Effects of Dynamic Neuromuscular Stabilization on Lower Limb Muscle Activity, Pain, and Disability in Individuals with Chronic Low Back Pain: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
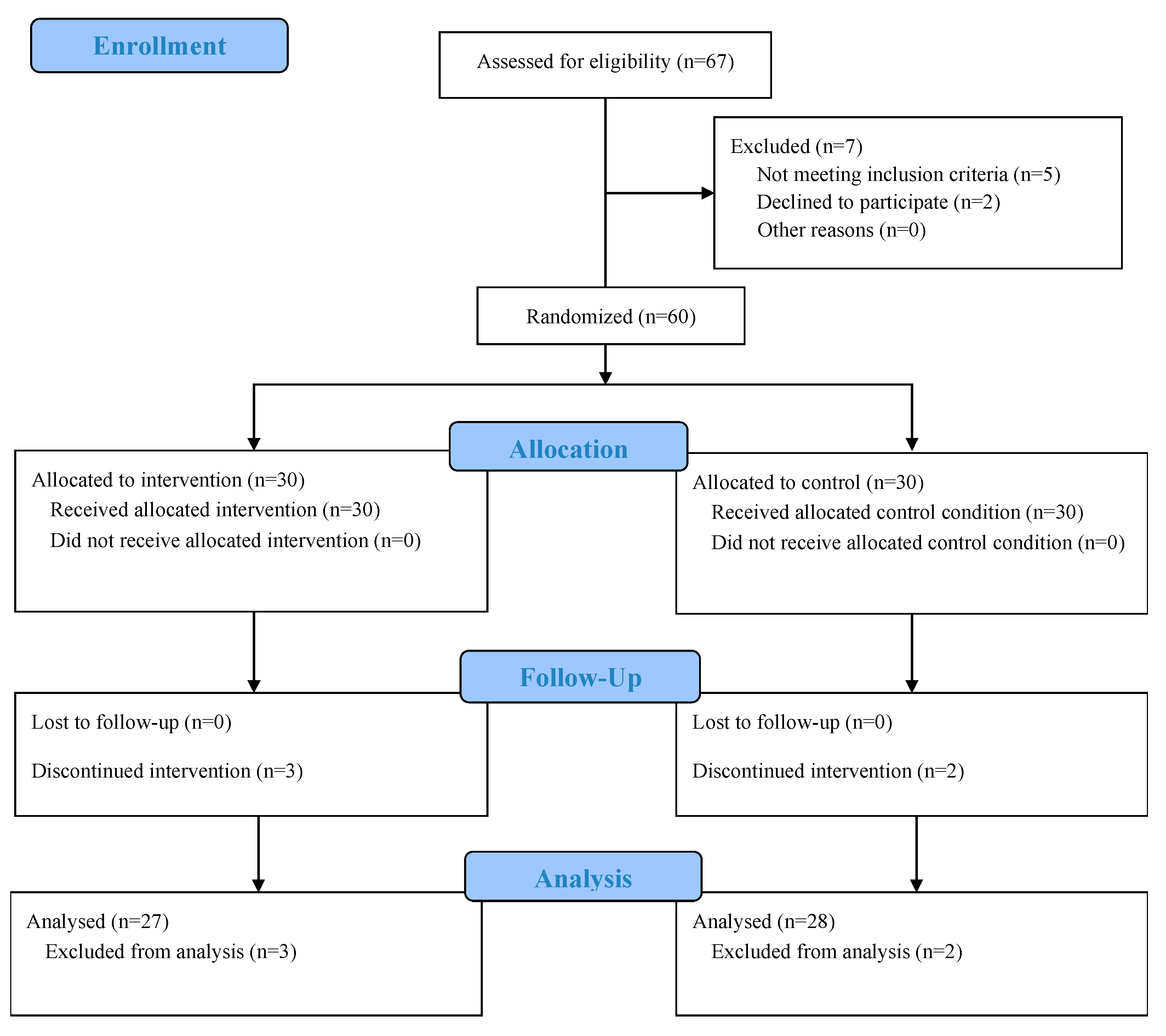
2.1. Participants and Design
2.2. Randomization and Blinding
2.3. Inclusion and Exclusion Criteria
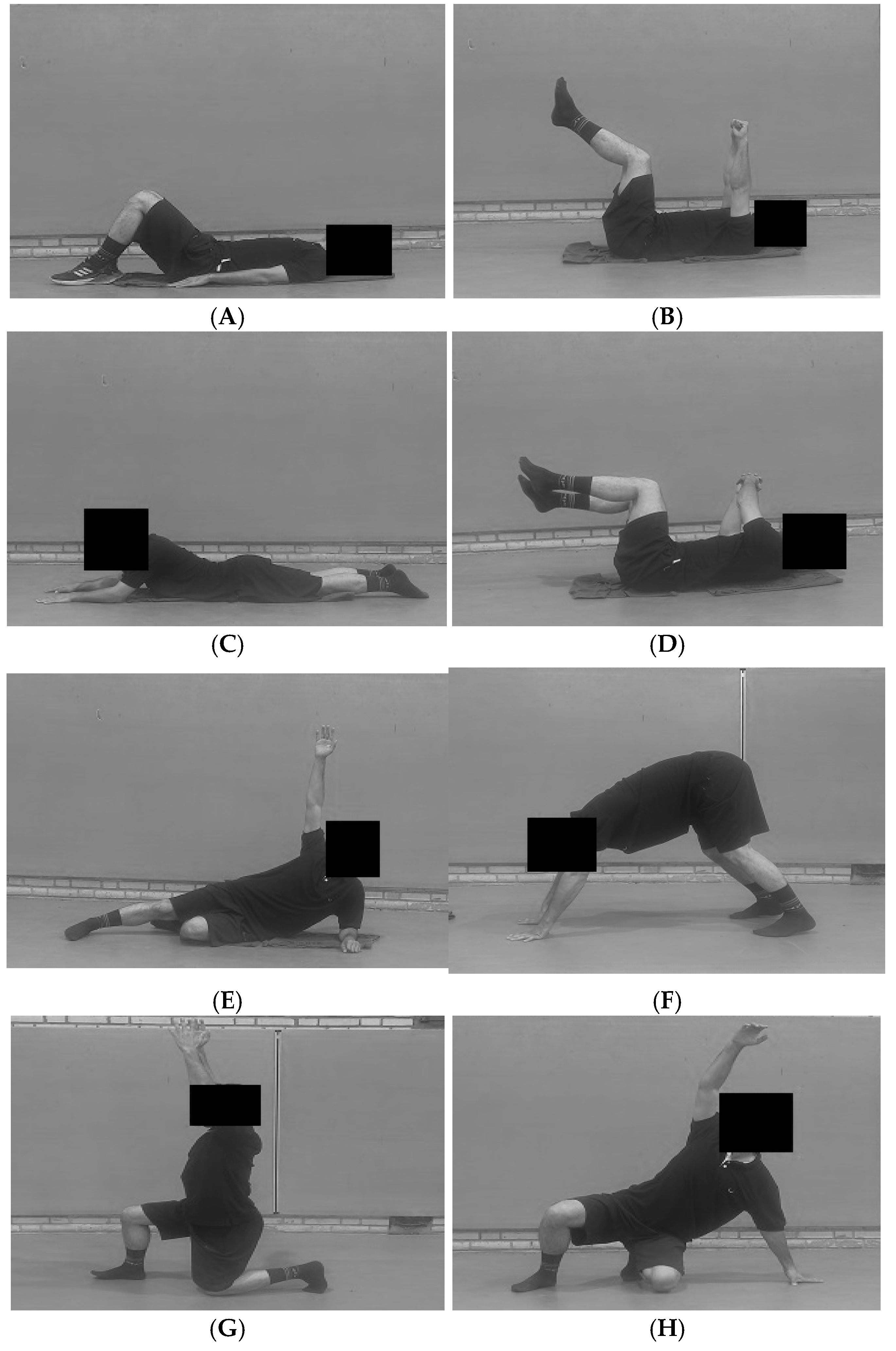
2.4. Intervention (DNS Protocol)
2.5. EMG Acquisition and Processing
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Participants
3.2. Clinical Outcomes
3.3. EMG Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLBP | Chronic Low Back Pain |
| LBP | Low Back Pain |
| EMG | Electromyography |
| DNS | Dynamic Neuromuscular Stabilization |
| VAS | Visual Analogue Scale |
| ODI | Oswestry Disability Index |
| MVIC | Maximal Voluntary Isometric Contraction |
| SENIAM | Surface Electromyography for Non-Invasive Assessment of Muscles |
| BMI | Body Mass Index |
| ANCOVA | Analysis of Covariance |
| SPSS | Statistical Package for the Social Sciences |
| G*Power | Power Analysis Software |
| TrA | transversus abdominis |
| TA | Tibialis Anterior |
| GC | Gastrocnemius |
| VL | Vastus Lateralis |
| VM | Vastus Medialis |
| RF | Rectus Femoris |
| ST | Semitendinosus |
| LR | Loading Response |
| MC | Mid-Stance |
| PO | Push-Off |
| SW | Swing |
| CGU | Czech Get Up |
Appendix A
Appendix A.1
| Exercise/Developmental Position | Week(s) | Description/Key Focus | Sets × Reps/Duration |
|---|---|---|---|
| Diaphragmatic Breathing | 1–2 | In supine and sitting positions, focus on 360-degree expansion of the abdominal wall and lower ribcage without chest elevation. Establish ideal Intra-Abdominal Pressure (IAP) regulation. | 3 Sets × 10 Breaths |
| Baby Rock (Supine 90–90) | Lie supine with hips and knees at 90 degrees. Maintain a stable lumbar spine (low back flat on the floor) and controlled breathing. | 3 Sets × 30-s Hold | |
| Prone on Elbows | 3–4 | From a prone position, lift the torso onto the elbows. Focus on maintaining a long, neutral spine and avoid scapular winging or excessive lumbar lordosis. | 3 Sets × 30-s Hold |
| Rolling | Initiate rotation from the core (using IAP and obliques), not by pushing with arms or legs. Movement should be smooth from supine to prone and back. | 2 Sets × 8–10 Rolls per side | |
| Side Lying & Oblique Sit | 5–6 | Stabilize the trunk in the frontal plane. Progress from lying on the side to supporting on one hand in the “oblique sit” position, lifting the hip off the floor. | 3 Sets × 20–30-s Hold per side |
| Tripod Position | Support on two hands and one foot/knee. Develop contralateral support patterns, focusing on co-contraction of the supporting shoulder and hip stabilizers. | 3 Sets × 20-s Hold per side | |
