Effect of Neurokinin-1 Receptor Antagonists on Experimental Postoperative Adhesion: A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Preclinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Literature Search
2.4. Study Selection
2.5. Data Extraction
2.6. Methodological Quality and Publication
2.7. Statistical Analyses
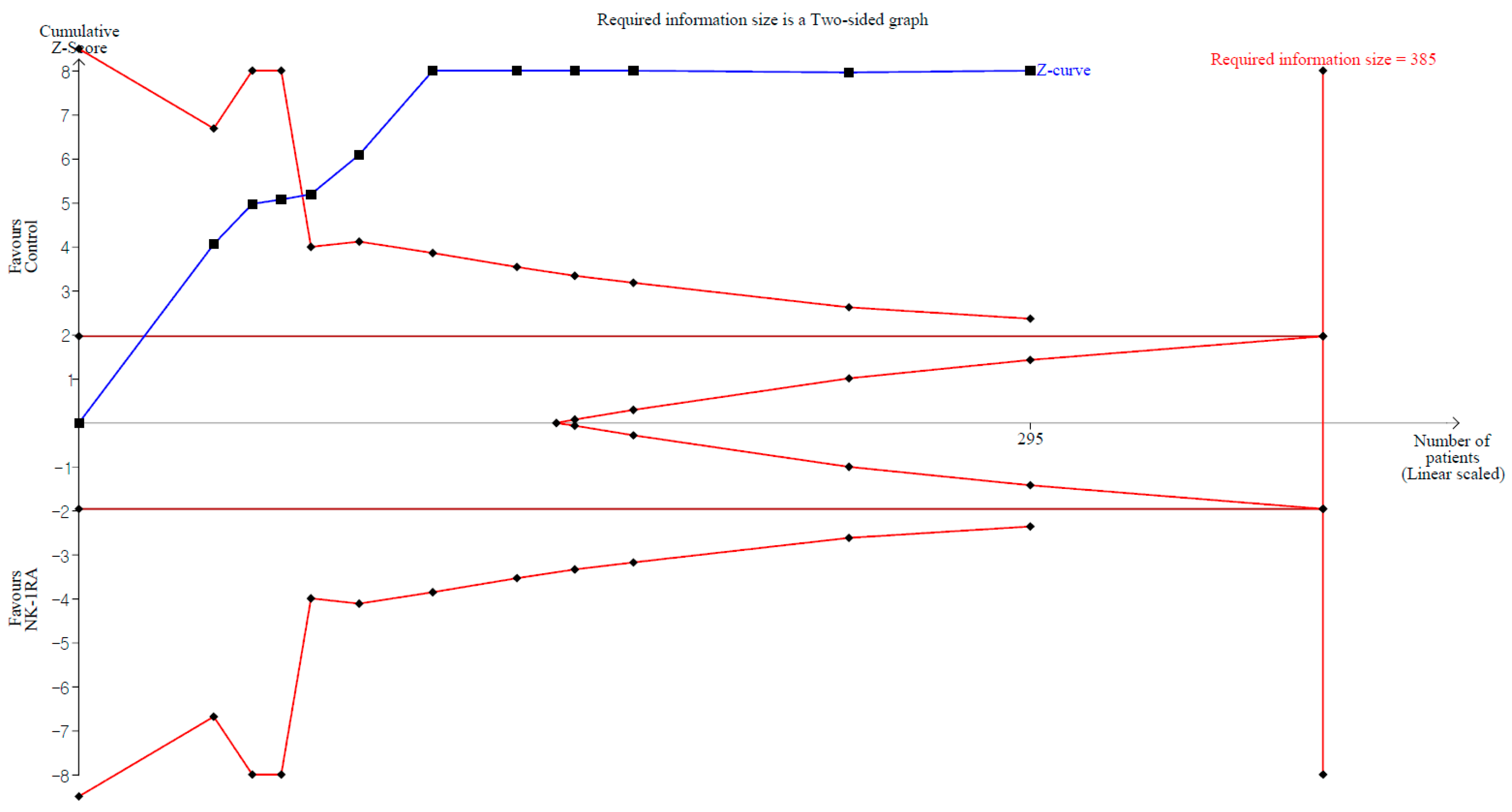
2.8. Trial Sequential Analysis
2.9. Certainty of Evidence
3. Results
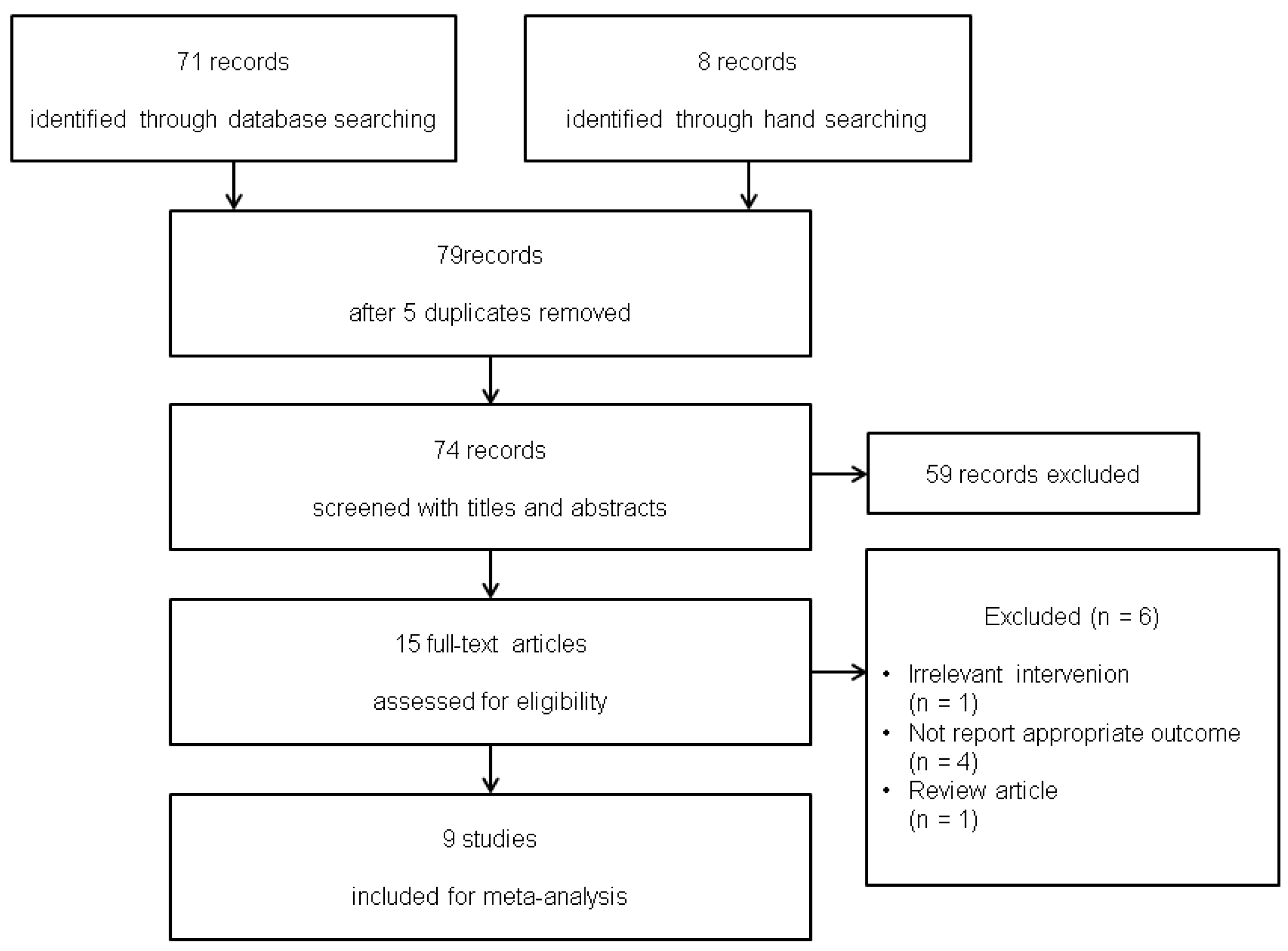
3.1. Study Selection
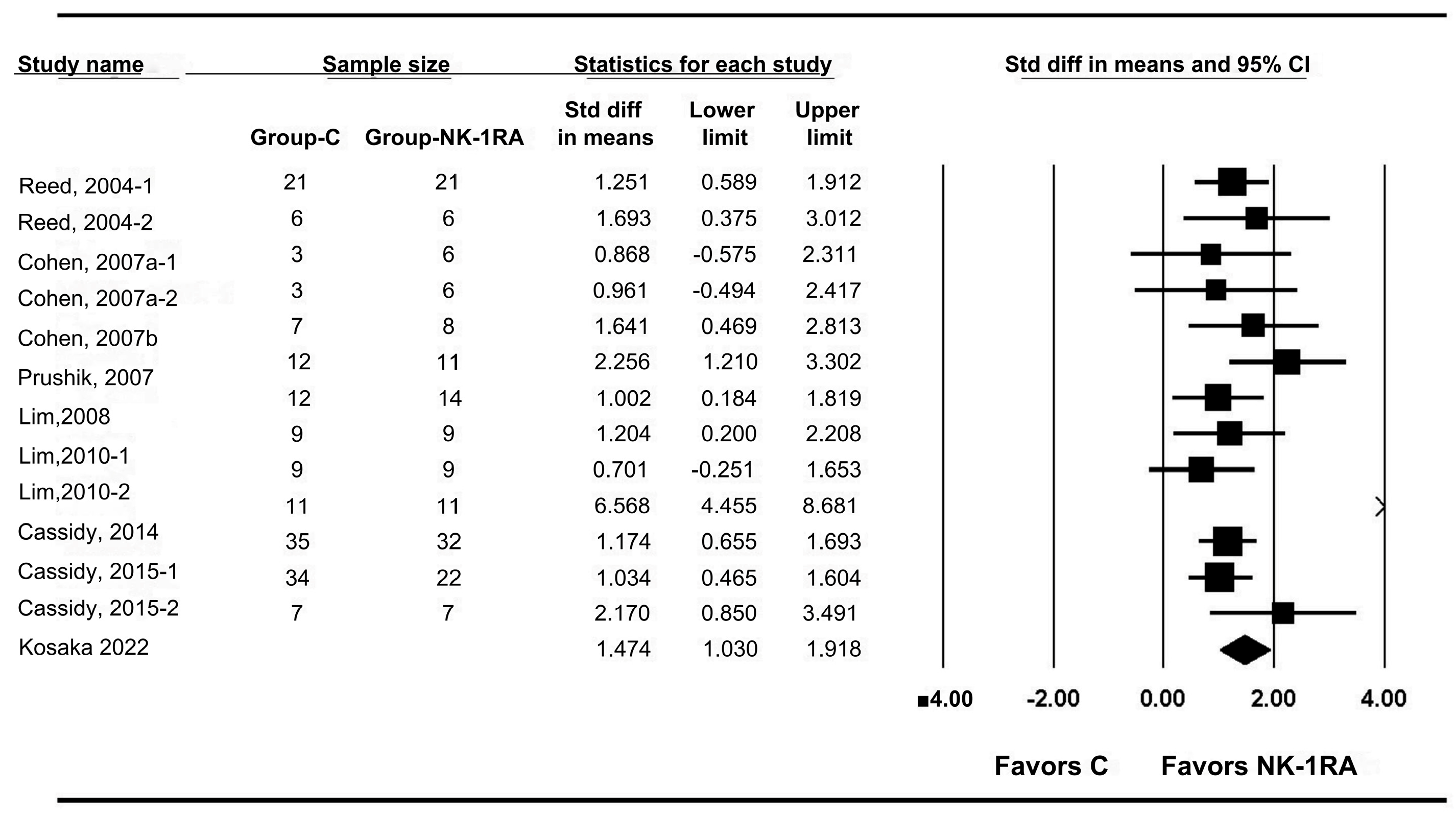
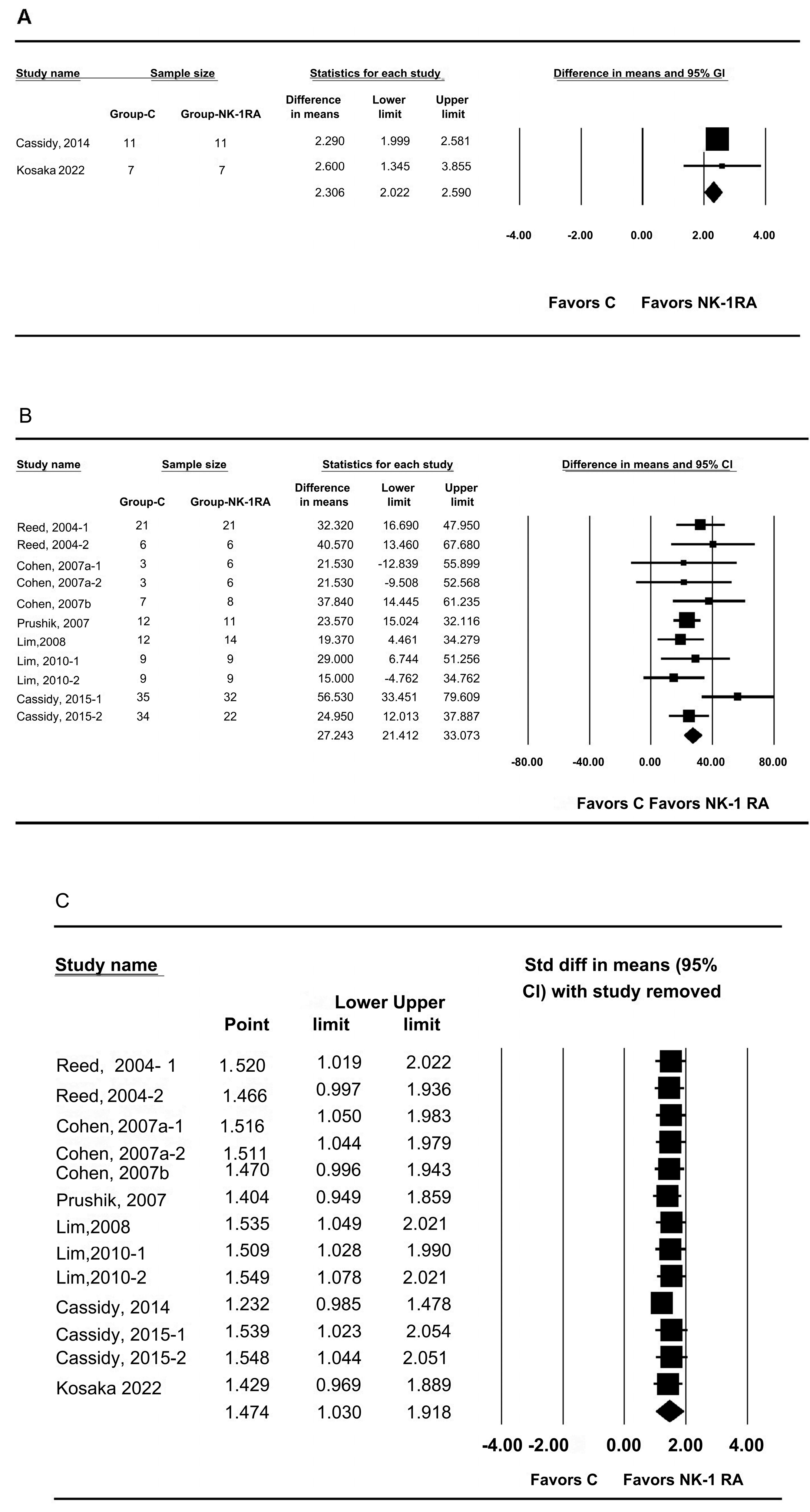
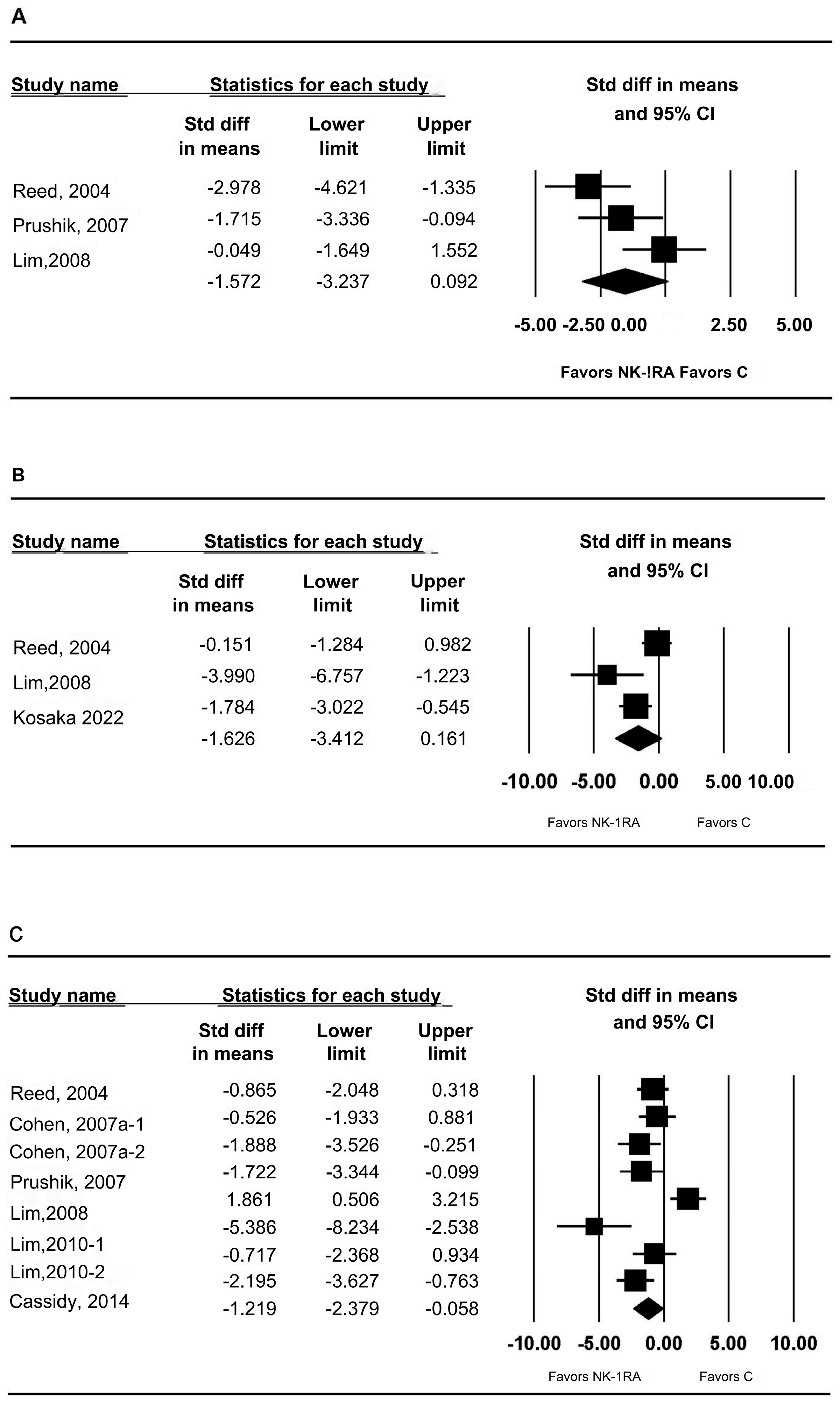
3.2. Macroscopic Adhesion Score
3.3. t-PA and PAI-1 mRNA Expression in Peritoneal Tissue
3.4. t-PA Activity in Peritoneal Tissue
3.5. Anastomotic Bursting Pressure
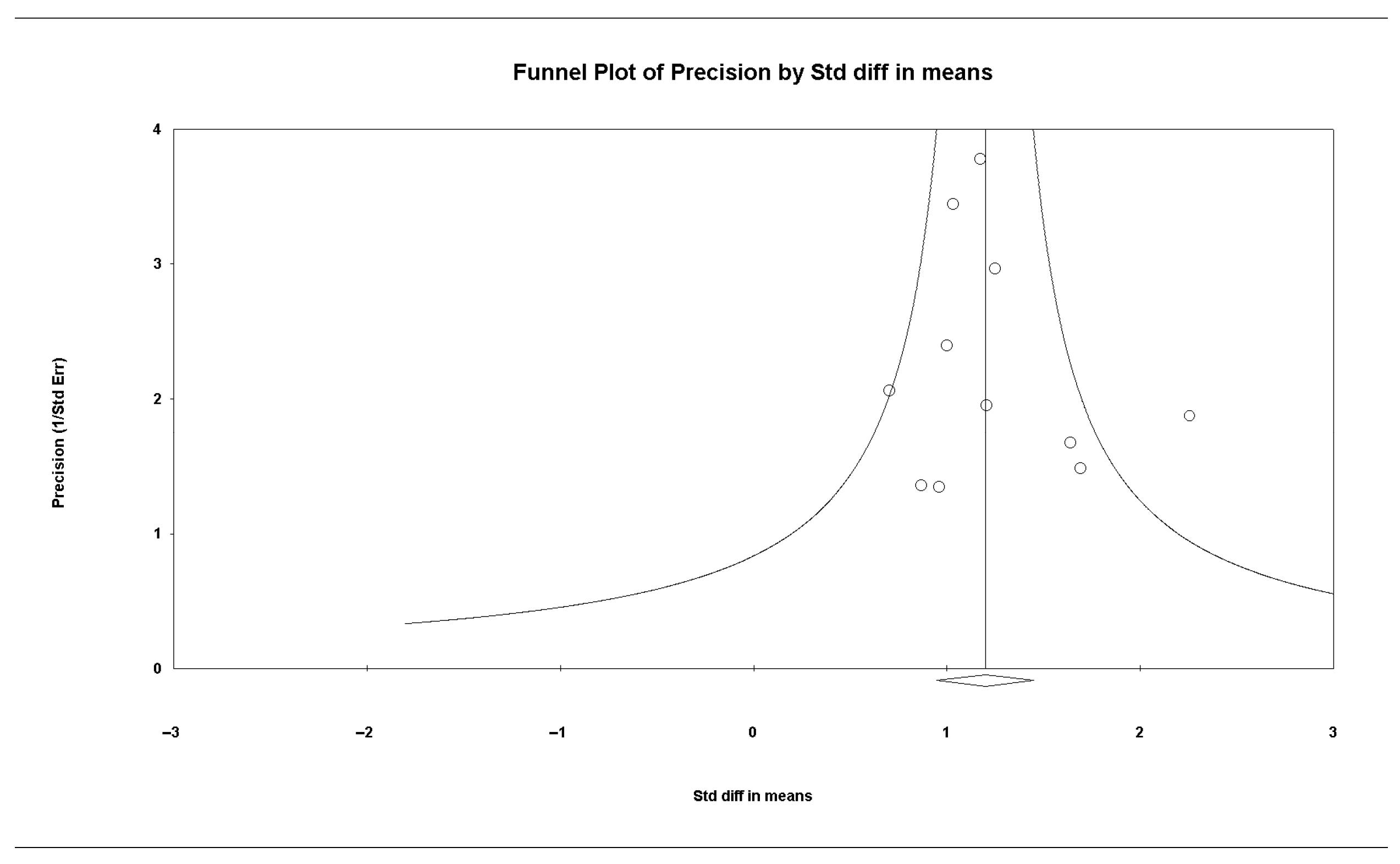
3.6. Publication Bias
3.7. Methodological Quality
3.8. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NK-1RA | Neurokinin-1 receptor antagonist |
| t-PA | tissue plasminogen activator |
| PAI-1 | plasminogen activator inhibitor-1 |
| SMD | standardized mean difference |
| MD | mean difference |
| CI | confidence interval |
| PrI | predictive interval |
| TSA | trial sequential analysis |
| RIS | required information size |
Appendix A
Appendix A.1. Search Term
| Pubmed | |
| #1 | General Surgery[MeSH Terms] |
| #2 | surg*[Title/Abstract] |
| #3 | oper*[Title/Abstract] |
| #4 | Surgical Procedures, Operative[MeSH Terms] |
| #5 | surgery [Subheading] |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | Tissue Adhesions[MeSH Terms] |
| #8 | adhesi*[Title/Abstract] |
| #9 | #7 OR #8 |
| #10 | #6 AND #9 |
| #11 | Neurokinin[Title/Abstract] |
| #12 | NK-1[Title/Abstract] |
| #13 | aprepitant[Title/Abstract] |
| #14 | Emend*[Title/Abstract] |
| #15 | Fosaprepitant*[Title/Abstract] |
| #16 | Netupitant*[Title/Abstract] |
| #17 | Akynzeo*[Title/Abstract] |
| #18 | Casopitant*[Title/Abstract] |
| #19 | Maropitant*[Title/Abstract] |
| #20 | Rolapitant*[Title/Abstract] |
| #21 | Tachykinin[Title/Abstract] |
| #22 | #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 |
| #23 | #10 AND #22 |
| Embase | |
| #1 | ‘general surgery’/exp OR ‘general surgery’ |
| #2 | surg*:ti,ab |
| #3 | oper*:ti,ab |
| #4 | ‘surgery’/exp OR ‘surgery’ |
| #5 | #1 OR #2 OR #3 OR #4 |
| #6 | ‘adhesion’/exp |
| #7 | adhesi*:ti,ab |
| #8 | ‘adhesion barrier’/exp |
| #9 | #6 OR #7 OR #8 |
| #10 | #5 AND #9 |
| #11 | ‘tachykinin receptor antagonist’/exp |
| #12 | neurokinin:ti,ab |
| #13 | ‘nk 1’:ti,ab |
| #14 | aprepitant:ti,ab |
| #15 | emend*:ti,ab |
| #16 | fosaprepitant*:ti,ab |
| #17 | netupitant*:ti,ab |
| #18 | akynzeo*:ti,ab |
| #19 | casopitant*:ti,ab |
| #20 | maropitant*:ti,ab |
| #21 | rolapitant*:ti,ab |
| #22 | #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 |
| #23 | #10 AND #22 |
| Web of Science | |
| #1 | TS = Surgery |
| #2 | TI = surg* |
| #3 | AB = surg* |
| #4 | TI = oper* |
| #5 | AB = oper* |
| #6 | #5 OR #4 OR #3 OR #2 OR #1 |
| #7 | TI = adhesi* |
| #8 | AB = adhesi* |
| #9 | TS = Tissue adhesion |
| #10 | #9 OR #8 OR #7 |
| #11 | #10 AND #6 |
| #12 | TS = Neurokinin |
| #13 | TI = NK-1 |
| #14 | AB = NK-1 |
| #15 | TI = NK1 |
| #16 | AB = NK1 |
| #17 | TI = aprepitant |
| #18 | AB = aprepitant |
| #19 | TI = Fosaprepitant |
| #20 | AB = Fosaprepitant |
| #21 | TI = Netupitant |
| #22 | AB = Netupitant |
| #23 | TI = Casopitant |
| #24 | AB = Casopitant |
| #25 | T I= Maropitant |
| #26 | AB = Maropitant |
| #27 | TI = Rolapitant |
| #28 | AB = Rolapitant |
| #29 | AB = Akynzeo |
| #30 | AB = Emend |
| #31 | #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 |
| #32 | #11 AND #31 |
Appendix B
Appendix B.1. Conference Proceedings Excluded
| No. | Reference No. | Brief Description | Reason for Exclusion |
