The Association and Significance of MDM2 and NF-κB Protein Expression in Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Evaluation of the Immunostaining
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
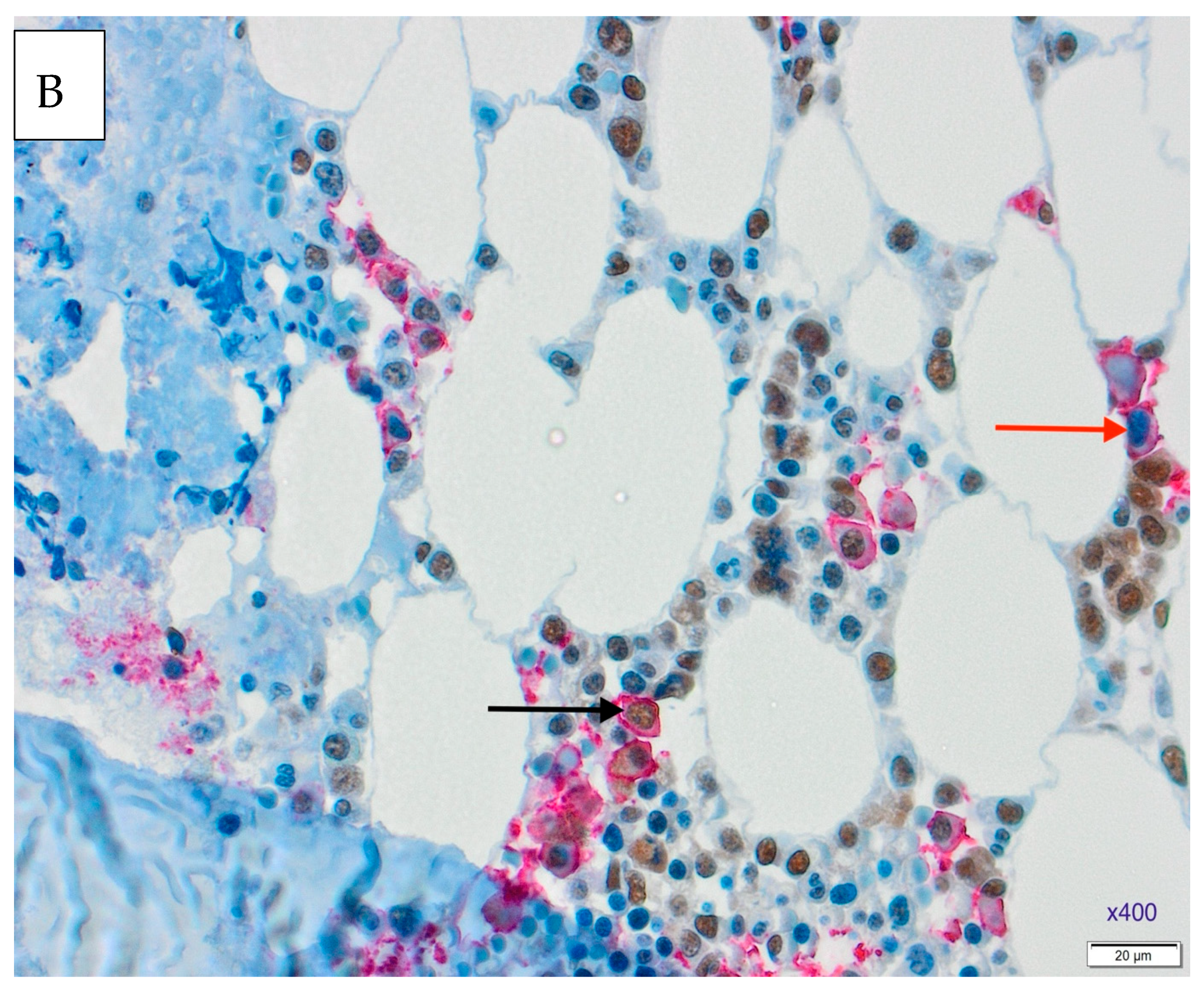
3.1. Results of Double Immunohistochemical Staining in the Bone Marrow
3.2. Expressions of NF-κB and MDM2 Proteins Before and After Therapy
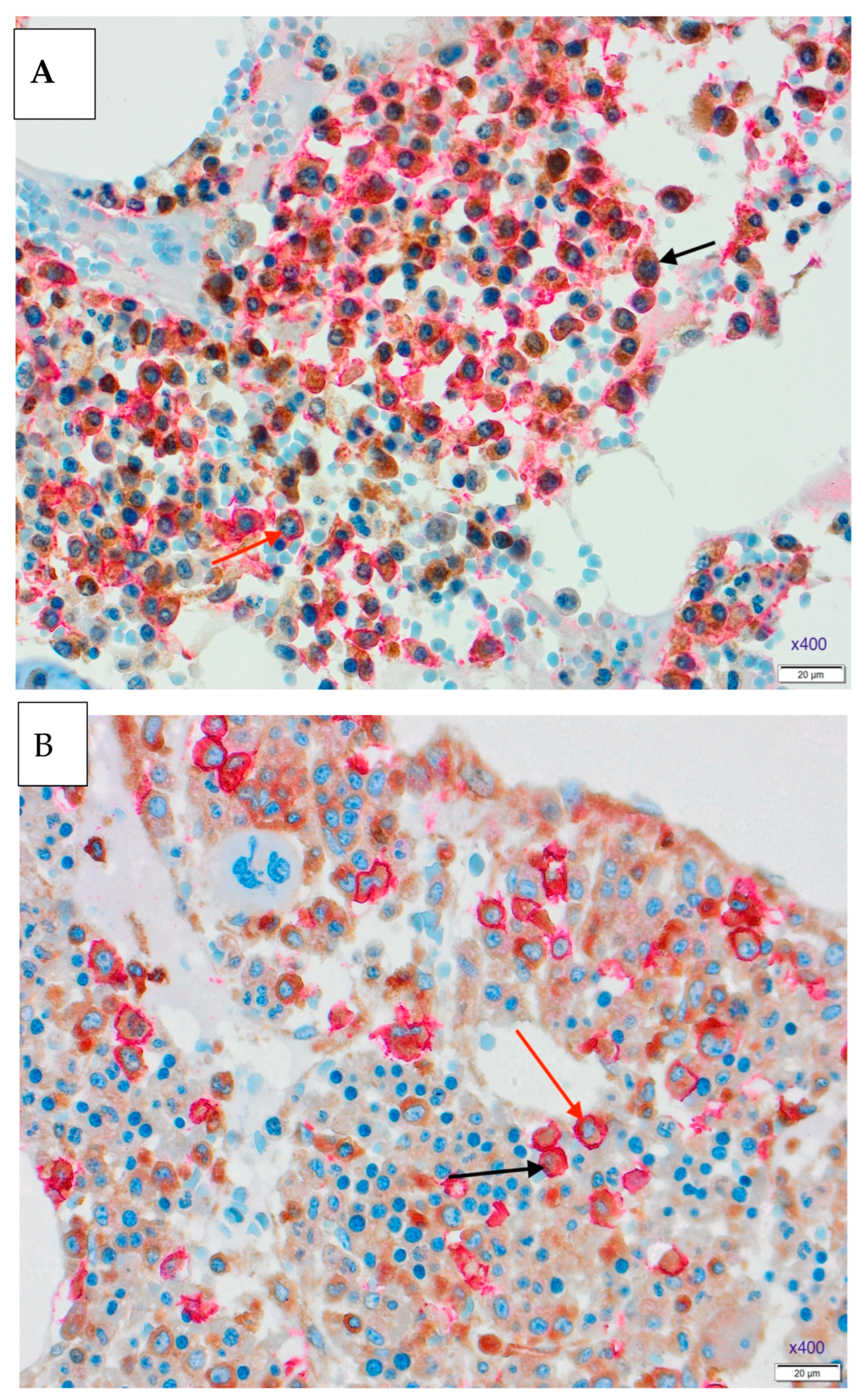
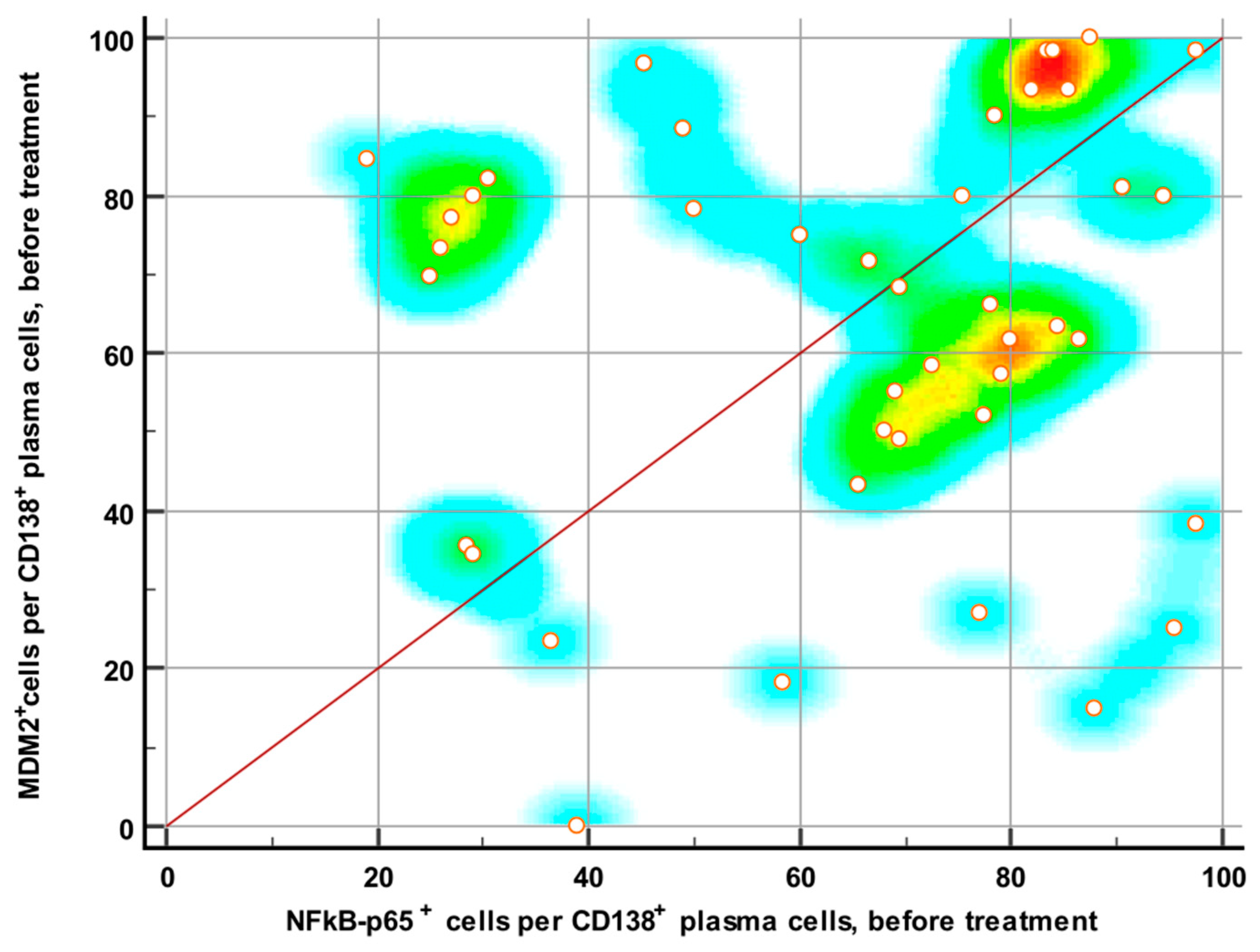
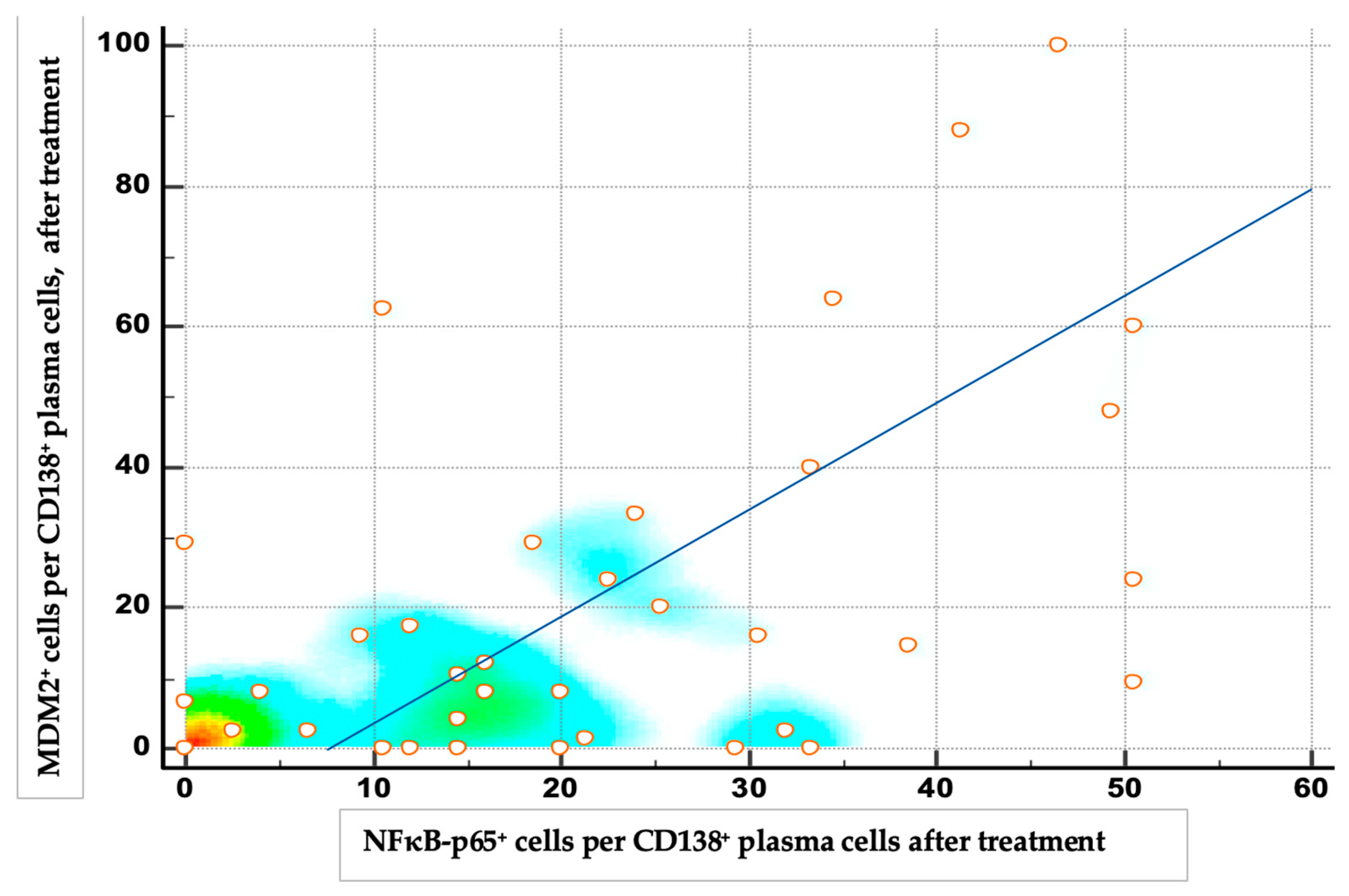
3.3. Expressions of NF-κB and MDM2 Proteins and Treatment Response
3.4. NF-κB and MDM2 Proteins Expression and Clinical/Prognostic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdi, J.; Rastgoo, N.; Li, L.; Chen, W.; Chang, H. Role of tumor suppressor p53 and micro-RNA interplay in multiple myeloma pathogenesis. J. Hematol. Oncol. 2017, 10, 169. [Google Scholar] [CrossRef]
- Zhang, Z.; He, G.; Lv, Y.; Liu, Y.; Niu, Z.; Feng, Q.; Hu, R.; Xu, J. HERC3 regulates epithelial-mesenchymal transition by directly ubiquitination degradation EIF5A2 and inhibits metastasis of colorectal cancer. Cell Death Dis. 2022, 13, 74. [Google Scholar] [CrossRef]
- Marine, J. MDM2 and MDMX in Cancer and Development. Curr. Top. Dev. Biol. 2011, 94, 45–75. [Google Scholar] [CrossRef]
