Postoperative Hypoalbuminemia as a Predictor of Early Mortality After Cementless Hemiarthroplasty for Hip Fractures
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgery and Rehabilitation
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhandari, M.; Swiontkowski, M. Management of acute hip fracture. N. Engl. J. Med. 2017, 377, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Page, P.R.J.; Poole, W.E.C.; Shah, K.; Upadhyay, P.K. Short or long intramedullary devices for hip fracture? A systematic review of the evidence. J. Orthop. 2020, 22, 377–382. [Google Scholar] [CrossRef]
- Veronese, N.; Maggi, S. Epidemiology and social costs of hip fracture. Injury 2018, 49, 1458–1460. [Google Scholar] [CrossRef]
- Hu, F.; Jiang, C.; Shen, J.; Tang, P.; Wang, Y. Preoperative predictors for mortality following hip fracture surgery: A systematic review and meta-analysis. Injury 2012, 43, 676–685. [Google Scholar] [CrossRef]
- Omsland, T.K.; Emaus, N.; Tell, G.S.; Magnus, J.H.; Ahmed, L.A.; Holvik, K.; Center, J.; Forsmo, S.; Gjesdal, C.G.; Schei, B.; et al. Mortality following the first hip fracture in Norwegian women and men (1999–2008). A NOREPOS study. Bone 1999, 63, 81–86. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Ehrenstein, V.; Szépligeti, S.K.; Lunde, A.; Lagerros, Y.T.; Westerlund, A.; Tell, G.S.; Sørensen, H.T. Thirty-five–year trends in first-time hospitalization for hip fracture, 1-year mortality, and the prognostic impact of comorbidity. Epidemiology 1980, 28, 898–905. [Google Scholar] [CrossRef]
- Smith, T.; Pelpola, K.; Ball, M.; Ong, A.; Myint, P.K. Pre-operative indicators for mortality following hip fracture surgery: A systematic review and meta-analysis. Age Ageing 2014, 43, 464–471. [Google Scholar] [CrossRef]
- Xing, F.; Luo, R.; Chen, W.; Zhou, X. The Risk-adjusted Charlson Comorbidity Index as a new predictor of one-year mortality rate in elderly Chinese patients who underwent hip fracture surgery. Orthop. Traumatol. Surg. Res. 2021, 107, 102860. [Google Scholar] [CrossRef] [PubMed]
