High-Sensitivity Troponin T as a Prognostic Factor of Conventional Echocardiographic Parameters in Cancer Patients: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. General Proceedings
2.2. Echocardiographic Evaluation
2.3. Definitions and Thresholds for Echocardiographic Parameters
2.4. High-Sensitivity Cardiac Troponin T Measurements
2.5. Statistical Methods
3. Results
3.1. Demographic and Clinical Characteristics of the Study Population
3.2. Cancer-Related Characteristics
3.3. Echocardiographic Evaluation and Clinical Events
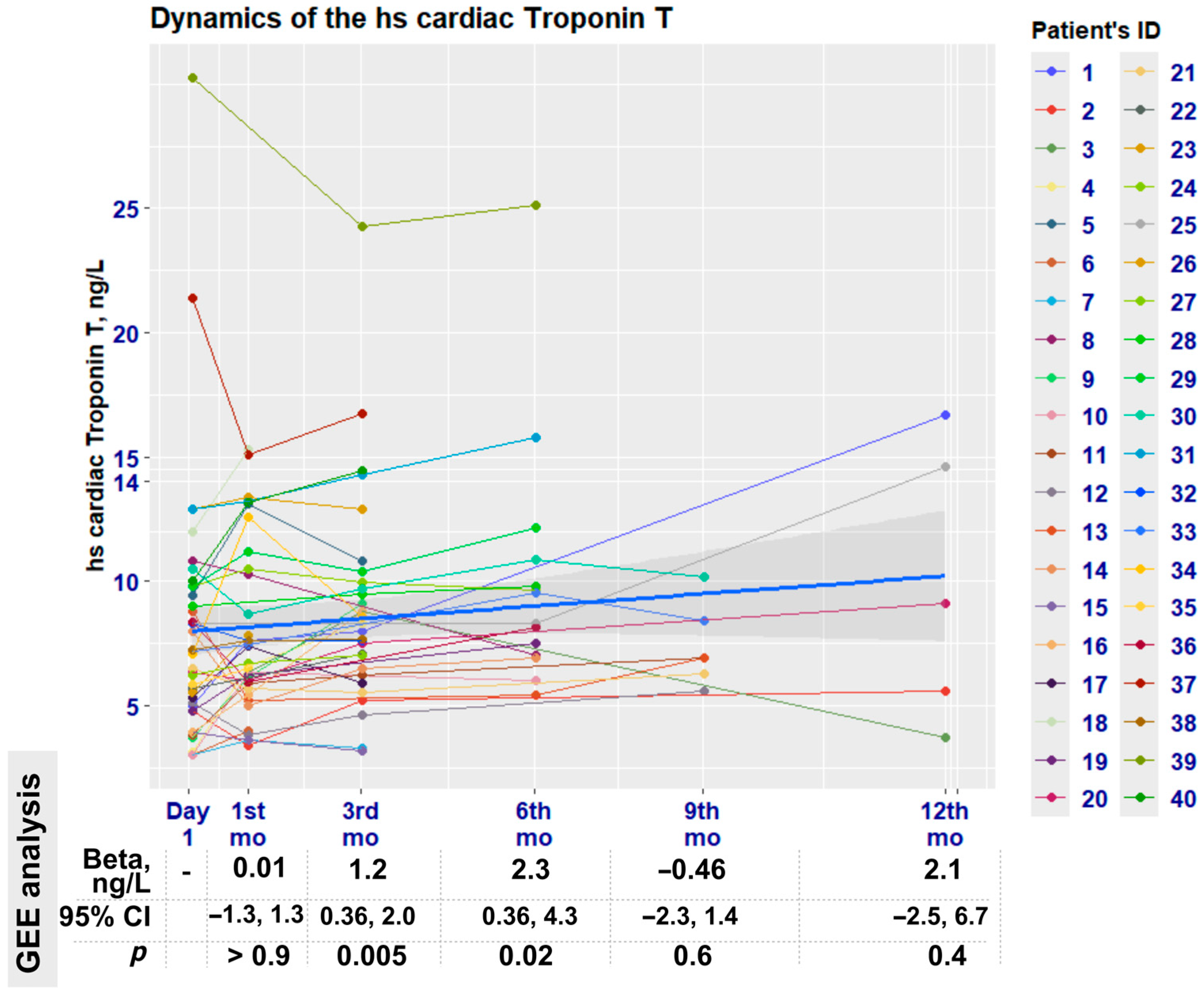
3.4. High-Sensitivity Cardiac Troponin Evaluation
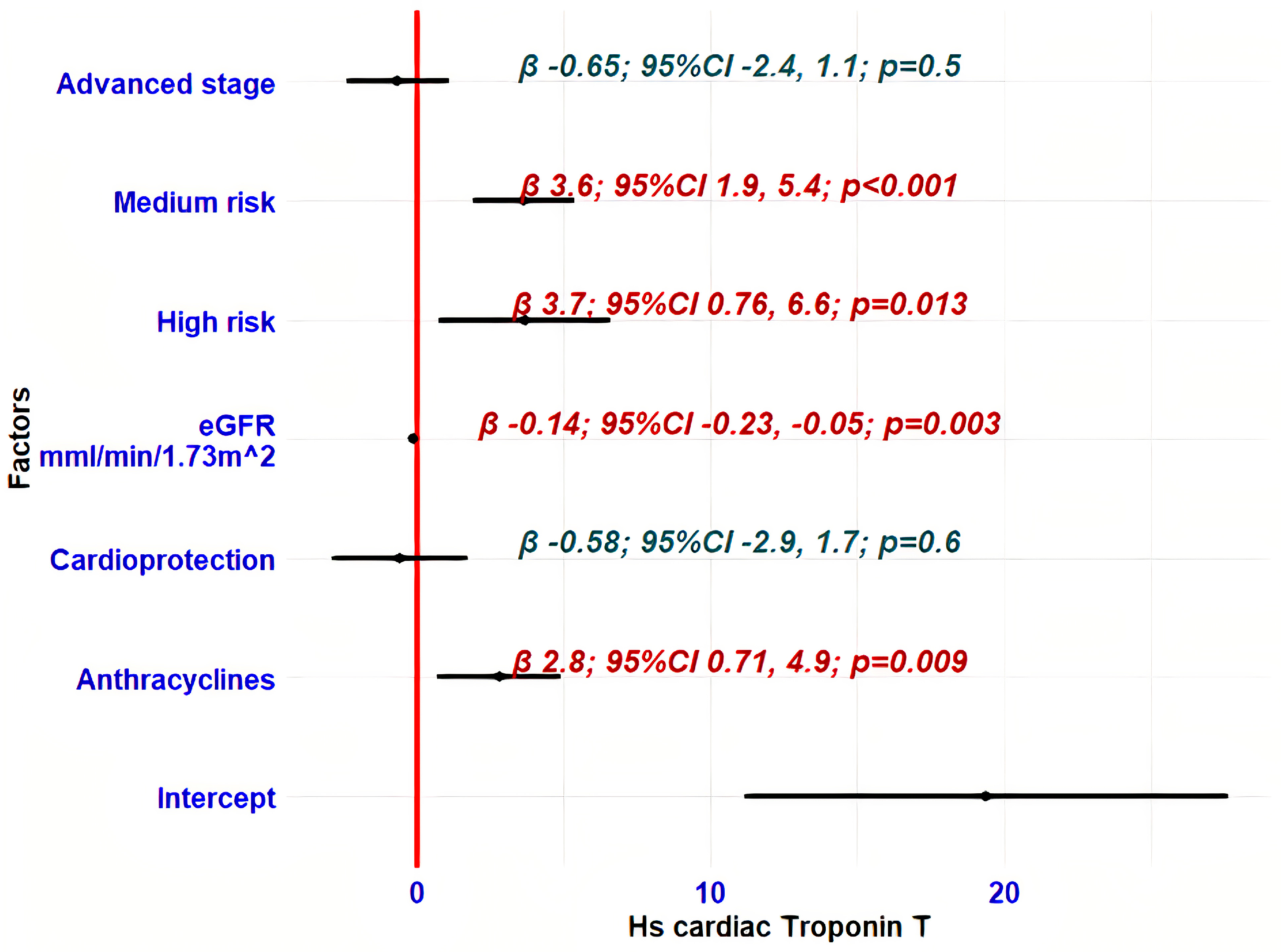
3.5. Determinants of hs-cTnT Variability During Oncological Treatment
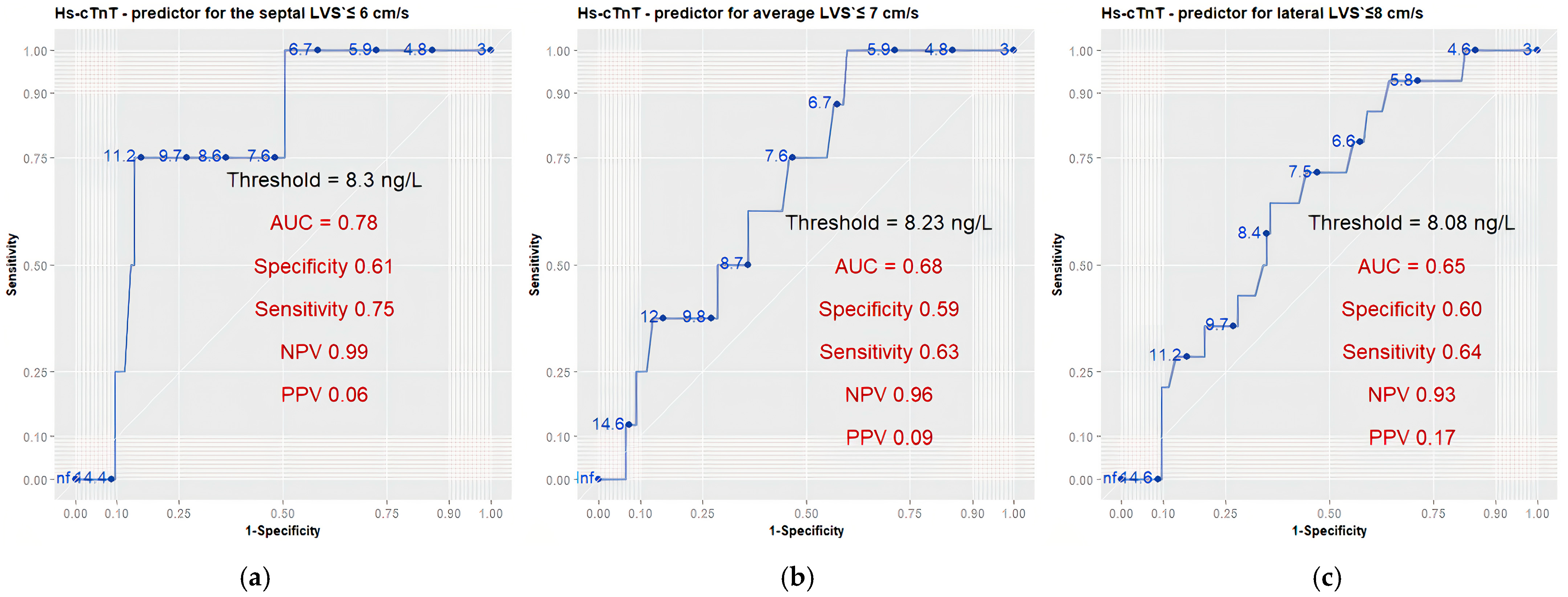
3.6. High-Sensitivity Cardiac Troponin T as a Prognostic Indicator of Left and Right Ventricular Function
4. Discussion
4.1. Early Changes in hs-cTnT Concentration
4.2. Rate of Abnormal hs-cTnT Levels
4.3. Factors Influencing hs-cTnT Increase
4.4. Predictive Ability of hs-cTnT and Cut-Off Values
4.5. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Average LVS′ | average value of Septal and Lateral LVS′ |
| BMI | body mass index |
| CTRCD | cancer therapy-related cardiac dysfunction |
| DecT | deceleration time |
| eGFR | estimated glomerular filtration rate |
| ESC | European Society of Cardiology |
| GEE | generalised estimating equations regression analysis |
| GLS | global longitudinal strain |
| GLM | generalised linear regression model |
| HER2 | human epidermal growth factor receptor 2 |
| HFA | Heart Failure Association of the European Society of Cardiology |
| Hs-cTn | high-sensitivity cardiac troponin |
| IC-OS | International Cardio-Oncology Society |
| IQR | interquartile range |
| IVRT | isovolumic relaxation time |
| Lateral LVS′ | tissue Doppler systolic S′ velocity, obtained at the lateral mitral annulus |
| LAV | left atrial volume |
| LV | left ventricular |
| LVe′ | early diastolic tissue Doppler e′ velocity |
| LVEDV | left ventricular end-diastolic volume |
| LVEF | left ventricular ejection fraction |
| LVMPI-PW | left ventricular myocardial performance index, measured by PW Doppler |
| MAPSE | mitral annular plane systolic excursion |
| RAAS | renin–angiotensin–aldosterone system |
| RAV | right atrial volume |
| ROC | receiver operating characteristic analysis |