- Moll, U.M.; Zaika, A. Disrupting the p53-mdm2 interaction as a potential therapeutic modality. Drug Resist. Updates 2000, 3, 217. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hsu, J.L.; Hung, M. Regulation of Ubiquitination-Mediated Protein Degradation by Survival Kinases in Cancer. Front. Oncol. 2012, 2, 20175. [Google Scholar] [CrossRef]
- Bose, I.; Ghosh, B. The p53-MDM2 network: From oscillations to apoptosis. arXiv 2007. [Google Scholar] [CrossRef] [PubMed]
- Moll, U.M.; Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar] [PubMed]
- Hou, H.; Sun, D.; Zhang, X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Q.; Li, Z.; Zhang, H. MDM2, current research status and prospects of tumor treatment. Cancer Cell Int. 2024, 24, 170. [Google Scholar] [CrossRef] [PubMed]
- Thut, C.J.; Goodrich, J.A.; Tjian, R. Repression of p53-mediated transcription by MDM2, a dual mechanism. Genes. Dev. 1997, 11, 1974. [Google Scholar] [CrossRef]
- Thomasová, D.; Mulay, S.R.; Bruns, H.; Anders, H. p53-Independent Roles of MDM2 in NF-κB Signaling: Implications for Cancer Therapy, Wound Healing, and Autoimmune Diseases. Neoplasia 2012, 14, 1097. [Google Scholar] [CrossRef]
- Muthumani, P.; Karthikeyan, A.; Dhandayuthapani, S.; Venkatesan, T.; Rathinavelu, A. Pro-angiogenic effects of MDM2 through HIF-1α and NF-κB mediated mechanisms in LNCaP prostate cancer cells. Mol. Biol. Rep. 2014, 41, 5533. [Google Scholar] [CrossRef]