| 1 | Brady 2014 [44], Brady 2015 [45] | Two proceedings reporting identical content; [44] retained temporarily. Later matched to Cassidy et al., 2015 [29] with nearly identical authors and study population but different outcome; insufficient data in proceeding to extract effect estimates. | Duplicate content; insufficient data |
| 2 | Kosaka 2011 [46], Kosaka 2012 [32] | Two proceedings with identical content; [47] retained temporarily. Subsequently published as [32] by overlapping authors with similar population and outcomes. | Duplicate content; subsequent peer-reviewed publication |
| 3 | Cassidy 2012 [48] | Compared an intervention outside the scope of the review. | Irrelevant intervention |
Appendix B.2. Articles Excluded at the Full-Text Review Stage
| No. | Reference No. | Reason for Exclusion |
| 1 | Reed 2008 [49] | Review article |
| 2 | Reed 2002 [50] | Did not report outcome of interest |
| 3 | Dehlin 2013 [51] | Did not report outcome of interest |
| 4 | Esposito 2013 [52] | Did not report outcome of interest |
| 5 | Gainsbury, 2011 [53] | Did not report outcome of interest |
| 6 | Azma 2014 [54] | Irrelevant intervention |
References
- Park, H.; Baek, S.; Kang, H.; Lee, D. Biomaterials to prevent post-operative adhesion. Materials 2020, 13, 3056. [Google Scholar] [CrossRef] [PubMed]
- Menzies, D. Postoperative adhesions: Their treatment and relevance in clinical practice. Ann. R. Coll. Surg. Engl. 1993, 75, 147–153. [Google Scholar]
- Ouaïssi, M.; Gaujoux, S.; Veyrie, N.; Denève, E.; Brigand, C.; Castel, B.; Duron, J.; Rault, A.; Slim, K.; Nocca, D. Post-operative adhesions after digestive surgery: Their incidence and prevention: Review of the literature. J. Visc. Surg. 2012, 149, e104–e114. [Google Scholar] [CrossRef]
- Ellis, H.; Moran, B.J.; Thompson, J.N.; Parker, M.C.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.; O’BRien, F.; et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: A retrospective cohort study. Lancet 1999, 353, 1476–1480. [Google Scholar] [CrossRef]
- Johns, A. Evidence-based prevention of post-operative adhesions. Hum. Reprod. Updat. 2001, 7, 577–579. [Google Scholar] [CrossRef]
- Torres-De La Roche, L.A.; Campo, R.; Devassy, R.; Sardo, A.D.S.; Hooker, A.; Koninckx, P.; Urman, B.; Wallwiener, M.; De Wilde, R.L. Adhesions and anti-adhesion systems highlights. Facts Views Vis. ObGyn 2019, 11, 137–149. [Google Scholar]
- Lee, H.K.; Hwang, J.; Jo, S.; Kim, J.K.; Lee, C.R.; Kang, S.-W.; Nam, K.-H.; Cho, S.-R. Adhesion reduction agent guardix-sg® versus megashield® for postoperative swallowing function analysis in thyroidectomy patients. Clin. Med. Insights Oncol. 2024, 18, 11795549241271715. [Google Scholar] [CrossRef]
- Scott, F.I.; Vajravelu, R.K.; Mamtani, R.; Bianchina, N.; Mahmoud, N.; Hou, J.K.; Wu, Q.; Wang, X.; Haynes, K.; Lewis, J.D. Association between statin use at the time of intra-abdominal surgery and postoperative adhesion-related complications and small-bowel obstruction. JAMA Netw. Open 2021, 4, e2036315. [Google Scholar] [CrossRef]
- Al-Chalabi, H.A.; Otubo, J.A. Value of a single intraperitoneal dose of heparin in prevention of adhesion formation: An experimental evaluation in rats. Int. J. Fertil. 1987, 32, 332–335. [Google Scholar] [PubMed]
- Hellebrekers, B.W.; Trimbos-Kemper, T.C.; Trimbos, J.M.; Emeis, J.J.; Kooistra, T. Use of fibrinolytic agents in the prevention of postoperative adhesion formation. Fertil. Steril. 2000, 74, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, J.S.; An, S.S.; Kang, H. Inhibition of thrombin-activated fibrinolysis inhibitor decreases postoperative adhesion. J. Surg. Res. 2015, 193, 560–566. [Google Scholar] [CrossRef]