- Capkin, S.; Guler, S.; Ozmanevra, R. C-reactive protein to albumin ratio may predict mortality for elderly population who undergo hemiarthroplasty due to hip fracture. J. Investig. Surg. 2021, 34, 1272–1277. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chou, C.H.; Su, H.H.; Tsai, Y.T.; Chiang, M.H.; Kuo, Y.J.; Chen, Y.P. Correlation between neutrophil-to-lymphocyte ratio and postoperative mortality in elderly patients with hip fracture: A meta-analysis. J. Orthop. Surg. Res. 2021, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Yang, L.; Jiang, W.; Chen, X.; Liu, Y. High platelet-to-lymphocyte ratio predicts poor survival of elderly patients with hip fracture. Int. Orthop. 2021, 45, 13–21. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, J.; Wang, J.; Wang, S.; Xia, J.; Wei, Y.; Wu, J.; Huang, G.; Chen, F.; Shi, J. Predictive values of the postoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio for the diagnosis of early periprosthetic joint infections: A preliminary study. J. Orthop. Surg. Res. 2020, 15, 571. [Google Scholar] [CrossRef]
- Bohl, D.D.; Shen, M.R.; Hannon, C.P.; Fillingham, Y.A.; Darrith, B.; Della Valle, C.J. Serum albumin predicts survival and postoperative course following surgery for geriatric hip fracture. J. Bone Jt. Surg. 2017, 99, 2110–2118. [Google Scholar] [CrossRef]
- Cabrerizo, S.; Cuadras, D.; Gomez-Busto, F.; Artaza-Artabe, I.; Marín-Ciancas, F.; Malafarina, V. Serum albumin and health in older people: Review and meta analysis. Maturitas 2015, 81, 17–27. [Google Scholar] [CrossRef]
- Kim, S.; McClave, S.A.; Martindale, R.G.; Miller, K.R.; Hurt, R.T. Hypoalbuminemia and clinical outcomes: What is the mechanism behind the relationship? Am. Surg. 2017, 83, 1220–1227. [Google Scholar] [CrossRef]