- Moyer, S.M.; Larsson, C.A.; Lozano, G. Mdm proteins: Critical regulators of embryogenesis and homoeostasis. J. Mol. Cell Biol. 2017, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Faruq, O.; Zhao, D.; Shrestha, M.; Vecchione, A.; Zacksenhaus, E.; Chang, H. Targeting an MDM2/MYC Axis to Overcome Drug Resistance in Multiple Myeloma. Cancers 2022, 14, 1592. [Google Scholar] [CrossRef]
- Roy, P.; Sarkar, U.A.; Basak, S. The NF-κB Activating Pathways in Multiple Myeloma. Biomedicines 2018, 6, 59. [Google Scholar] [CrossRef]
- Davis, L.N.; Walker, Z.J.; Reiman, L.T.; Parzych, S.E.; Stevens, B.M.; Jordan, C.T.; Forsberg, P.A.; Sherbenou, D.W. MYC Inhibition Potentiates CD8+ T Cells Against Multiple Myeloma and Overcomes Immunomodulatory Drug Resistance. Clin. Cancer Res. 2024, 30, 3023. [Google Scholar] [CrossRef]
- Mao, H.; Zhao, X.; Sun, S. NF-κB in inflammation and cancer. Cell. Mol. Immunol. 2025, 22, 811–839. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.H.; Shin, E.M.; Tergaonkar, V.; Chng, W.J. Targeting NF-κB Signaling for Multiple Myeloma. Cancers 2020, 12, 2203. [Google Scholar] [CrossRef]
- Mondello, P.; Cuzzocrea, S.; Navarra, M.; Mian, M. Bone marrow micro-environment is a crucial player for myelomagenesis and disease progression. Oncotarget 2017, 8, 20394. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-κB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View. Int. J. Mol. Sci. 2020, 21, 3070. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Jana, A.; Krett, N.L.; Guzman, G.; Khalid, A.; Ozden, O.; Staudacher, J.J.; Bauer, J.; Baik, S.H.; Carroll, T.; Yazici, C.; et al. NFkB is essential for activin-induced colorectal cancer migration via upregulation of PI3K-MDM2 pathway. Oncotarget 2017, 8, 37377. [Google Scholar] [CrossRef]