- Neagoe, O.C.; Ionica, M.; Mazilu, O. Use of methylene blue in the prevention of recurrent intra-abdominal postoperative adhesions. J. Int. Med Res. 2018, 46, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, E.; Yilmazlar, A.; Berhuni, S.; Yilmazlar, T. The effectiveness of local anesthetics in preventing postoperative adhesions in rat models. Tech. Coloproctology 2010, 14, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Oncel, M.; Kurt, N.; Remzi, F.H.; Sensu, S.S.; Vural, S.; Gezen, C.F.; Cincin, T.G.; Olcay, E. The effectiveness of systemic antibiotics in preventing postoperative, intraabdominal adhesions in an animal model. J. Surg. Res. 2001, 101, 52–55. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Bmj 2009, 339, b2700. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. Syrcle’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Choi, G.J.; Kang, H. Heterogeneity in meta-analyses: An unavoidable challenge worth exploring. Korean J. Anesthesiol. 2025, 78, 301–314. [Google Scholar] [CrossRef]
- Kang, H. Statistical considerations in meta-analysis. Hanyang Med Rev. 2015, 35, 23–32. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kang, H. Trial sequential analysis: Novel approach for meta-analysis. Anesthesia Pain Med. 2021, 16, 138–150. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; de Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef]
- Reed, K.L.; Fruin, A.B.; Gower, A.C.; Stucchi, A.F.; Leeman, S.E.; Becker, J.M. A neurokinin 1 receptor antagonist decreases postoperative peritoneal adhesion formation and increases peritoneal fibrinolytic activity. Proc. Natl. Acad. Sci. USA 2004, 101, 9115–9120. [Google Scholar] [CrossRef]
- Cohen, P.A.; Aarons, C.B.; Gower, A.C.; Stucchi, A.F.; Leeman, S.E.; Becker, J.M.; Reed, K.L. The effectiveness of a single intraperitoneal infusion of a neurokinin-1 receptor antagonist in reducing postoperative adhesion formation is time dependent. Surgery 2007, 141, 368–375. [Google Scholar] [CrossRef]
- Cohen, P.A.; Gower, A.C.; Stucchi, A.F.; Leeman, S.E.; Becker, J.M.; Reed, K.L. A neurokinin-1 receptor antagonist that reduces intraabdominal adhesion formation increases peritoneal matrix metalloproteinase activity. Wound Repair Regen. 2007, 15, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Morrill, J.M.; Prushik, S.G.; Reed, K.L.; Gower, A.C.; Leeman, S.E.; Stucchi, A.F.; Becker, J.M. An fda approved neurokinin-1 receptor antagonist is effective in reducing intraabdominal adhesions when administered intraperitoneally, but not orally. J. Gastrointest. Surg. 2008, 12, 1754–1761. [Google Scholar] [CrossRef]
- Lim, R.; Stucchi, A.F.; Morrill, J.M.; Reed, K.L.; Lynch, R.; Becker, J.M. The efficacy of a hyaluronate-carboxymethylcellulose bioresorbable membrane that reduces postoperative adhesions is increased by the intra-operative co-administration of a neurokinin 1 receptor antagonist in a rat model. Surgery 2010, 148, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, M.R.; Sherburne, A.C.; Heydrick, S.J.; Stucchi, A.F. Combined intraoperative administration of a histone deacetylase inhibitor and a neurokinin-1 receptor antagonist synergistically reduces intra-abdominal adhesion formation in a rat model. Surgery 2015, 157, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Prushik, S.G.; Aarons, C.B.; Matteotti, R.; Reed, K.L.; Gower, A.C.; Leeman, S.E.; Stucchi, A.F.; Becker, J.M. A neurokinin 1 receptor antagonist decreases adhesion reformation after laparoscopic lysis of adhesions in a rat model of adhesion formation. Surg. Endosc. 2007, 21, 1790–1795. [Google Scholar] [CrossRef]
- Cassidy, M.R.; Sheldon, H.K.; Gainsbury, M.L.; Gillespie, E.; Kosaka, H.; Heydrick, S.; Stucchi, A.F. The neurokinin 1 receptor regulates peritoneal fibrinolytic activity and postoperative adhesion formation. J. Surg. Res. 2014, 191, 12–18. [Google Scholar] [CrossRef]
- Kosaka, H.; Kaibori, M.; Chu, D.I.; Stucchi, A.F.; Sekimoto, M. Role of substance p–dependent chemotactic signaling in postoperative adhesion formation. J. Surg. Res. 2022, 270, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.; Costain, D.J.; McAlister, V.C.; Lee, T.D. Prevention of experimental postoperative peritoneal adhesions by N,O-carboxymethyl chitosan. Surgery 1996, 120, 866–870. [Google Scholar] [CrossRef]