- Malafarina, V.; Reginster, J.Y.; Cabrerizo, S.; Bruyere, O.; Kanis, J.A.; Martinez, J.A.; Zulet, B.A. Nutritional status and nutritional treatment are related to outcomes and mortality in older adults with hip fracture. Nutrients 2018, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, Y.; Zhang, B.F. Elevated albumin: A protective factor against mortality in geriatric hip fracture patients. J. Orthop. Surg. Res. 2025, 20, 485. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Zheng, H.; Wang, X.; Liu, Z.; Sun, T. Prognostic role of serum albumin, total lymphocyte count, and mini nutritional assessment on outcomes after geriatric hip fracture surgery: A meta-analysis and systematic review. J. Arthroplast. 2019, 34, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.J.; Guerrero, E.M.; Tainter, D.; Dial, B.; Milton-Cole, R.; Blair, J.A.; Alexander, J.; Swamy, P.; Kuramoto, L.; Guy, P.; et al. Prognostic factors of in-hospital complications after hip fracture surgery: A scoping review. Osteoporos. Int. 2019, 30, 1339–1351. [Google Scholar] [CrossRef]
- Sofu, H.; Üçpunar, H.; Çamurcu, Y.; Duman, S.; Konya, M.N.; Gürsu, S.; Şahin, V. Predictive factors for early hospital readmission and one year mortality in elder patients following surgical treatment of a hip fracture. Turk. J. Trauma Emerg. Surg. 2017, 23, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Nijmeijer, W.S.; Hegeman, J.H.; Witteveen, A.; Groothuis-Oudshoorn, C.G.M. Systematic review and meta-analysis of preoperative predictors for early mortality following hip fracture surgery. Osteoporos. Int. 2024, 35, 561–574. [Google Scholar] [CrossRef]
- Hommel, A.; Ulander, K.; Bjorkelund, K.B.; Norrman, P.O.; Wingstrand, H.; Thorngren, K.G. Influence of optimised treatment of people with hip fracture on time to operation, length of hospital stay, reoperations and mortality within 1 year. Injury 2008, 39, 1164–1174. [Google Scholar] [CrossRef]