- Zhuang, C.; Miao, Z.; Wu, Y.; Guo, Z.; Li, J.; Yao, J.; Xing, C.; Sheng, C.; Zhang, W. Double-edged swords as cancer therapeutics: Novel, orally active, small molecules simultaneously inhibit p53-MDM2 interaction and the NF-κB pathway. J. Med. Chem. 2014, 57, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Hideshima, T.; Raje, N.; Kumar, S.; Ishitsuka, K.; Yasui, H.; Shiraishi, N.; Ribatti, D.; Nico, B.; Vacca, A.; et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006, 66, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Grubesic, A.; Valkovic, T.; Babarovic, E.; Hadzisejdic, I.; Vranic, L.; Belancic, A.; Skrtic, A.; Jonjic, N. Anti-Angiogenic Effect of Bortezomib Treatment in Multiple Myeloma is Associated with Lower NF-KB and OPN Expression. J. Biol. Regul. Homeost. Agents 2023, 37, 6535–6543. [Google Scholar]
- Dimopoulos, M.A.; Merlini, G.; Bridoux, F.; Leung, N.; Mikhael, J.; Harrison, S.J.; Kastritis, E.; Garderet, L.; Gozzetti, A.; van de Donk, N.W.C.J.; et al. Management of multiple myeloma-related renal impairment: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2023, 24, e293–e311. [Google Scholar] [CrossRef]
- Mıkhael, J.; Singh, E.; Rice, M.S. Real-world renal function among patients with multiple myeloma in the United States. Blood Cancer J. 2021, 11, 99. [Google Scholar] [CrossRef]