| RV | right ventricular |
| RVA | right ventricular end-diastolic area |
| RVFAC | right ventricular fractional area changes |
| RVS′ | right ventricular systolic S′ tissue Doppler velocity at the lateral tricuspid annulus |
| RVMPI-TDI | right ventricular myocardial performance index, measured by tissue Doppler |
| SD | standard deviation |
| Septal LVS′ | tissue Doppler systolic S′ velocity, obtained at the septal mitral annulus |
| TAPSE | tricuspid annular plane systolic excursion |
| VEGF | vascular endothelial growth factor |
References
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Transl. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Colombo, N.; Boeri, M.; Lamantia, G.; Civelli, M.; Peccatori, F.; Martinelli, G.; Fiorentini, C.; et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004, 109, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Biasillo, G.; Salvatici, M.; Sandri, M.T.; Cipolla, C.M. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert. Rev. Mol. Diagn. 2017, 17, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Mincu, R.I.; Mahabadi, A.A.; Settelmeier, S.; Al-Rashid, F.; Rassaf, T.; Totzeck, M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: A meta-analysis. Eur. J. Heart Fail. 2020, 22, 350–361. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Sherwood, M.W.; Kristin Newby, L. High-Sensitivity Troponin Assays: Evidence, Indications, and Reasonable Use. J. Am. Heart Assoc. 2014, 3, e000403. [Google Scholar] [CrossRef]
- Pavo, N.; Raderer, M.; Hülsmann, M.; Neuhold, S.; Adlbrecht, C.; Strunk, G.; Goliasch, G.; Gisslinger, H.; Steger, G.G.; Hejna, M.; et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart 2015, 101, 1874–1880. [Google Scholar] [CrossRef]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Cohen, V.; Gosavi, S.; Carver, J.R.; Wiegers, S.E.; Martin, R.P.; et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011, 107, 1375–1380. [Google Scholar] [CrossRef]
- Zardavas, D.; Suter, T.M.; Veldhuisen, D.J.V.; Steinseifer, J.; Noe, J.; Lauer, S.; Al-Sakaff, N.; Piccart-Gebhart, M.J.; Azambuja, E.d. Role of Troponins I and T and N-Terminal Prohormone of Brain Natriuretic Peptide in Monitoring Cardiac Safety of Patients with Early-Stage Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer Receiving Trastuzumab: A Herceptin Adjuvant Study Cardiac Marker Substudy. J. Clin. Oncol. 2017, 35, 878–884. [Google Scholar] [CrossRef]