- Kim, S.M.; Moon, Y.W.; Lim, S.J.; Yoon, B.K.; Min, Y.K.; Lee, D.Y.; Park, Y.S. Prediction of survival, second fracture, and functional recovery following the first hip fracture surgery in elderly patients. Bone 2012, 50, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ning, T.; Wu, H.; Liu, H.; Wang, H.; Li, X.; Cao, Y. Prognostic nomogram for risk of mortality after hip fracture surgery in geriatrics. Injury 2022, 53, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Söderqvist, A.; Ekström, W.; Ponzer, S.; Pettersson, H.; Cederholm, T.; Dalén, N.; Hedström, M.; Tidermark, J. Prediction of mortality in elderly patients with hip fractures: A two-year prospective study of 1,944 patients. Gerontology 2009, 55, 496–504. [Google Scholar] [CrossRef]
- Bilsel, K.; Erdil, M.; Gulabi, D.; Elmadag, M.; Cengiz, O.; Sen, C. Factors affecting mortality after hip fracture surgery: A retrospective analysis of 578 patients. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 895–900. [Google Scholar] [CrossRef]
- Forssten, M.P.; Mohammad Ismail, A.; Ioannidis, I.; Wretenberg, P.; Borg, T.; Cao, Y.; Ribeiro, M.A.F.; Mohseni, S. The mortality burden of frailty in hip fracture patients: A nationwide retrospective study of cause-specific mortality. Eur. J. Trauma Emerg. Surg. 2023, 49, 1467–1475. [Google Scholar] [CrossRef]
- Venishetty, N.; Jose, J.; Purudappa, P.P.A.; Mounasamy, V.; Sambandam, S. Factors that influence the mortality of patients following hip hemiarthroplasty. Arthroplasty 2024, 6, 36. [Google Scholar] [CrossRef]
- Giummarra, M.J.; Ekegren, C.L.; Gong, J.; Simpson, P.; Cameron, P.A.; Edwards, E.; Gabbe, B.J. Twelve month mortality rates and independent living in people aged 65 years or older after isolated hip fracture: A prospective registry-based study. Injury 2020, 51, 420–428. [Google Scholar] [CrossRef]
- Sim, S.D.; Sim, Y.E.; Tay, K.; Howe, T.S.; Png, M.A.; Chang, C.C.P.; Abdullah, H.R.; Koh, J.S.B. Preoperative hypoalbuminemia: Poor functional outcomes and quality of life after hip fracture surgery. Bone 2021, 143, 115567. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Schwartz, N.; Sakhnini, A.; Bisharat, N. Predictive modeling of inpatient mortality in departments of internal medicine. Intern. Emerg. Med. 2018, 13, 205–211. [Google Scholar] [CrossRef]