- Mohyuddin, G.R.; Koehn, K.; Shune, L.; Aziz, M.; Abdallah, A.-O.; McClune, B.; Ganguly, S.; McGuirk, J.; Kambhampati, S. Renal insufficiency in multiple myeloma: A systematic review and meta-analysis of all randomized trials from 2005–2019. Leuk. Lymphoma 2021, 62, 1386. [Google Scholar] [CrossRef]
- Mezzano, S.A.; Barría, M.; Droguett, M.A.; Burgos, M.E.; Ardiles, L.G.; Flores, C.; Egido, J. Tubular NF-κB and AP-1 activation in human proteinuric renal disease. Kidney Int. 2001, 60, 1366. [Google Scholar] [CrossRef] [PubMed]
- Wardle, E.N. Antagonism of Nuclear Factor Kappa B. Nephron J. 2002, 90, 239. [Google Scholar] [CrossRef] [PubMed]
- Schiano, C.; Soricelli, A.; Nigris Fde Napoli, C. New challenges in integrated diagnosis by imaging and osteo-immunology in bone lesions. Expert. Rev. Clin. Immunol. 2018, 15, 289. [Google Scholar] [CrossRef] [PubMed]


| Subjects N = 42 (%) | |
|---|---|
| Gender | Male—17 (40.5) Female—25 (59.5) |
| Paraprotein type | IgG kappa—19 (45.3) IgG lambda—4 (9.5) IgA kappa—6 (14.3) IgA lambda—3 (7.1) FLC * kappa—3 (7.1) FLC * lambda—7 (16.7) |
| International staging system (ISS) | ISS I—14 (33.3) ISS II—10 (23.9) ISS III—18 (42.8) |
| Anemia | Present—21 (50.0) Absent—21 (50.0) |
| Hypercalcemia | Present—3 (7.2) Absent—39 (92.8) |
| Bone lesions | Present—31 (74.0) Absent—11 (26.0) |