- Lv, X.; Pan, C.; Guo, H.; Chang, J.; Gao, X.; Wu, X.; Zhi, X.; Ren, C.; Chen, Q.; Jiang, H.; et al. Early diagnostic value of high-sensitivity cardiac troponin T for cancer treatment-related cardiac dysfunction: A meta-analysis. ESC Heart Fail. 2023, 10, 2170–2182. [Google Scholar] [CrossRef]
- Kang, Y.; Xu, X.; Cheng, L.; Li, L.; Sun, M.; Chen, H.; Pan, C.; Shu, X. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur. J. Heart Fail. 2014, 16, 300–308. [Google Scholar] [CrossRef]
- Ky, B.; Putt, M.; Sawaya, H.; French, B.; Januzzi, J.L., Jr.; Sebag, I.A.; Plana, J.C.; Cohen, V.; Banchs, J.; Carver, J.R.; et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014, 63, 809–816. [Google Scholar] [CrossRef]
- Song, F.Y.; Shi, J.; Guo, Y.; Zhang, C.J.; Xu, Y.C.; Zhang, Q.L.; Shu, X.H.; Cheng, L.L. Assessment of biventricular systolic strain derived from the two-dimensional and three-dimensional speckle tracking echocardiography in lymphoma patients after anthracycline therapy. Int. J. Cardiovasc. Imaging 2017, 33, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Bannister, C.; Tam To, B.; Patel, T.; Yap, R.; Cannata, A.; Bromage, D.; Mcdonagh, T. The use of high sensitivity troponin T as a biomarker of anthracycline cardiotoxicity. Eur. Heart J. 2023, 44, ehad655-2718. [Google Scholar] [CrossRef]
- Mahjoob, M.P.; Sheikholeslami, S.A.; Dadras, M.; Mansouri, H.; Haghi, M.; Naderian, M.; Sadeghi, L.; Tabary, M.; Khaheshi, I. Prognostic Value of Cardiac Biomarkers Assessment in Combination with Myocardial 2D Strain Echocardiography for Early Detection of Anthracycline-Related Cardiac Toxicity. Cardiovasc. Hematol. Disord. Drug Targets 2020, 20, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated with Anthracyclines, Taxanes, and Trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603. [Google Scholar] [CrossRef]
- Demissei, B.G.; Hubbard, R.A.; Zhang, L.; Smith, A.M.; Sheline, K.; McDonald, C.; Narayan, V.; Domchek, S.M.; Demichele, A.; Shah, P.; et al. Changes in Cardiovascular Biomarkers with Breast Cancer Therapy and Associations with Cardiac Dysfunction. J. Am. Heart Assoc. 2020, 9, e014708. [Google Scholar] [CrossRef] [PubMed]