- Krielen, P.; Stommel, M.W.J.; Pargmae, P.; Bouvy, N.D.; Bakkum, E.A.; Ellis, H.; Parker, M.C.; Griffiths, E.A.; van Goor, H.; Broek, R.P.G.T. Adhesion-related readmissions after open and laparoscopic surgery: A retrospective cohort study (SCAR update). Lancet 2020, 395, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Capmas, P.; Payen, F.; Lemaire, A.; Fernandez, H. Adhesions in abdomino-pelvic surgeries: A real economic impact? PLoS ONE 2022, 17, e0276810. [Google Scholar] [CrossRef]
- Holmdahl, L.; Falkenberg, M.; Ivarsson, M.; Risberg, B. Plasminogen activators and inhibitors in peritoneal tissue. APMIS 1997, 105, 25–30. [Google Scholar] [CrossRef]
- Dizerega, G.S.; Campeau, J.D. Peritoneal repair and post-surgical adhesion formation. Hum. Reprod. Updat. 2001, 7, 547–555. [Google Scholar] [CrossRef]
- Metwali, A.; Blum, A.M.; Elliott, D.E.; Setiawan, T.; Weinstock, J.V. Cutting edge: Hemokinin has substance p-like function and expression in inflammation. J. Immunol. 2004, 172, 6528–6532. [Google Scholar] [CrossRef]
- Schäffer, M.; Beiter, T.; Becker, H.D.; Hunt, T.K. Neuropeptides: Mediators of inflammation and tissue repair? Arch. Surg. 1998, 133, 1107–1116. [Google Scholar] [CrossRef]
- Murphy, P.G.; Hart, D.A. Plasminogen activators and plasminogen activator inhibitors in connective tissues and connective tissue cells: Influence of the neuropeptide substance P on expression. Biochim. Biophys. Acta 1993, 1182, 205–214. [Google Scholar] [CrossRef]
- Riemma, G.; De Franciscis, P.; La Verde, M.; Ravo, M.; Fumiento, P.; Fasulo, D.D.; Della Corte, L.; Ronsini, C.; Torella, M.; Cobellis, L. Impact of the hemostatic approach after laparoscopic endometrioma excision on ovarian reserve: Systematic review and network meta-analysis of randomized controlled trials. Int. J. Gynecol. Obstet. 2023, 162, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Abu Hilal, M.; Underwood, T.; Taylor, M.G.; Hamdan, K.; Elberm, H.; Pearce, N.W. Bleeding and hemostasis in laparoscopic liver surgery. Surg. Endosc. 2010, 24, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brady, M.T.; King, E.G.; Keenan, B.; Heydrick, S.; Cassidy, M.R.; Stucchi, A. Co-administration of valproic acid (vpa), an histone deacetylase inhibitor, and a neurokinin-1 receptor antagonist (nk-1ra) that reduces intraabdominal adhesion formation in a rat surgical model downregulates the expression of the early growth response (egr) genes 1 and 3. J. Am. Coll. Surg. 2014, 219, S15. [Google Scholar] [CrossRef]
- Brady, M.T.; Keenan, B.J.; King, E.G.; Heydrick, S.; Cassidy, M.R.; Stucchi, A. Co-Administration of valproic acid (vpa), a Histone Deacetylase Inhibitor, and a neurokinin-1 receptor antagonist (nk-1ra) Reduces Intraabdominal Adhesion Formation in a Rat Surgical Model and also Downregulates the Expression of the early growth response (egr) Genes 1 and 3 both In Vivo and in Human Mesothelial Cells. Available online: https://www.gastrojournal.org/article/S0016-5085(15)33930-5/pdf (accessed on 21 October 2025).
- Kosaka, H.; Gainsbury, M.; Sheldon, H.; Cassidy, M.R.; Stucchi, A.; Becker, J.M. A neurokinin-1 receptor (nk1r) antagonist (nk-1ra) that reduces postoperative adhesions reduces the adhesion related chemokines cxcl1(kc) and cxcl2 (mip-2) and their receptor, cxcr2. J. Am. Coll. Surg. 2011, 213, S18–S19. [Google Scholar] [CrossRef]
- Kosaka, H.; Cassidy, M.R.; Stucchi, A.F.; Becker, J.M. A neurokinin-1 receptor (nk1r) antagonist (nk-1ra) that reduces postoperative adhesions reduces the adhesion related chemokines cxcl1(kc) and cxcl2 (mip-2) and their receptor, cxcr2. Gastroenterology 2012, 142, S1107. [Google Scholar] [CrossRef]
- Cassidy, M.R.; Gallant, J.J.; Sherburne, A.C.; Gainsbury, M.L.; Sheldon, H.K.; Stucchi, A.F. Synergistic reduction of postoperative adhesions by combined intraoperative administration of a histone deacetylase inhibitor and a neurokinin-1 receptor antagonist is achieved by targeting different mechanisms in adhesiogenesis. J. Am. Coll. Surg. 2012, 215, S22. [Google Scholar] [CrossRef]