| Kneeling to Squat | 7–8 | Practice transitioning from a high-kneeling position to a deep squat while maintaining an upright torso, neutral spine, and stable IAP. | 2 Sets × 10–12 Reps |
| Czech Get Up (CGU)—Partial | Practice smooth transitions through the foundational DNS positions (e.g., from supine to oblique sit to tripod). Focus on the quality and control of each transition. | 2 Sets × 3–5 Transitions per side |
Appendix A.2
References
- Rahimi, A.; Arab, A.M.; Nourbakhsh, M.R.; Hosseini, S.M.; Forghany, S. Lower limb kinematics in individuals with chronic low back pain during walking. J. Electromyogr. Kinesiol. 2020, 51, 102404. [Google Scholar] [CrossRef]
- McIntosh, G.; Hall, H. Low back pain (acute). BMJ Clin. Evid. 2011, 1102. [Google Scholar]
- Cramer, H.; Mehling, W.E.; Saha, F.J.; Dobos, G.; Lauche, R. Postural awareness and its relation to pain: Validation of an innovative instrument measuring awareness of body posture in patients with chronic pain. BMC Musculoskelet. Disord. 2018, 19, 109. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.A.; van den Hoorn, W.; Klyne, D.M.; Hodges, P.W. Postural control of the trunk in individuals with and without low back pain during unstable sitting: A protocol for a systematic review with an individual participant data meta-analysis. PLoS ONE 2022, 17, e0268381. [Google Scholar] [CrossRef] [PubMed]
- Najafi Ghagholestani, B.; Gandomi, F.; Asar, S. The Effect of Eight Weeks Aquatic and Dynamic Neuromuscular Stabilization Exercises on Postural Sways and Foot Pressure Distribution Symmetry in Patients with Nonspecific Chronic Low Back Pain. J. Sport Rehabil. 2022, 10, 143–163. [Google Scholar] [CrossRef]
- Koch, C.; Hänsel, F. Chronic Non-specific Low Back Pain and Motor Control During Gait. Front. Psychol. 2018, 9, 2236. [Google Scholar] [CrossRef]
- Southwell, D.J.; Hills, N.F.; McLean, L.; Graham, R.B. The acute effects of targeted abdominal muscle activation training on spine stability and neuromuscular control. J. Neuroeng. Rehabil. 2016, 13, 19. [Google Scholar] [CrossRef]
- Farahpour, N.; Younesian, H.; Bahrpeyma, F. Electromyographic activity of erector spinae and external oblique muscles during trunk lateral bending and axial rotation in patients with adolescent idiopathic scoliosis and healthy subjects. Clin. Biomech. 2015, 30, 411–417. [Google Scholar] [CrossRef]
- Ippersiel, P.; Dussault-Picard, C.; Mohammadyari, S.G.; De Carvalho, G.B.; Chandran, V.D.; Pal, S.; Dixon, P.C. Muscle coactivation during gait in children with and without cerebral palsy. Gait Posture 2024, 108, 110–116. [Google Scholar] [CrossRef]
- Sung, W.; Abraham, M.; Plastaras, C.; Silfies, S.P. Trunk motor control deficits in acute and subacute low back pain are not associated with pain or fear of movement. Spine J. 2015, 15, 1772–1782. [Google Scholar] [CrossRef]