| Renal impairment | Present—15 (35.7) Absent—27 (64.3) |
| Therapy | VCD *—32 (76.1) VD *—10 (23.8) |
| Response to treatment | CR *—17 (40.6) VGPR *—12 (28.5) PR *—12 (28.5) PD *—1 (2.3) |
| Treatment Response | N = 42 (%) | Δ NFκB (%) Median (IQR) | Δ MDM2 (%) Median (IQR) |
|---|---|---|---|
| CR | 17 (40.6) | −54.4 (−76.0–−24.4) | −51.7 (−80.5–−18.6) |
| VGPR | 12 (28.5) | −58.1 (−77.4–−21.8) | −66.5 (−81–−44.44) |
| PR | 12 (28.5) | −32.4 (−52.7–−22.5) | −28.6 (−51.8–2.0) |
| PD | 1 (2.3) | −29.4 (29.4–−29.4) | −1.6 (−1.6–−1.6) |
| p | / | 0.461 | 0.051 |
| Parameter | Before Therapy Median (IQR) | After Therapy Median (IQR) | p * |
|---|---|---|---|
| NFkB (%) | 71 (45.3–84) | 16 (4–32) | <0.001 |
| MDM2 (%) | 68.95 (49–82) | 8 (0–24) | <0.001 |
| Haemoglobin (g/L) * | 101.8 ± 22.7 | 118.2 ± 14.8 | 0.052 |
| Creatinine (μmol/L) | 91.5 (67–127) | 75.0 (64–100) | 0.002 |
| Calcium (mmol/L) | 2.35 (2.21–2.50) | 2.30 (2.21–2.36) | 0.079 |
| LD * (U/L) | 165 (137–194) | 189.5 (176.0–206) | 0.039 |
| Albumin (g/L) | 36.5 (33–42) | 60 (49.1–63.6) | <0.001 |
| B2MG * (mg/L) | 4.3 (2.7–8.4) | 3.0 (22–3.9) | <0.001 |
| Parameter | NFKB | MDM2 | ||||
|---|---|---|---|---|---|---|
| Univariate Regression | Multivariate Regression | Univariate Regression | ||||
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Creatinine (decrease) | 0.963 (0.927–0.999) | 0.049 | 0.979 (0.956–1.00) | 0.017 | 1.016 (0.994–1.038) | 0.159 |