- Reed, K.L.; Stucchi, A.F.; Leeman, S.E.; Becker, J.M. Inhibitory effects of a neurokinin-1 receptor antagonist on postoperative peritoneal adhesion formation. Ann. N. Y. Acad. Sci. 2008, 1144, 116–126. [Google Scholar] [CrossRef]
- Reed, K.L.; Fruin, A.B.; Bishop-Bartolomei, K.K.; Gower, A.C.; Nicolaou, M.; Stucchi, A.F.; Leeman, S.E.; Becker, J.M. Neurokinin-1 receptor and substance p messenger rna levels increase during intraabdominal adhesion formation. J. Surg. Res. 2002, 108, 165–172. [Google Scholar] [CrossRef]
- Dehlin, H.M.; Manteufel, E.J.; Monroe, A.L.; Reimer, M.H., Jr.; Levick, S.P. Substance p acting via the neurokinin-1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. Int. J. Cardiol. 2013, 168, 4643–4651. [Google Scholar] [CrossRef]
- Esposito, A.J.; Heydrick, S.J.; Cassidy, M.R.; Gallant, J.; Stucchi, A.F.; Becker, J.M. Substance p is an early mediator of peritoneal fibrinolytic pathway genes and promotes intra-abdominal adhesion formation. J. Surg. Res. 2013, 181, 25–31. [Google Scholar] [CrossRef]
- Gainsbury, M.L.; Sheldon, H.K.; Mitra, S.; Abdou, R.; Chu, D.I.; Reed, K.L.; Stucchi, A.F.; Becker, J.M. Hypoxia upregulates neurokinin-1 receptor (nk-1r) signaling in rat peritoneal mesothelial cells (rpmcs): A key early event in intraabdominal adhesion formation. Gastroenterology 2011, 140, S1045. [Google Scholar] [CrossRef]
- Azma, T.; Tuluc, F.; Ito, T.; Aoyama-Mani, C.; Kawahito, S.; Kinoshita, H. Mechanisms of action of anesthetics for the modulation of perioperative thrombosis: Evidence for immune mechanisms from basic and clinical studies. Curr. Pharm. Des. 2014, 20, 5779–5793. [Google Scholar] [CrossRef] [PubMed]
| First Author, Publication Year | Animal | Surgery | Group | Definition | |
|---|---|---|---|---|---|
| Reed, 2004 [24] | Male Wistar rats | Intraperitoneal ischemic buttons | Control 1 (n = 21) | Sterile saline; same schedule as Experiment 1, substituting saline for CJ-12,255 | |
| Experiment 1 (n = 21) | CJ-12,255; IP injection (2.5 mg/kg) twice daily for 2 days before surgery and for 7 days after surgery; IP lavage of 0.75 mg on the day of surgery | ||||
| Control 2 (n = 6) | Sterile saline; same schedule as Experiment 2, substituting saline for CJ-12,255 | ||||
| Experiment 2 | CJ-12,255; IP delivery (10 mg/kg) via an ALZET osmotic pump | ||||
| Cohen, 2007a [25] | Male Wistar rats | Intraperitoneal ischemic buttons | Control 1-1 (n = 6) | Sterile saline; same lavage schedule as Experiments 1-1/1-2, substituting saline for CJ-12,255 | |
| Experiment 1-1 (n = 6) | CJ-12,255; intraoperative IP lavage (5 mg/kg) | ||||
| Experiment 1-2 (n = 6) | CJ-12,255; intraoperative IP lavage (25 mg/kg) | ||||
| Control 2 (n = 4) | Sterile saline; same schedule as Experiment 2, substituting saline for CJ-12,255 | ||||
| Experiment 2 (n = 4) | CJ-12,255; IP injection (25 mg/kg) 1 h after surgery | ||||
| Control 3 (n = 7) | Sterile saline; same schedule as Experiment 3, substituting saline for CJ-12,255 | ||||
| Experiment 3 (n = 7) | CJ-12,255; IP injection (25 mg/kg) 5 h after surgery | ||||
| Control 4 (n = 4) | Sterile saline; same schedule as Experiment 4, substituting saline for CJ-12,255 | ||||
| Experiment 4 (n = 4) | CJ-12,255; IP injection (25 mg/kg) 12 h after surgery | ||||
| Control 5 (n = 5) | Sterile saline; same schedule as Experiment 5, substituting saline for CJ-12,255 | ||||
| Experiment 5 (n = 5) | CJ-12,255; IP injection (25 mg/kg) 24 h after surgery | ||||
| Cohen, 2007b [26] | Male Wistar rats | Intraperitoneal ischemic buttons | Control 1 (n = 8) | Sterile water; same schedule as Experiment 1, substituting water for CJ-12,255; euthanized 1 day after surgery | |
| Experiment 1 (n = 7) | CJ-12,255; intraoperative IP lavage (25 mg/kg); euthanized 1 day after surgery | ||||
| Sham 1 (n = 7) | No surgery; euthanized 1 day after surgery | ||||