- Mok, N.W.; Brauer, S.G.; Hodges, P.W. Hip strategy for balance control in quiet standing is reduced in people with low back pain. Spine 2004, 29, E107–E112. [Google Scholar] [CrossRef]
- Mientjes, M.I.; Frank, J.S. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin. Biomech. 1999, 14, 710–716. [Google Scholar] [CrossRef]
- Lima, M.; Ferreira, A.S.; Reis, F.J.J.; Paes, V.; Meziat-Filho, N. Chronic low back pain and back muscle activity during functional tasks. Gait Posture 2018, 61, 250–256. [Google Scholar] [CrossRef]
- Waiteman, M.C.; Chia, L.; Ducatti, M.H.M.; Bazett-Jones, D.M.; Pappas, E.; de Azevedo, F.M.; Briani, R.V. Trunk Biomechanics in Individuals with Knee Disorders: A Systematic Review with Evidence Gap Map and Meta-analysis. Sports Med. Open 2022, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Kobesova, A.; Kolar, P. Dynamic neuromuscular stabilization & sports rehabilitation. Int. J. Sports Phys. Ther. 2013, 8, 62–73. [Google Scholar] [PubMed]
- Mahdieh, L.; Zolaktaf, V.; Karimi, M.T. Effects of dynamic neuromuscular stabilization (DNS) training on functional movements. Hum. Mov. Sci. 2020, 70, 102568. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, H.; Choudhary, A.; Sharma, M. Effectiveness of Dynamic Neuromuscular Stabilization Technique in Neurological Conditions: An Updated Review. J. Health Allied Sci. NU 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Liu, H.; He, Y.; Li, Y.; Shen, P.; Chen, Y.; Huang, J.; Liu, C. Effect of different isometric trunk extension intensities on the muscle stiffness of the lumbar and lower limbs. Front. Physiol. 2024, 14, 1337170. [Google Scholar] [CrossRef]
- Mousavi, S.M.S.; Mirsafaei Rizi, R. Effect of Central Stability and Dynamic Neuromuscular Stabilization Exercises on Pain, Flexibility, Balance, Muscle Endurance and Quality of Life in Men with Nonspecific Chronic Low Back Pain. J. Guilan Univ. Med. Sci. 2022, 31, 136–149. [Google Scholar] [CrossRef]
- Abuín-Porras, V.; Clemente-Suárez, V.J.; Jaén-Crespo, G.; Navarro-Flores, E.; Pareja-Galeano, H.; Romero-Morales, C. Effect of Physiotherapy Treatment in the Autonomic Activation and Pain Perception in Male Patients with Non-Specific Subacute Low Back Pain. J. Clin. Med. 2021, 10, 1793. [Google Scholar] [CrossRef]
- Hei, P.; Zhang, Z.; Wei, J.; Lan, C.; Wang, X.; Jing, X.; Chen, X.; Wu, Z. The effect of dynamic neuromuscular stabilization technique combined with Kinesio Taping on neuromuscular function and pain self-efficacy in individuals with chronic nonspecific low back pain: A randomized trial. Medicine 2025, 104, e41265. [Google Scholar] [CrossRef]
- Kaushik, M.; Ahmad, I. Bridging Dynamic Neuromuscular Stabilization Synergism with Movement Control Impairment Related Non-Specific Low Back Pain: Scoping Review. J. Musculoskelet. Neuronal Interact. 2024, 24, 420–432. [Google Scholar]