- Bisoc, A.; Ciurescu, D.; Rădoi, M.; Tântu, M.M.; Rogozea, L.; Sweidan, A.J.; Bota, D.A. Elevations in High-Sensitive Cardiac Troponin T and N-Terminal Prohormone Brain Natriuretic Peptide Levels in the Serum Can Predict the Development of Anthracycline-Induced Cardiomyopathy. Am. J. Ther. 2020, 27, e142–e150. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pei, X.L.; Song, F.Y.; Guo, Y.; Zhang, Q.L.; Shu, X.H.; Hsi, D.H.; Cheng, L.L. Early anthracycline-induced cardiotoxicity monitored by echocardiographic Doppler parameters combined with serum hs-cTnT. Echocardiography 2017, 34, 1593–1600. [Google Scholar] [CrossRef]
- Souza, T.F.d.; Silva, T.Q.A.C.; Costa, F.O.; Shah, R.; Neilan, T.G.; Velloso, L.; Nadruz, W.; Brenelli, F.; Sposito, A.C.; Matos-Souza, J.R.; et al. Anthracycline Therapy Is Associated with Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc. Imaging 2018, 11, 1045–1055. [Google Scholar] [CrossRef]
- Gilbert, G.; Plummer, C.; Plummer, R.; Maier, R.; Austin, D. Cardiac troponins and anthracycline cardiotoxicity: A sub study of the PROACT trial; are all assays made equal? Eur. Heart J. Suppl. 2025, 27, suaf083-130. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Chahal, N.S.; Lim, T.K.; Jain, P.; Chambers, J.C.; Kooner, J.S.; Senior, R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: A population-based study. Eur. J. Echocardiogr. 2010, 11, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Dodos, F.; Halbsguth, T.; Erdmann, E.; Hoppe, U.C. Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin. Res. Cardiol. 2008, 97, 318–326. [Google Scholar] [CrossRef]
- Finke, D.; Romann, S.W.; Heckmann, M.B.; Hund, H.; Bougatf, N.; Kantharajah, A.; Katus, H.A.; Müller, O.J.; Frey, N.; Giannitsis, E.; et al. High-sensitivity cardiac troponin T determines all-cause mortality in cancer patients: A single-centre cohort study. ESC Heart Fail. 2021, 8, 3709–3719. [Google Scholar] [CrossRef]
- Romann, S.W.; Finke, D.; Heckmann, M.B.; Hund, H.; Giannitsis, E.; Katus, H.A.; Frey, N.; Lehmann, L.H. Cardiological parameters predict mortality and cardiotoxicity in oncological patients. ESC Heart Fail. 2024, 11, 366–377. [Google Scholar] [CrossRef]