| Control 2 (n = 8) | Sterile water; same schedule as Experiment 2, substituting water for CJ-12,255; euthanized 7 days after surgery | ||||
| Experiment 2 (n = 7) | CJ-12,255; intraoperative IP lavage (25 mg/kg); euthanized 7 days after surgery | ||||
| Prushik, 2007 [30] | Male Wistar rats | Laparoscopic lysis of adhesions 7 days after intraperitoneal ischemic buttons | Control 1 (n = 12) | Sterile water; same schedule as Experiment 1, substituting water for CJ-12,255; euthanized 7 days after surgery | |
| Experiment 1 (n = 11) | CJ-12,255; intraoperative IP lavage (25 mg/kg); euthanized 7 days after surgery | ||||
| Self-control 1 (n = 23) | No injection; adhesions assessed during laparoscopy | ||||
| Control 2 (n = 4) | Sterile water; same schedule as Experiment 2, substituting water for CJ-12,255; euthanized 1 day after surgery | ||||
| Experiment 2 (n = 4) | CJ-12,255; intraoperative IP lavage (25 mg/kg); euthanized 1 day after surgery | ||||
| Lim, 2008 [27] | Male Wistar rats | Intraperitoneal ischemic buttons | Control 1 (n = 12) | Dimethyl sulfoxide (DMSO); same schedule as Experiment 1, substituting DMSO for aprepitant | |
| Experiment 1 (n = 14) | Aprepitant; intraoperative IP lavage (50 mg/kg) | ||||
| Control 2 (n = 7) | 2% carboxymethylcellulose (CMC); oral gavage schedule as in Experiment 2 without aprepitant | ||||
| Experiment 2 (n = 8) | Emend (aprepitant suspended in 2% CMC); single oral gavage (50 mg/kg) 3 h preoperatively | ||||
| Control 3 (n = 14) | 2% carboxymethylcellulose (CMC); oral gavage schedule as in Experiment 2 without aprepitant | ||||
| Experiment 3 (n = 13) | Emend (aprepitant suspended in 2% CMC); oral gavage (50 mg/kg), five preoperative doses and then every 12 h over 60 h | ||||
| Lim, 2010 [28] | Male Wistar rats | Intraperitoneal ischemic buttons | Control 1 (n = 9) | Saline; same schedule as Experiment 1-1, substituting saline for CJ-12,255 | |
| Experiment 1-1 (n = 9) | CJ-12,255; IP lavage (25 mg/kg) before abdominal closure | ||||
| Experiment 1-2 (n = 9) | 1 × 1 cm HA/CMC pieces + saline; same procedure as Experiment 1-3, substituting saline for CJ-12,255 | ||||
| Experiment 1-3 (n = 9) | 1 × 1 cm HA/CMC pieces + CJ-12,255; IP lavage (25 mg/kg) before abdominal closure | ||||
| Control 2 a (n = 12) | Unilateral HA/CMC + saline; same schedule as Experiment 2a, substituting saline for CJ-12,255 | ||||
| Experiment 2 a (n = 12) | Unilateral HA/CMC + CJ-12,255; IP lavage (25 mg/kg) before abdominal closure | ||||
| Cassidy, 2014 [31] | Male wild-type mice/homozygous NK-1R−/− mice | Laparotomy with cecal cautery | Control 1 (n = 22; combined with Experiment 1) | Wild type; normal saline; same schedule as Experiment 1, substituting saline for CJ-12,255 | |
| Experiment 1 | Wild type; CJ-12,255; intraoperative IP administration (25 mg/kg) | ||||
| Control 2 a (n = 21; combined with Experiment 2) | Homozygous NK-1R−/− mice; same schedule as Experiment 2, substituting saline for CJ-12,255 | ||||
| Experiment 2 a | Homozygous NK-1R−/− mice; CJ-12,255; intraoperative IP administration (25 mg/kg) | ||||
| Cassidy, 2015 [29] | Male Wistar rats | Intraperitoneal ischemic buttons | Control 1 (n = 9) | Normal saline; same schedule as Experiment 1-1, substituting saline for CJ-12,255 | |
| Experiment 1-1 | Sodium valproate (VPA); IP administration (25 mg/kg) at the time of surgery | ||||
| Experiment 1-2 | CJ-12,255; IP administration (50 mg/kg) at the time of surgery | ||||
| Experiment 1-3 | Sodium valproate (VPA) + CJ-12,255; IP administration at the time of surgery: VPA (25 mg/kg) plus CJ-12,255 (50 mg/kg) | ||||
| Control 2 (n = 6) | To assess normal wound healing; normal saline; same schedule as Experiment 2, substituting saline for CJ-12,255 | ||||
| Experiment 2 (n = 6) | To assess normal wound healing; sodium valproate (VPA) + CJ-12,255; IP administration at the time of surgery: VPA (25 mg/kg) plus CJ-12,255 (50 mg/kg) | ||||