- Rabieezadeh, A.; Mahdavinejad, R.; Sedehi, M.; Adimi, M. The effects of an 8-week dynamic neuromuscular stabilization exercise on pain, functional disability, and quality of life in individuals with non-specific chronic low back pain: A randomized clinical trial with a two-month follow-up study. BMC Sports Sci. Med. Rehabil. 2024, 16, 161. [Google Scholar] [CrossRef]
- Hosseini, H.; Rahimi, A.; Javanshir, K.; Taghipour, M.; Daryabor, A.; Naimi, S.S. The Effect of 8 Weeks of Dynamic Neuromuscular Stabilization Exercises on Sonographic and Clinical Outcomes in Patients with Non-Specific Low Back Pain. Iran. Red Crescent Med. J. 2025, 27, 1–9. [Google Scholar] [CrossRef]
- Hediyeh, H.; Abbas, R.; Khodabakhsh, J.; Mohammad, T.; Aliyeh, D.; Sedigheh Sadat, N. Effect of Dynamic Neuromuscular Stabilization Exercises on Activity of External Oblique Muscle in Low Back Pain. J. Mod. Rehabil. 2024, 19, 90–97. [Google Scholar] [CrossRef]
- Singh, V.; Anwar, Z.; Khera, K. Effectiveness of Dynamic Neuromuscular Stabilization (DNS) Exercises Among Subjects with Musculoskeletal Problems, Balance and Gait Abnormalities—A Pilot Study. J. Neonatal Surg. 2025, 14, 164–181. [Google Scholar]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Huang, H.; Xie, H.; Zhang, G.; Xiao, W.; Ge, L.; Chen, S.; Zeng, Y.; Wang, C.; Li, H. Effects of dynamic neuromuscular stabilization training on the core muscle contractility and standing postural control in patients with chronic low back pain: A randomized controlled trial. BMC Musculoskelet. Disord. 2025, 26, 213. [Google Scholar] [CrossRef]
- Williams, J.R. The Declaration of Helsinki and public health. Bull. World Health Organ. 2008, 86, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Kolářová, B.; Krobot, A.; Polehlová, K.; Hluštík, P.; Richards, J.D. Effect of Gait Imagery Tasks on Lower Limb Muscle Activity with Respect to Body Posture. Percept. Mot. Skills 2016, 122, 411–431. [Google Scholar] [CrossRef]
- Haltmar, H.; Janura, M.; Kolářová, B. Muscle activity and lower body kinematics change when performing motor imagery of gait. Sci. Rep. 2025, 15, 191. [Google Scholar] [CrossRef]
- Temporiti, F.; Casirati, C.; Adamo, P.; Leo, D.; Marino, G.; Grappiolo, G.; Gatti, R. Indoor and outdoor 10-Meter Walk Test and Timed Up and Go in patients after total hip arthroplasty: A reliability and comparative study. Arch. Physiother. 2024, 14, 90–95. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H.J. Standards for Surface Electromyography: The European Project Surface EMG for Non-Invasive Assessment of Muscles (SENIAM). Enschede: Roessingh Research and Development. 2007. Volume 10. Available online: https://www.researchgate.net/publication/228486725_Standards_for_suface_electromyography_The_European_project_Surface_EMG_for_non-invasive_assessment_of_muscles_SENIAM (accessed on 24 September 2025).