- Prayogo, A.A.; Suryantoro, S.D.; Savitri, M.; Hendrata, W.M.; Wijaya, A.Y.; Pikir, B.S. High Sensitivity Troponin T as Complementary Modality for Determining Doxorubicin Regimen Cardiotoxicity in Non-Hodgkin Lymphoma Patients. Adv. Pharm. Bull. 2020, 12, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Blaes, A.; Rehman, A.; Vock, D.; Luo, X.; Menge, M.; Yee, D.; Missov, E.; Duprez, D. Utility of high-sensitivity cardiac troponin T in patients receiving anthracycline chemotherapy. Vasc. Health Risk Manag. 2015, 591, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018, 23, 725–734. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Barbieri, A.; Camilli, M.; Bisceglia, I.; Mantovani, F.; Ciampi, Q.; Zito, C.; Canale, M.L.; Khoury, G.; Antonini-Canterin, F.; Carerj, S.; et al. Current use of echocardiography in cardio-oncology: Nationwide real-world data from an ANMCO/SIECVI joint survey. Eur. Heart J. Imaging Methods Pract. 2024, 2, qyae081. [Google Scholar] [CrossRef]
- Sade, L.E.; Joshi, S.S.; Cameli, M.; Cosyns, B.; Delgado, V.; Donal, E.; Edvardsen, T.; Carvalho, R.F.; Manka, R.; Podlesnikar, T.; et al. Current clinical use of speckle-tracking strain imaging: Insights from a worldwide survey from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1583–1592. [Google Scholar] [CrossRef]
- Al Saikhan, L.; Alobaida, M.; Bhuva, A.; Chaturvedi, N.; Heasman, J.; Hughes, A.D.; Jones, S.; Eastwood, S.; Manisty, C.; March, K.; et al. Imaging Protocol, Feasibility, and Reproducibility of Cardiovascular Phenotyping in a Large Tri-Ethnic Population-Based Study of Older People: The Southall and Brent Revisited (SABRE) Study. Front. Cardiovasc. Med. 2020, 7, 591946. [Google Scholar] [CrossRef] [PubMed]
- Dalen, H.; Thorstensen, A.; Vatten, L.J.; Aase, S.A.; Stoylen, A. Reference Values and Distribution of Conventional Echocardiographic Doppler Measures and Longitudinal Tissue Doppler Velocities in a Population Free From Cardiovascular Disease. Circ. Cardiovasc. Imaging 2010, 3, 614–622. [Google Scholar] [CrossRef]
- Ichikawa, N.; Nishizaki, Y.; Miyazaki, S.; Nojima, M.; Kataoka, K.; Kasahara, R.; Takei, J.; Asano, T.; Komiyama, N. Efficacy of mitral annular velocity as an alternative marker of left ventricular global longitudinal strain to detect the risk of cancer therapy-related cardiac disorders. Echocardiography 2024, 41, e15877. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, H.; Kondo, T.; Sugiyama, J.; Kurimoto, K.; Nishino, Y.; Kawada, M.; Hirayama, M.; Tsuji, Y. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer 2017, 24, 774–782. [Google Scholar] [CrossRef]