| Kosaka, 2022 [32] | Female wild-type BALB/c mice; BALB/c NKT cell–deficient mice; BALB/c IFN-γ−/− mice | Laparotomy with cecal cautery | Control 1 (n = 7) | Laparotomy | Saline |
| Experiment 1-1 (n = 7) | Laparotomy | SB225002; continuous IP infusion (800 μg) for 24 h after surgery using an ALZET 2001D osmotic pump | |||
| Experiment 1-2 (n = 7) | Laparotomy | IP injection of CJ-12,255 (2.5 mg/kg) immediately after cecal cauterization | |||
| Control 2 (n = 7) | Laparotomy + cauterization | Saline | |||
| Experiment 2-1 (n = 7) | Laparotomy + cauterization | SB225002; continuous IP infusion (800 μg) for 24 h after surgery using an ALZET 2001D osmotic pump | |||
| Experiment 2-2 (n = 7) | Laparotomy + cauterization | CJ-12,255; IP injection (2.5 mg/kg) immediately after cecal cauterization | |||
| First Author, Publication Year | Macroscopic Adhesion Score | Other Outcome Variables |
|---|---|---|
| Reed, 2004 [24] | Percentage of ischemic buttons with attached adhesions | Peritoneal tPA and PAI-1 mRNA expression; tPA-mediated fibrinolytic activity |
| Cohen, 2007a [25] | Percentage of ischemic buttons with attached adhesions | Peritoneal tPA and PAI-1 expression; fibrinolytic activity in peritoneal fluid; anastomotic integrity and healing (burst pressure) |
| Cohen, 2007b [26] | Percentage of ischemic buttons with attached adhesions | Matrix metalloproteinases (MMPs): total activity; MMP-2, -3, -7, -8, -9; TIMPs |
| Prushik, 2007 [30] | Percentage of ischemic buttons with attached adhesions | Fibrinolytic activity (tPA activity); tPA mRNA |
| Lim, 2008 [27] | Percentage of ischemic buttons with attached adhesions | tPA and PAI-1; tPA-mediated fibrinolytic activity |
| Lim, 2010 [28] | Percentage of ischemic buttons with attached adhesions | Peritoneal fibrinolytic activity |
| Cassidy, 2014 [31] | Adhesion scoring scale (0 = no adhesions; 1 = one thin, filmy adhesion; 2 = more than one thin adhesion; 3 = thick adhesion with a focal point; 4 = thick adhesion with planar attachment or more than one thick adhesion with a focal point; 5 = very thick, vascularized adhesion or more than one planar adhesion) | Fibrinolytic activity (tPA activity) |
| Cassidy, 2015 [29] | Percentage of ischemic buttons with attached adhesions | Anastomotic burst pressure |
| Kosaka, 2022 [32] | Adhesion scoring scale (0 = no adhesions; 1 = one thin, filmy adhesion; 2 = more than one thin adhesion; 3 = thick adhesion with a focal point; 4 = thick adhesion with planar attachment or more than one thick adhesion with a focal point; 5 = very thick, vascularized adhesion or more than one planar adhesion) | Cytokine/chemokine RNA expression; chemokine levels in abdominal lavage fluid |
| First Author and Publication Year | Random Allocation Stated | Husbandry Conditions Reported | Compliance with Animal Welfare Regulations | Potential Conflict of Interest | Peer Reviewed | Score |
|---|---|---|---|---|---|---|
| Reed 2004 [24] | 1 | 1 | 1 | 0 (a) | 1 | 4 |
| Cohen 2007a [25] | 1 | 1 | 1 | 0 (a) | 1 | 4 |
| Cohen 2007b [26] | 1 | 1 | 1 | 0 (a) | 1 | 4 |
| Prushik 2007 [30] | 1 | 1 | 1 | 0 (a) | 1 | 4 |
| Lim 2008 [27] | 0 | 1 | 1 | 1 | 1 | 4 |
| Lim 2010 [28] | 1 | 1 | 1 | 0 (a) | 1 | 4 |
| Cassidy 2014 [31] | 1 | 1 | 1 | 0 (a) | 1 | 4 |
| Cassidy 2015 [29] | 0 | 1 | 1 | 0 (a) | 1 | 3 |
| Kosaka 2022 [32] | 0 | 1 | 1 | 0 (a) | 1 | 3 |
| First Author, Publication Year | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Reed, 2004 [24] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Yes | Yes | Yes | Unclear f |
| Cohen, 2007a [25] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Yes | Yes | Yes | Unclear f |
| Cohen, 2007b [26] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Yes | Yes | Yes | Unclear f |
| Prushik, 2007 [30] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Unclear e | Yes | Yes | Unclear f |
| Lim, 2008 [27] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Unclear e | Yes | Yes | Yes |