- Makaracı, Y.; Ruiz-Cárdenas, J.D.; Pamuk, Ö.; Nas, K.; Demiray, Z.; Duysak, H.; Gruet, M. Kinesio Taping does not Enhance Jump Performance and Muscle Activity in Female Athletes. Int. J. Sports Med. 2025, 46, 271–280. [Google Scholar] [CrossRef]
- De Luca, C.J.; Adam, A.; Wotiz, R.; Gilmore, L.D.; Nawab, S.H. Decomposition of surface EMG signals. J. Neurophysiol. 2006, 96, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, F.; Moffat, M.; Gutierrez, G. Neuromuscular control during performance of a dynamic balance task in subjects with and without ankle instability. Int. J. Sports Phys. Ther. 2015, 10, 520–529. [Google Scholar]
- Burd, N.A.; Andrews, R.J.; West, D.W.; Little, J.P.; Cochran, A.J.; Hector, A.J.; Cashaback, J.G.; Gibala, M.J.; Potvin, J.R.; Baker, S.K.; et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J. Physiol. 2012, 590, 351–362. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Kobesova, A.; Davidek, P.; Morris, C.E.; Andel, R.; Maxwell, M.; Oplatkova, L.; Safarova, M.; Kumagai, K.; Kolar, P. Functional postural-stabilization tests according to Dynamic Neuromuscular Stabilization approach: Proposal of novel examination protocol. J. Bodyw. Mov. Ther. 2020, 24, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Choi, D.H.; Shin, C.S. The Effect of Strength Training Targeting Medial Thigh Muscles on Neuromuscular and Biomechanical Risk Factors for Anterior Cruciate Ligament Injury: A Randomized Controlled Trial. Sports Med. Open 2025, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Chester, R.; Smith, T.O.; Sweeting, D.; Dixon, J.; Wood, S.; Song, F. The relative timing of VMO and VL in the aetiology of anterior knee pain: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2008, 9, 64. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Z.; Li, Y.; Zhu, X.; Fan, J.; Meng, L.; Zhang, Q. Effects of dynamic neuromuscular stabilization/vojta therapy on pain intensity and physical function in individuals with low back pain: A systematic review and meta-analysis. Iran. J. Public Health 2024, 53, 1910–1923. [Google Scholar] [CrossRef]
- Alvani, E.; Ziya, M.; Sahebozamani, M. The effect of dynamic neuromuscular stability (DNS) training on dynamic balance and functional disability in athletes with non-specific chronic low back pain. J. Res. Sport Rehabil. 2020, 8, 127–138. [Google Scholar] [CrossRef]
- Karartı, C.; Özsoy, İ.; Özyurt, F.; Basat, H.Ç.; Özsoy, G.; Özüdoğru, A. The effects of dynamic neuromuscular stabilization approach on clinical outcomes in older patients with chronic nonspecific low back pain: A randomized, controlled clinical trial. Somatosens. Mot. Res. 2023, 40, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Soundararajan, K.; Kishen, T.J.; Janardhan, S.; Cr, S.K. Comparison of yoga and dynamic neuromuscular stabilization exercise in chronic low back pain on magnetic resonance imaging of lumbar multifidus-protocol for a randomized controlled trial. Contemp. Clin. Trials Commun. 2022, 28, 100937. [Google Scholar] [CrossRef] [PubMed]