- Foley, E.F.; Borlase, B.C.; Dzik, W.H.; Bistrian, B.R.; Benotti, P.N. Albumin supplementation in the critically ill: A prospective, randomized trial. Arch. Surg. 1990, 125, 739. [Google Scholar] [CrossRef] [PubMed]
- Liumbruno, G.; Bennardello, F.; Lattanzio, A.; Piccoli, P.; Rossettias, G. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009, 7, 216. [Google Scholar] [CrossRef]
- Huh, J.W.; Seo, H.E.; Lee, D.H.; Yoo, J.H. Risk factors of the 2-year mortality after bipolar hemiarthroplasty for displaced femoral neck fracture. Hip Pelvis 2023, 35, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, K.U. Risk factors for postoperative pneumonia in the elderly following hip fracture surgery: A systematic review and meta-analysis. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 21514593221083825. [Google Scholar] [CrossRef]
- Wiedermann, C.J. Hypoalbuminemia as surrogate and culprit of infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef] [PubMed]
- Kishawi, D.; Schwarzman, G.; Mejia, A.; Hussain, A.K.; Gonzalez, M.H. Low preoperative albumin levels predict adverse outcomes after total joint arthroplasty. J. Bone Jt. Surg. Am. 2020, 102, 889–895. [Google Scholar] [CrossRef]
- Curran, S.; Apruzzese, P.; Kendall, M.C.; De Oliveira, G. The impact of hypoalbuminemia on postoperative outcomes after outpatient surgery: A national analysis of the NSQIP database. Can. J. Anaesth. 2022, 69, 1099–1106. [Google Scholar] [CrossRef]

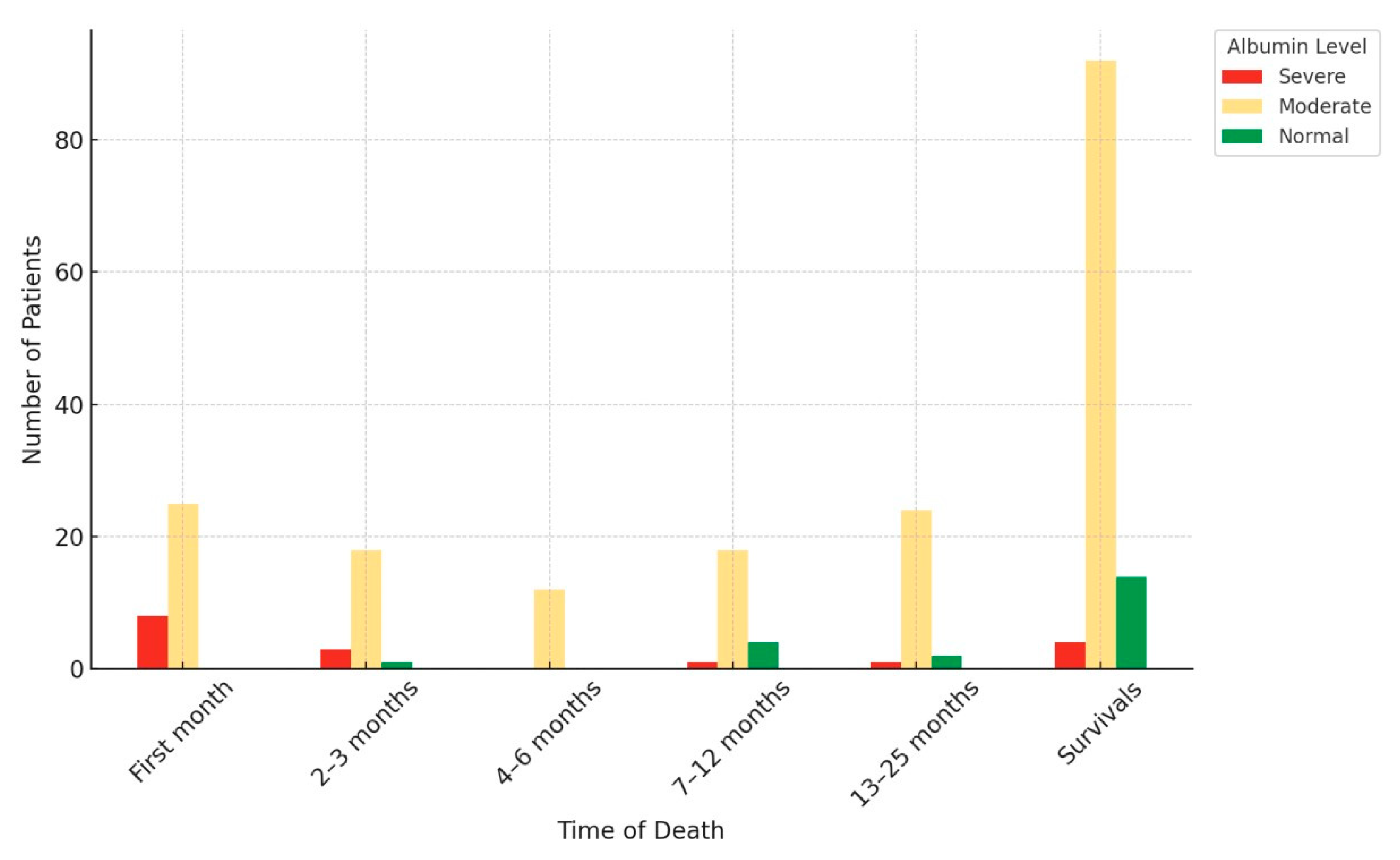
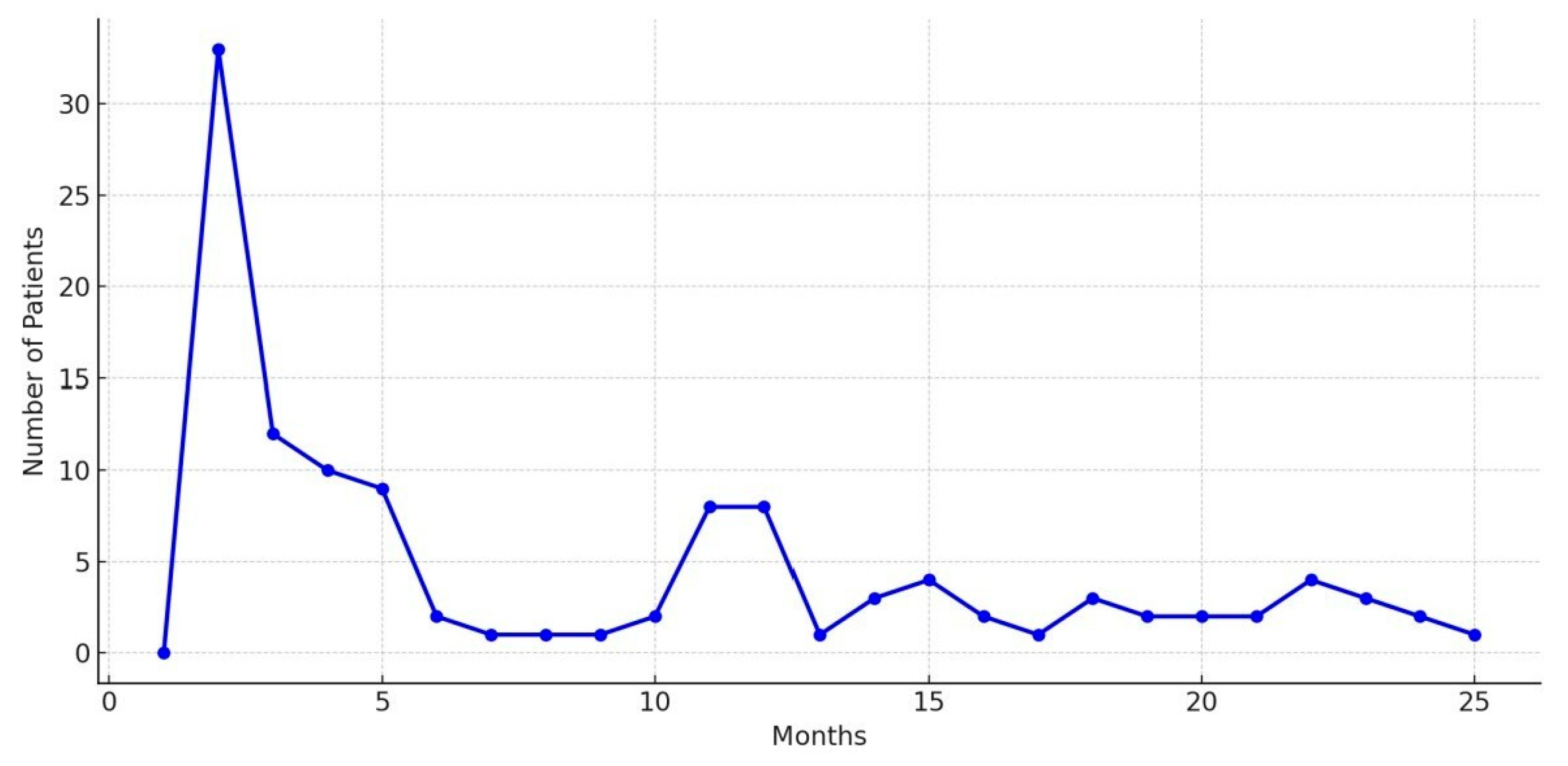
| Characteristic | Group 1 (n = 160) | Group 2 (n = 67) | p Value |
|---|---|---|---|
| Age, yr, med (min–max) | 81 (64–97) | 86 (65–96) | 0.001 a |
| Gender, n (%) | 0.875 b | ||
| Male | 51 (31.9) | 20 (29.9) | |
| Female | 109 (68.1) | 47 (70.1) | |