- Ben Kridis, W.; Sghaier, S.; Charfeddine, S.; Toumi, N.; Daoud, J.; Kammoun, S.; Khanfir, A. A Prospective Study About Trastuzumab-induced Cardiotoxicity in HER2-positive Breast Cancer. Am. J. Clin. Oncol. 2020, 43, 510–516. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n = 40 1 |
|---|---|
| Age, yrs | |
| Mean, (SD) | 54, (12) |
| Min, Max | 31, 74 |
| Gender | |
| Female | 36 (90%) |
| Male | 4 (10%) |
| Smokers (active or former) | 18 (45%) |
| BMI, mean (SD) kg/m2 | 26.2, (3.9) |
| Diabetes | 4 (10%) |
| Hypertension | 17 (43%) |
| Dyslipidaemia | 5 (13%) |
| eGFR, mean (SD), min mL/min/1.73 m2 | 97.3 (16.4) |
| Haemoglobin, mean (SD), min g/L | 131 (10.5) |
| Cardioprotective medications | 21 (53%) |
| RAAS inhibitor (RAASi) | 18 (45%) |
| RAASi—started before the recruitment | 13 (32,5%) |
| Beta blockers (BB) | 15 (38%) |
| BB—started before the recruitment | 10 (25%) |
| Beta-blockers + RAAS inhibitors | 12 (30%) |
| BB + RAASi—started before recruitment | 2 (5%) |
| Statins | 5 (12.5%) |
| HFA-ICOS cardiotoxicity risk level (n = 31) | |
| Low | 16 (52%) |
| Medium | 10 (32%) |
| High | 5 (16%) |
| Follow-up time, median, (IQR) days | 360, (162, 478) |
| Number of follow-up visits, median (IQR) | 4, (3.75, 5.0) |
| Characteristic | n = 40 1 |
|---|---|
| Cancer type | |
| Breast cancer | 31 (77%) |
| Colon or rectal cancer | 7 (18%) |
| Pancreatic cancer | 1 (2.5%) |
| Stomach cancer | 1 (2.5%) |
| Cancer stage | |
| I | 6 (15%) |
| II | 16 (40%) |
| III | 11 (27.5%) |
| IV | 7 (17.5%) |
| Chemotherapy | |
| Adjuvant | 26 (65%) |
| Neoadjuvant + adjuvant | 2 (5.0%) |
| Neoadjuvant | 12 (30%) |
| Previous chemotherapy | 4 (10%) |
| Previous chest radiotherapy | 3 (7.5%) |
| Drugs in the systemic treatment protocols | |
| Anthracyclines 2 | 19 (48%) |
| Cumulative doxorubicin equivalent dose per BSA, mean, (SD) mg/m2 | 123, (43) |
| Trastuzumab | 6 (15%) |
| Pertuzumab + trastuzumab | 4 (10%) |
| Bevacizumab | 4 (10%) |
| 5-Fluorouracil/capecitabine | 9 (22.5%) |
| Taxane | 31 (78%) |
| Cyclophosphamide | 21 (53%) |
| Endocrine therapy | 17 (43%) |
| Types of systemic cancer treatment | |
| Anthracycline-based chemotherapy | 17 (43%) |
| Anthracyclines + trastuzumab | 2 (5.0%) |
| Non-anthracycline-based chemotherapy + trastuzumab | 8 (20%) |
| Other chemotherapy regimens | 13 (33%) |
| Chemotherapy duration, median (IQR), days | 106 (65, 116) |
| HER2-targeted therapy completion on median (IQR) day | 454 (365, 514) |
| Radiotherapy | 16 (40%) |
