleOprita, A. V., Nitipir, C., Achim, E., & Grama, F. A. (2025). Synchronous Versus Metachronous Multiple Malignant Tumors Involving the Digestive Tract: Predictors of Survival from a Single-Center Retrospective Study. Medicina, 61(11), 1962. https://doi.org/10.3390/medicina61111962