| Side, n (%) | 0.772 b | ||
| Right | 85 (53.1) | 37 (55.2) | |
| Left | 75 (46.9) | 30 (44.8) | |
| Fracture site, n (%) | 0.307 b | ||
| Femoral neck | 91 (56.9) | 43 (64.2) | |
| Intertrochanteric fracture | 69 (43.1) | 24 (35.8) | |
| Hospitalization duration, days, median (min–max) | 6 (2–30) | 6 (2–27) | 0.617 a |
| Intensive care status, n (%) | 0.009 b | ||
| No | 88 (55) | 24 (35.8) | |
| Yes | 72 (45) | 43 (64.2) | |
| Intensive care duration, days, median (min–max) | 1 (0–15) | 2 (0–25) | 0.006 a |
| Follow-up duration, months, median (min–max) | 21 (1–33) | 2 (1–5) | 0.001 a |
| Time from trauma to surgery, days, median (min–max) | 1 (0–27) | 1 (1–7) | 0.278 a |
| Operation duration, min, median (min–max) | 75 (30–150) | 70 (40–145) | 0.233 a |
| ASA classification, n (%) | 0.002 b | ||
| ASA 2 | 29 (18.1) | 4 (6) | |
| ASA 3 | 113 (70.6) | 45 (67.2) | |
| ASA 4 | 18 (11.3) | 18 (26.9) | |
| Charlson Comorbidity Index, median (min–max) | 5 (1–12) | 6 (3–10) | 0.002 a |
| Hypertension, n (%) | 70 (44) | 48 (71.6) | 0.001 b |
| COPD, n (%) | 20 (12.5) | 28 (41.8) | 0.001 b |
| Chronic heart disease, n (%) | 22 (14) | 39 (58.2) | 0.001 b |
| EF, median (min–max) | 60 (30–65) | 55 (30–65) | 0.032 a |
| Chronic renal disease, n (%) | 9 (5.6) | 16 (23.9) | 0.001 b |
| Ischemic stroke, n (%) | 19 (11.8) | 20 (29.8) | 0.001 b |
| Diabetes mellitus, n (%) | 26 (16.3) | 29 (43.3) | 0.001 b |
| Cancer, n (%) | 4 (2.5) | 9 (13.4) | 0.003 c |
| Dementia, n (%) | 12 (7.5) | 23 (34.3) | 0.001 b |
| Osteoporosis, n (%) | 107 (66.9) | 55 (82.1) | 0.021 a |
| HHS (mean ± SD) | 80 (70–89) | 68 (45–74) | 0.001 a |
| Complications, n (%) | |||
| Dislocation | 4 (2.5) | 2 (3) | 0.385 c |
| Infection | 9 (5.65) | 6 (8.9) |
| Parameters | Group 1 (n = 160) | Group 2 (n = 67) | p |
|---|---|---|---|
| Preoperative NLR median (min–max) | 7.24 (0.03–33.3) | 8.67 (1.86–29.2) | 0.472 a |
| Postoperative NLR median (min–max) | 8.88 (1.88–40) | 8.7 (1.71–33.4) | 0.680 a |