| Left-chest radiotherapy | 8 (20%) |
| Total dose > 50 Gy | 4 (10%) |
| Radiotherapy completion on median (IQR) day | 183 (155, 270) |
| Echocardiographic Functional Parameters Baseline vs. Maximum Deviations, n = 40 | p Value | ||
|---|---|---|---|
| LVEF, % | Baseline | Minimal | p < 0.001 1 |
| Mean, (SD) | 64.6, (4.6) | 57, (6) | |
| Min, Max | 57.0, 75.0 | 44, 67 | |
| Median time (IQR), days | - | 171 (85, 233) | |
| MAPSE, mm | Baseline | Minimal | p < 0.001 1 |
| Mean, (SD) | 12.94, (1.74) | 11.25 (1.648) | |
| Median time (IQR), days | - | 85 (29, 171) | |
| Septal LVS′, cm/s | Baseline | Minimal | p < 0.001 2 |
| Median (IQR) | 8.00 (7.00, 8.90) | 6.80 (6.00, 7.00) | |
| Median time (IQR), days | 85 (29, 171) | ||
| Lateral LVS′, cm/s | Baseline | Minimal | p < 0.001 2 |
| Median (IQR) | 9.20 (8.30, 10.50) | 7.80 (7.30, 8.50) | |
| Median time (IQR), days | 85 (85, 171) | ||
| Average LVS′, cm/s | Baseline | Minimal | p < 0.001 2 |
| Median (IQR) | 8.50 (8.00, 9.50) | 7.40 (6.85, 8.10) | |
| Median time (IQR), days | 85 (57, 171) | ||
| LVMPI (PW) | Baseline | Maximum | p < 0.001 2 |
| Mean, (SD) | 0.43, (0.10) | 0.54, (0.12) | |
| Median time (IQR), days | 128 (29, 374) | ||
| RVFAC, % | Baseline | Minimal | p < 0.001 1 |
| Mean, (SD) | 46, (6) | 38.6, (5.2) | |
| Median time (IQR), days | 85 (85, 171) | ||
| RVS′, cm/s | Baseline | Minimal | p < 0.001 1 |
| Mean, (SD) | 12.53, (2.16) | 10.60, (1.66) | |
| Median time (IQR), days | 85 (29, 374) | ||
| TAPSE, mm | Baseline | Minimal | p < 0.001 1 |
| Mean, (SD) | 19.2, (3.3) | 16.56 (3.12) | |
| Median time (IQR), days | 128 (85, 171) | ||
| RVMPI (TDI) | Baseline | Maximum | p = 0.011 2 |
| Median (IQR) | 0.38 (0.32, 0.51) | 0.48 (0.37, 0.57) | |
| Median time (IQR), days | 85 (29, 107) | ||
| LVe′, cm/s | Baseline | Minimum | p < 0.001 2 |
| Median (IQR) | 9.80 (8.80, 11.30) | 8.30 (7.20, 10.50) | |
| Median time (IQR), days | 171 (85, 213) | ||
| LV E/e′ | Baseline | Maximum | p < 0.001 2 |
| Median (IQR) | 7.95 (6.40, 8.90) | 8.45 (7.40, 9.60) | |
| Median time (IQR), days | 85 (85, 171) | ||
| DecT, msec | Baseline | Maximum | p < 0.001 2 |
| Median (IQR) | 200 (170, 218) | 231 (219, 260) | |
| Median time (IQR), days | 171 (71, 284) | ||
| hs-cTnT | Baseline n = 40 | 1st Month n = 37 | 3rd Month n = 29 | 6th Month n = 14 | 9th Month n = 6 | 12th Month n = 5 |
|---|---|---|---|---|---|---|