| Albumin (increase) | 0.982 (0.942–1.024) | 0.395 | / | / | 1.017 (0.991–1.045) | 0.213 |
| β2 microglobulin (reduction) | 0.991 (0.961–1.022) | 0.568 | / | / | 0.999 (0.978–1.020) | 0.918 |
| LD (reduction) | 0.987 (0.958–1.017) | 0.385 | / | / | 0.991 (0.970–1.011) | 0.360 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanić Damić, M.; Grubešić, A.; Perić, Z.; Jonjić, N.; Seili-Bekafigo, I.; Hauser, G.; Jajaš, N.; Valković, T. The Association and Significance of MDM2 and NF-κB Protein Expression in Multiple Myeloma. Medicina 2025, 61, 1948. https://doi.org/10.3390/medicina61111948
Stanić Damić M, Grubešić A, Perić Z, Jonjić N, Seili-Bekafigo I, Hauser G, Jajaš N, Valković T. The Association and Significance of MDM2 and NF-κB Protein Expression in Multiple Myeloma. Medicina. 2025; 61(11):1948. https://doi.org/10.3390/medicina61111948
Chicago/Turabian StyleStanić Damić, Marija, Aron Grubešić, Zinaida Perić, Nives Jonjić, Irena Seili-Bekafigo, Goran Hauser, Nina Jajaš, and Toni Valković. 2025. "The Association and Significance of MDM2 and NF-κB Protein Expression in Multiple Myeloma" Medicina 61, no. 11: 1948. https://doi.org/10.3390/medicina61111948
APA StyleStanić Damić, M., Grubešić, A., Perić, Z., Jonjić, N., Seili-Bekafigo, I., Hauser, G., Jajaš, N., & Valković, T. (2025). The Association and Significance of MDM2 and NF-κB Protein Expression in Multiple Myeloma. Medicina, 61(11), 1948. https://doi.org/10.3390/medicina61111948