| Preoperative albumin median (min–max) | 38 (25–48) | 36 (21–48) | 0.001 a |
| Postoperative albumin median (min–max) | 31 (21–40) | 29 (17–37) | 0.001 a |
| Preoperative hemoglobin median (min–max) | 12.45 (9–17) | 12.5 (10–16) | 0.477 a |
| Postoperative hemoglobin median (min–max) | 10.2 (7–14) | 10.1 (7–13) | 0.418 a |
| Albumin < 29.5 | 44 (27.5) | 39 (58.2) | 0.001 b |
| Albumin > 29.6 | 116 (72.5) | 28 (41.8) |
| Variables | β | SE | p Value | OR | 95% CI |
|---|---|---|---|---|---|
| Gender | −0.095 | 0.316 | 0.764 | 0.909 | 0.489–1.691 |
| Time from trauma to surgery | −0.114 | 0.09 | 0.204 | 0.892 | 0.747–1.064 |
| Preoperative NLR | 0.009 | 0.023 | 0.689 | 1.009 | 0.965–1.05 |
| Postoperative NLR | −0.015 | 0.023 | 0.512 | 0.985 | 0.941–1.031 |
| Fracture site | −0.306 | 0.301 | 0.308 | 0.736 | 0.408–1.327 |
| Operation duration | −0.008 | 0.007 | 0.244 | 0.992 | 0.980–1.005 |
| Preoperative hemoglobin | −0.078 | 0.094 | 0.405 | 0.925 | 0.770–1.111 |
| Postoperative hemoglobin | −0.102 | 0.103 | 0.321 | 0.903 | 0.737–1.105 |
| Hospitalization duration | 0.017 | 0.031 | 0.581 | 1.017 | 0.958–1.080 |
| Age | 0.061 | 0.02 | 0.003 | 1.063 | 1.022–1.106 |
| EF | −0.059 | 0.024 | 0.013 | 0.943 | 0.900–0.988 |
| ASA 3 | 1.06 | 0.562 | 0.059 | 2.887 | 0.960–8.682 |
| ASA 4 | 1.981 | 0.629 | 0.002 | 7.25 | 2.113–24.872 |
| Preoperative albumin | −0.156 | 0.039 | 0.001 | 0.856 | 0.793–0.924 |
| Postoperative albumin | −0.208 | 0.045 | 0.001 | 0.812 | 0.744–0.887 |
| CCI | 0.247 | 0.092 | 0.007 | 1.28 | 1.068–1.535 |
| Intensive care status | 0.784 | 0.3 | 0.009 | 2.19 | 1.216–3.945 |
| Intensive care duration | 0.124 | 0.047 | 0.008 | 1.132 | 1.033–1.240 |
| Variables | β | SE | p Value | OR | 95% CI |
|---|---|---|---|---|---|
| 1st month | |||||
| Constant | 6.789 | 3.556 | 0.056 | 887.859 | |
| Postoperative albumin | −0.190 | 0.075 | 0.011 | 0.827 | 0.714–0.958 |
| 3rd month | |||||
| Constant | 4.235 | 3.07 | 0.167 | 69.05 | |