| Parameter | DNS (n = 27) | DNS (n = 27) | p (Between-Groups) |
|---|---|---|---|
| Age (years) | 23.9 ± 1.3 | 23.6 ± 1.4 | 0.453 |
| Sex (M/F) | 14 (51.9%)/13 (48.1%) | 14 (51.9%)/13 (48.1%) | 0.579 |
| Height (cm) | 175.2 ± 5.4 | 174.4 ± 6.4 | 0.628 |
| Weight (kg) | 74.2 ± 7.2 | 74.2 ± 7.6 | 0.972 |
| BMI (kg/m2) | 24.1 ± 2.0 | 24.3 ± 2.1 | 0.780 |
| Muscle | Electrode Location | Electrode Orientation |
|---|---|---|
| Tibialis Anterior (TA) | At 1/3 on the line between the tip of the fibula and the tip of the medial malleolus. | Along the line between the fibula and the medial malleolus. |
| Gastrocnemius (GC) | On the most prominent bulge of the muscle. | Parallel to the line between the head of the fibula and the heel. |
| Vastus Lateralis (VL) | At 2/3 on the line from the Anterior Superior Iliac Spine (ASIS) to the lateral side of the patella. | In the direction of the muscle fibers. |
| Vastus Medialis (VM) | At 80% of the distance on the line between the ASIS and the joint space in front of the anterior border of the medial collateral ligament. | In an oblique direction, parallel to the muscle fibers. |
| Rectus Femoris (RF) | At 50% on the line from the ASIS to the superior part of the patella. | Along the line between the ASIS and the patella. |
| Biceps Femoris (BF) | At 50% on the line between the ischial tuberosity and the lateral epicondyle of the tibia. | Along the line between the ischial tuberosity and the fibular head. |
| Semitendinosus (ST) | At 50% on the line between the ischial tuberosity and the medial epicondyle of the tibia. | In the direction of the line between the ischial tuberosity and the tibia. |
| Gluteus Medius (GM) | At 50% on the line from the iliac crest to the greater trochanter of the femur. | Parallel to the direction of the fibers, pointing towards the greater trochanter. |
| Outcome | DNS (EMM ± SE) | Control (EMM ± SE) | F(1, 52) | p-Value | ηp2 | Levene’s p |
|---|---|---|---|---|---|---|
| VAS (0–10) | 3.0 ± 0.2 | 6.1 ± 0.2 | 76.66 | <0.001 | 0.596 | 0.976 |
| ODI (%) | 15.7 ± 1.5 | 34.3 ± 1.5 | 71.47 | <0.001 | 0.579 | <0.001 |
| Muscles | Phases | Treatment (DNS, n = 27) Mean ± SD | 95% CI | Control (n = 28) Mean ± SD | 95% CI | Sig. (2-Tailed) |
|---|---|---|---|---|---|---|
| TA | LR | 88.9 ± 25.6 | 78.7–99.0 | 80.1 ± 24.6 | 70.5–89.6 | 0.200 |
| MC | 97.5 ± 28.2 | 86.3–108.7 | 85.8 ± 24.3 | 76.3–95.2 | 0.104 | |
| PO | 90.3 ± 47.5 | 71.5–109.1 | 87.0 ± 27.5 | 76.3–97.7 | 0.752 | |
| SW | 84.2 ± 19.6 | 76.4–91.9 | 76.5 ± 21.7 | 68.1–85.0 | 0.179 | |
| GC | LR | 86.4 ± 32.3 | 73.6–99.2 | 89.0 ± 24.6 | 79.4–98.6 | 0.741 |
| MC | 85.5 ± 32.0 | 73.6–97.3 | 79.2 ± 19.6 | 71.8–86.7 | 0.387 | |
| PO | 90.4 ± 25.4 | 80.5–100.4 | 85.3 ± 30.2 | 73.6–97.1 | 0.501 | |
| SW | 84.5 ± 24.1 | 75.0–94.0 | 95.0 ± 30.4 | 83.4–106.7 | 0.163 | |
| VL | LR | 64.5 ± 21.5 | 56.0–73.0 | 74.6 ± 24.4 | 65.9–84.8 | 0.110 |