| Lim, 2010 [28] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Yes | Yes | Yes | Unclear f |
| Cassidy, 2014 [31] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Yes | Yes | Yes | Unclear f |
| Cassidy, 2015 [29] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Unclear e | Yes | Yes | Unclear f |
| Kosaka, 2022 [32] | Unclear a | Yes | Unclear b | Yes | Unclear c | Unclear d | Yes | Yes | Yes | Unclear f |
| Item | Description of domain | |||||||||
| Item 1 | Describe the methods used, if any, to generate the allocation sequence in sufficient detail to allow an assessment whether it should produce comparable groups. | |||||||||
| Item 2 | Describe all the possible prognostic factors or animal characteristics, if any, that are compared in order to judge whether or not intervention and control groups were similar at the start of the experiment. | |||||||||
| Item 3 | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen before or during enrolment. | |||||||||
| Item 4 | Describe all measures used, if any, to house the animals randomly within the animal room. | |||||||||
| Item 5 | Describe all measures used, if any, to blind trial caregivers and researchers from knowing which intervention each animal received. Provide any information relating to whether the intended blinding was effective. | |||||||||
| Item 6 | Describe whether or not animals were selected at random for outcome assessment, and which methods to select the animals, if any, were used. | |||||||||
| Item 7 | Describe all measures used, if any, to blind outcome assessors from knowing which intervention each animal received. Provide any information relating to whether the intended blinding was effective. | |||||||||
| Item 8 | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized animals), reasons for attrition or exclusions, and any re-inclusions in analyses for the review. | |||||||||
| Item 9 | State how selective outcome reporting was examined and what was found. | |||||||||
| Item 10 | State any important concerns about bias not covered by other domains in the tool. | |||||||||
| Study Limitations | Imprecision | Inconsistency | Indirectness | Publication Bias | GRADE | |
|---|---|---|---|---|---|---|
| Macroscopic adhesion | Serious a | Not serious | Serious c | Not serious | Not serious | ⨁⨁◯◯ Low |
| t-PA mRNA expression | Serious a | Serious b | Serious c | Not serious | NA | ⨁◯◯◯ Very low |
| PAI-1 mRNA expression | Serious a | Serious b | Serious c | Not serious | NA | ⨁◯◯◯ Very low |
| t-PA activity | Serious a | Not serious | Serious c | Not serious | NA | ⨁⨁◯◯ Low |
| Anastomotic bursting pressure | Serious a | Serious b | Not serious | Not serious | NA | ⨁⨁◯◯ Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.J.; Choi, G.J.; Choi, Y.S.; Kim, B.G.; Kang, H. Effect of Neurokinin-1 Receptor Antagonists on Experimental Postoperative Adhesion: A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Preclinical Studies. Medicina 2025, 61, 1933. https://doi.org/10.3390/medicina61111933
Hwang HJ, Choi GJ, Choi YS, Kim BG, Kang H. Effect of Neurokinin-1 Receptor Antagonists on Experimental Postoperative Adhesion: A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Preclinical Studies. Medicina. 2025; 61(11):1933. https://doi.org/10.3390/medicina61111933
Chicago/Turabian StyleHwang, Hyeon Joung, Geun Joo Choi, Yoo Shin Choi, Beom Gyu Kim, and Hyun Kang. 2025. "Effect of Neurokinin-1 Receptor Antagonists on Experimental Postoperative Adhesion: A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Preclinical Studies" Medicina 61, no. 11: 1933. https://doi.org/10.3390/medicina61111933
APA StyleHwang, H. J., Choi, G. J., Choi, Y. S., Kim, B. G., & Kang, H. (2025). Effect of Neurokinin-1 Receptor Antagonists on Experimental Postoperative Adhesion: A Systematic Review and Meta-Analysis with Trial Sequential Analysis of Preclinical Studies. Medicina, 61(11), 1933. https://doi.org/10.3390/medicina61111933