| Postoperative albumin | −0.207 | 0.066 | 0.002 | 0.813 | 0.715–0.925 |
| 6th month | |||||
| Constant | 3.469 | 2.911 | 0.233 | 32.108 | |
| Postoperative albumin | −0.180 | 0.061 | 0.003 | 0.835 | 0.741–0.941 |
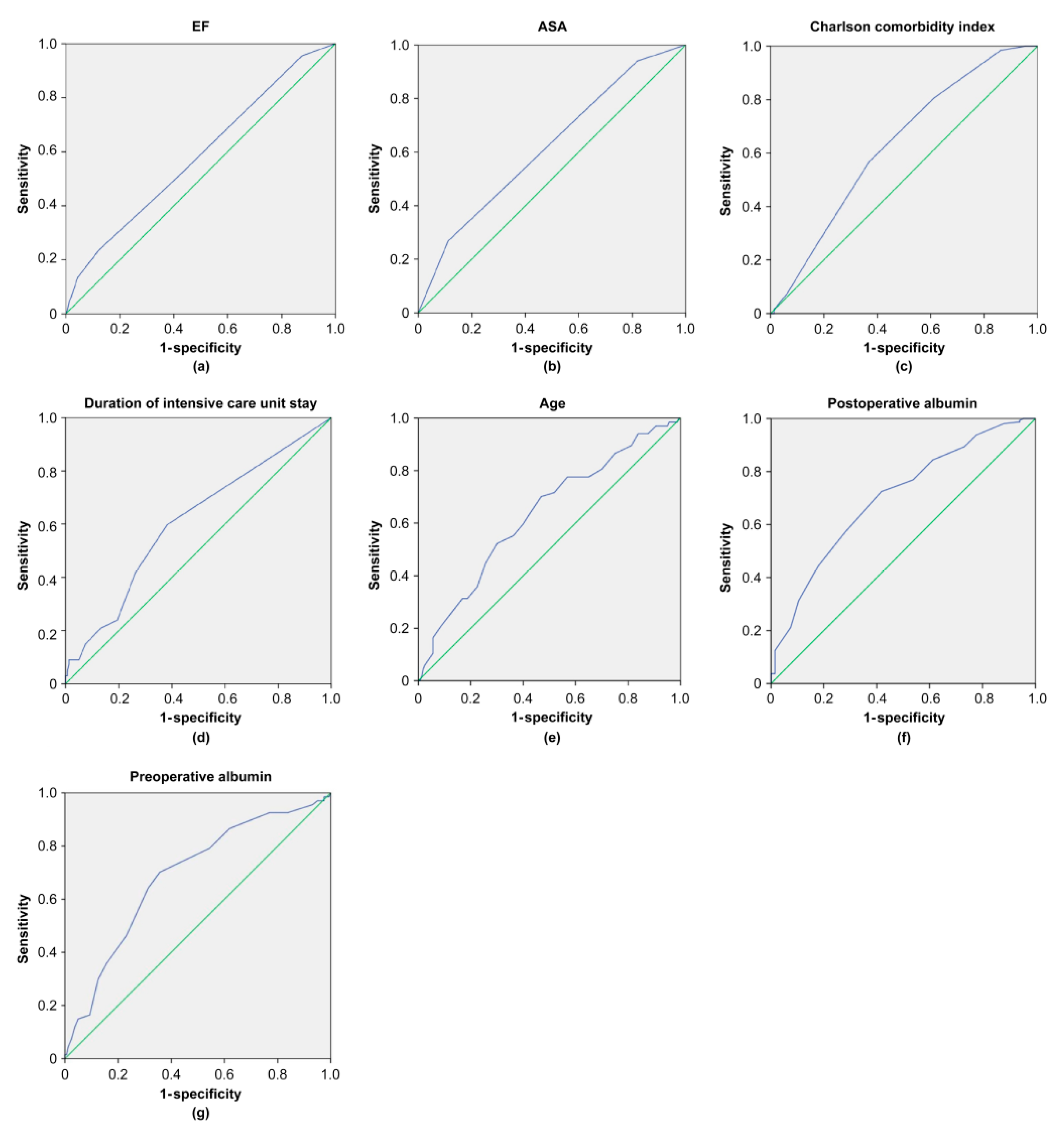
| Risk Factor | AUC (95% CI) | p Value | Cutoff | Sensitivity (%) | Specificity (%) | J |
|---|---|---|---|---|---|---|
| Preoperative albumin | 0.688 (0.613–0.764) | 0.001 | 3.75 | 70.1 | 64.4 | 0.345 |
| Postoperative albumin | 0.700 (0.626–0.774) | 0.001 | 2.95 | 72.5 | 58.2 | 0.307 |
| Age | 0.634 (0.555–0.714) | 0.001 | 81.5 | 70.1 | 53.1 | 0.233 |
| EF | 0.585 (0.503–0.666) | 0.044 | 52.5 | 23.9 | 87.5 | 0.114 |
| ASA | 0.618 (0.538–0.697) | 0.005 | 3.5 | 26.9 | 88.7 | 0.156 |
| CCI | 0.629 (0.554–0.704) | 0.002 | 5.5 | 56.7 | 63.1 | 0.198 |
| Intensive care duration | 0.608 (0.527–0.689) | 0.01 | 1.5 | 59.7 | 61.9 | 0.216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melez, M.; Altun, İ.; Ünlü, Ö.C.; Okur, K.T.; Ozan, F. Postoperative Hypoalbuminemia as a Predictor of Early Mortality After Cementless Hemiarthroplasty for Hip Fractures. Medicina 2025, 61, 1936. https://doi.org/10.3390/medicina61111936
Melez M, Altun İ, Ünlü ÖC, Okur KT, Ozan F. Postoperative Hypoalbuminemia as a Predictor of Early Mortality After Cementless Hemiarthroplasty for Hip Fractures. Medicina. 2025; 61(11):1936. https://doi.org/10.3390/medicina61111936
Chicago/Turabian StyleMelez, Muhammed, İbrahim Altun, Ömer Can Ünlü, Kürşat Tuğrul Okur, and Fırat Ozan. 2025. "Postoperative Hypoalbuminemia as a Predictor of Early Mortality After Cementless Hemiarthroplasty for Hip Fractures" Medicina 61, no. 11: 1936. https://doi.org/10.3390/medicina61111936
APA StyleMelez, M., Altun, İ., Ünlü, Ö. C., Okur, K. T., & Ozan, F. (2025). Postoperative Hypoalbuminemia as a Predictor of Early Mortality After Cementless Hemiarthroplasty for Hip Fractures. Medicina, 61(11), 1936. https://doi.org/10.3390/medicina61111936