| MC | 76.1 ± 26.8 | 65.7–86.5 | 75.3 ± 24.2 | 66.7–84.0 | 0.918 | |
| PO | 71.8 ± 29.3 | 60.2–83.4 | 77.9 ± 30.2 | 66.2–89.6 | 0.449 | |
| SW | 66.1 ± 21.8 | 57.4–74.7 | 75.4 ± 30.2 | 63.7–87.1 | 0.196 | |
| VM | LR | 70.2 ± 23.4 | 61.7–78.7 | 78.4 ± 21.2 | 70.3–86.5 | 0.183 |
| MC | 75.9 ± 28.1 | 64.7–87.0 | 70.3 ± 16.0 | 64.0–76.5 | 0.365 | |
| PO | 78.4 ± 30.7 | 66.1–90.8 | 72.5 ± 24.9 | 62.0–82.9 | 0.707 | |
| SW | 68.4 ± 26.4 | 58.3–78.5 | 80.9 ± 27.2 | 70.2–91.5 | 0.091 | |
| RF | LR | 67.1 ± 18.7 | 59.7–74.5 | 79.2 ± 25.4 | 69.4–89.1 | 0.051 |
| MC | 76.2 ± 25.3 | 66.1–86.4 | 73.9 ± 16.5 | 67.7–80.1 | 0.685 | |
| PO | 74.8 ± 26.2 | 64.7–84.9 | 78.6 ± 19.5 | 71.5–85.7 | 0.542 | |
| SW | 69.3 ± 21.4 | 61.0–77.7 | 81.1 ± 28.8 | 70.0–92.3 | 0.090 | |
| BF | LR | 74.1 ± 21.4 | 65.8–82.4 | 75.9 ± 27.7 | 65.3–86.5 | 0.791 |
| MC | 86.9 ± 29.5 | 75.5–98.2 | 82.0 ± 30.6 | 70.4–93.6 | 0.552 | |
| PO | 78.9 ± 36.3 | 65.5–92.3 | 78.9 ± 26.7 | 68.7–89.1 | 0.999 | |
| SW | 76.6 ± 22.6 | 68.1–85.0 | 79.7 ± 31.6 | 67.7–91.6 | 0.679 | |
| ST | LR | 82.0 ± 30.9 | 70.1–93.9 | 82.5 ± 31.8 | 70.2–94.9 | 0.947 |
| MC | 79.0 ± 32.0 | 66.7–91.3 | 66.8 ± 24.1 | 57.5–76.2 | 0.116 | |
| PO | 86.0 ± 34.8 | 73.0–98.9 | 89.4 ± 32.3 | 77.3–101.6 | 0.703 | |
| SW | 75.8 ± 29.1 | 64.6–86.9 | 79.7 ± 23.4 | 70.9–88.5 | 0.583 | |
| GM | LR | 72.9 ± 26.1 | 63.0–82.9 | 77.9 ± 30.2 | 66.8–89.0 | 0.517 |
| MC | 74.9 ± 32.3 | 62.7–87.2 | 67.7 ± 16.8 | 61.2–74.2 | 0.302 | |
| PO | 79.9 ± 30.7 | 68.0–91.9 | 81.7 ± 20.1 | 74.9–88.5 | 0.802 | |
| SW | 67.3 ± 25.5 | 57.8–76.9 | 76.7 ± 20.9 | 69.7–83.8 | 0.141 |
| Muscle | Gait Phase | Adjusted Mean ± SE (DNS) [95% CI] | Adjusted Mean ± SE (Control) [95% CI] | F(1, 52) | p-Value | ηp2 |
|---|---|---|---|---|---|---|
| TA | LR | 88.6 ± 7.3 [73.9–103.2] | 104.6 ± 7.1 [90.2–119.0] | 2.406 | 0.127 | 0.044 |
| GC | 106.0 ± 13.7 [78.5–133.6] | 85.5 ± 13.4 [58.4–112.6] | 1.138 | 0.291 | 0.021 | |
| VL | 70.8 ± 4.5 [61.6–80.0] | 64.0 ± 4.5 [54.9–73.0] | 1.084 | 0.303 | 0.020 | |
| VM | 80.7 ± 4.3 [72.1–89.4] | 68.1 ± 4.2 [59.6–76.5] | 4.351 | 0.042 * | 0.077 | |
| RF | 80.1 ± 4.7 [70.5–89.8] | 80.9 ± 4.7 [71.5–90.4] | 0.014 | 0.908 | 0.000 | |
| BF | 81.6 ± 4.8 [71.9–91.3] | 85.9 ± 4.7 [76.4–95.4] | 0.405 | 0.527 | 0.008 | |
| ST | 84.0 ± 4.3 [75.2–92.7] | 84.9 ± 4.2 [76.3–93.5] | 0.025 | 0.875 | 0.000 | |
| GM | 69.5 ± 4.3 [60.8–78.1] | 77.8 ± 4.2 [69.3–86.3] | 1.909 | 0.173 | 0.035 | |
| TA | MC | 78.5 ± 4.1 [70.3–86.7] | 95.2 ± 4.0 [87.2–103.3] | 8.263 | 0.006 * | 0.137 |
| GC | 79.6 ± 4.0 [71.4–87.8] | 83.5 ± 4.0 [75.4–91.5] | 0.442 | 0.509 | 0.008 | |
| VL | 74.2 ± 4.0 [66.1–82.3] | 75.7 ± 3.9 [67.8–83.6] | 0.070 | 0.793 | 0.001 | |
| VM | 72.1 ± 5.3 [61.3–82.9] | 85.2 ± 5.2 [74.7–95.8] | 3.033 | 0.088 | 0.055 | |
| RF | 72.0 ± 4.5 [62.8–81.1] | 83.6 ± 4.4 [74.7–92.6] | 3.323 | 0.074 | 0.060 | |
| BF | 77.0 ± 5.3 [66.3–87.8] | 91.0 ± 5.2 [80.5–101.6] | 3.493 | 0.067 | 0.063 | |
| ST | 76.6 ± 4.9 [66.7–86.4] | 83.3 ± 4.8 [73.6–92.9] | 0.925 | 0.341 | 0.017 | |
| GM | 73.8 ± 5.5 [62.7–84.9] | 76.2 ± 5.4 [65.4–87.1] | 0.099 | 0.754 | 0.002 | |
| TA | PO | 79.2 ± 5.2 [68.6–89.8] | 88.3 ± 5.1 [77.9–98.7] | 1.510 | 0.225 | 0.028 |
| GC | 90.0 ± 4.5 [80.8–99.1] | 86.1 ± 4.4 [77.1–95.1] | 0.372 | 0.544 | 0.007 | |
| VL | 68.8 ± 3.4 [61.9–75.7] | 74.3 ± 3.3 [67.6–81.1] | 1.314 | 0.257 | 0.025 | |
| VM | 72.8 ± 4.0 [64.6–80.9] | 70.0 ± 3.9 [62.0–77.9] | 0.244 | 0.624 | 0.005 | |
| RF | 65.6 ± 5.2 [55.1–76.2] | 85.2 ± 5.1 [74.8–95.5] | 7.022 | 0.011 * | 0.119 | |
| BF | 68.0 ± 3.7 [60.5–75.5] | 84.9 ± 3.6 [77.6–92.3] | 10.489 | 0.002 * | 0.168 | |
| ST | 76.9 ± 4.5 [67.7–86.0] | 83.9 ± 4.4 [74.9–92.9] | 1.211 | 0.276 | 0.023 | |
| GM | 72.9 ± 4.8 [63.2–82.6] | 72.4 ± 4.7 [62.8–81.9] | 0.006 | 0.939 | 0.000 | |
| TA | SW | 82.3 ± 4.6 [73.0–91.5] | 86.3 ± 4.5 [77.2–95.4] | 0.388 | 0.536 | 0.007 |
| GC | 84.0 ± 5.8 [72.3–95.7] | 85.0 ± 5.7 [73.5–96.5] | 0.014 | 0.905 | 0.000 | |
| VL | 70.2 ± 5.4 [59.2–81.2] | 78.9 ± 5.3 [68.1–89.7] | 1.276 | 0.264 | 0.024 | |
| VM | 66.2 ± 4.3 [57.6–74.9] | 75.9 ± 4.2 [67.5–84.4] | 2.516 | 0.119 | 0.046 | |
| RF | 69.4 ± 4.4 [60.4–78.3] | 74.5 ± 4.3 [65.8–83.3] | 0.673 | 0.416 | 0.013 | |
| BF | 68.0 ± 4.0 [59.9–76.1] | 74.4 ± 3.9 [66.5–82.4] | 1.268 | 0.265 | 0.024 | |
| ST | 76.7 ± 4.3 [67.9–85.5] | 80.0 ± 4.2 [71.4–88.6] | 0.288 | 0.594 | 0.006 | |
| GM | 75.7 ± 5.1 [65.4–85.9] | 69.4 ± 5.0 [59.3–79.5] | 0.756 | 0.389 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezazadeh, F.; Aali, S.; Imani, F.; Sheikhalizadeh, H.; Ouergui, I.; Enoiu, R.-S.; Ardigò, L.P.; Badicu, G. Effects of Dynamic Neuromuscular Stabilization on Lower Limb Muscle Activity, Pain, and Disability in Individuals with Chronic Low Back Pain: A Randomized Controlled Trial. Medicina 2025, 61, 1961. https://doi.org/10.3390/medicina61111961
Rezazadeh F, Aali S, Imani F, Sheikhalizadeh H, Ouergui I, Enoiu R-S, Ardigò LP, Badicu G. Effects of Dynamic Neuromuscular Stabilization on Lower Limb Muscle Activity, Pain, and Disability in Individuals with Chronic Low Back Pain: A Randomized Controlled Trial. Medicina. 2025; 61(11):1961. https://doi.org/10.3390/medicina61111961
Chicago/Turabian StyleRezazadeh, Farhad, Shirin Aali, Fariborz Imani, Hamed Sheikhalizadeh, Ibrahim Ouergui, Razvan-Sandu Enoiu, Luca Paolo Ardigò, and Georgian Badicu. 2025. "Effects of Dynamic Neuromuscular Stabilization on Lower Limb Muscle Activity, Pain, and Disability in Individuals with Chronic Low Back Pain: A Randomized Controlled Trial" Medicina 61, no. 11: 1961. https://doi.org/10.3390/medicina61111961
APA StyleRezazadeh, F., Aali, S., Imani, F., Sheikhalizadeh, H., Ouergui, I., Enoiu, R.-S., Ardigò, L. P., & Badicu, G. (2025). Effects of Dynamic Neuromuscular Stabilization on Lower Limb Muscle Activity, Pain, and Disability in Individuals with Chronic Low Back Pain: A Randomized Controlled Trial. Medicina, 61(11), 1961. https://doi.org/10.3390/medicina61111961