| Median (IQR) | 6.8 (4.8, 9.6) | 6.6 (5.9, 10.3) | 8.3 (6.5, 10.0) | 8.9 (7.0, 10.9) | 6.9 (6.3, 8.4) | 9.1 (5.6, 14.6) |
| Min, Max | 3.0, 30.2 | 3.4, 15.3 | 3.2, 24.3 | 5.4, 25.1 | 5.6, 10.2 | 3.7, 16.7 |
| Prognostic Factors | Beta | 95% CI | p-Value |
|---|---|---|---|
| Age | 0.19 | 0.07, 0.31 | 0.002 |
| Cancer therapy | |||
| Trastuzumab | 1.3 | −3.4, 0.81 | 0.2 |
| Anthracyclines | 2.2 | −0.38, 4.7 | 0.095 |
| Other | −1.0 | −3.2, 1.3 | 0.4 |
| Cardioprotective treatment | 2.1 | −0.30, 4.5 | 0.086 |
| Haemoglobin | −0.05 | −0.12, 0.02 | 0.14 |
| Cancer stage | |||
| Early stage | reference | ||
| Advanced stage | 3.6 | 1.2, 5.9 | 0.003 |
| eGFR, mL/min/1.73 m2 | −0.14 | 0.22, −0.06 | <0.001 |
| HFA-ICOS cardiotoxicity risk | |||
| Low | — | — | |
| Medium | 5.2 | 1.2, 9.1 | 0.010 |
| High | 3.9 | 2.4, 5.5 | <0.001 |
| Echocardiographic Variable | Univariate Model | Multivariate Model 1 | ||||
|---|---|---|---|---|---|---|
| hs-cTnT Beta, ng/L | 95% CI | p | hs-cTnT 1 Beta, ng/L | 95% CI | p | |
| LVEF, % | 0.12 | −0.09, 0.33 | 0.3 | - | - | - |
| RVFAC, % | −0.02 | −0.30, 0.26 | >0.9 | - | - | - |
| Average LVS′, cm/s | −0.07 | −0.12, −0.02 | 0.008 | −0.07 | −0.14, 0.00 | 0.036 |
| Septal LVS′, cm/s | −0.06 | −0.11, −0.02 | 0.005 | −0.06 3 | −0.12, 0.01 | 0.088 |
| Lateral LVS′, cm/s | −0.08 | −0.14, −0.01 | 0.030 | −0.09 | −0.17, −0.01 | 0.033 |
| RVS′, cm/s | −0.08 | −0.16, 0.01 | 0.083 | - | - | - |
| LV e′, cm/s | −0.23 | −0.34, −0.12 | <0.001 | −0.06 4 | −0.16, 0.05 | 0.3 |
| LV E/A | −0.02 | −0.03, −0.01 | <0.001 | 0.00 | −0.02, 0.03 | 0.8 |
| LV E/e′ | 0.24 | 0.09, 0.39 | 0.002 | 0.07 5 | −0.02, 0.15 | 0.11 |
| LV DecT | 2.9 | 1.2, 4.7 | <0.001 | 1.5 6 | 0.25, 2.7 | 0.018 |
| LV systolic dysfunction | 1.07 2 | 0.96, 1.19 | 0.2 | - | - | - |
| ROC Analysis of hs-cTnT Thresholds for Prediction of Echocardiographic Parameters Abnormal Values | ||||||
|---|---|---|---|---|---|---|
| Echo Parameters | hs-cTnT Threshold 1 | Specificity 1 | Sensitivity 1 | NPV 1 | PPV 1 | AUC 1 |
| Septal LVS′ ≤ 6 cm/s | 8.3 (6.95, 9.67) | 0.61 (0.45, 0.73) | 0.75 (0.5, 1.0) | 0.99 (0.98, 1.0) | 0.06 (0.05, 0.08) | 0.78 (0.59, 0.98) |
| Average LVS′ ≤ 7 cm/s | 8.23 (6.88, 9.71) | 0.59 (0.44, 0.73) | 0.63 (0.38, 1.0) | 0.96 (0.95, 0.97) | 0.09 (0.08, 0.09) | 0.68 (0.53, 0.84) |
| Lateral LVS′ ≤ 8 cm/s | 8.08 (6.73, 9.62) | 0.60 (0.44, 0.73) | 0.64 (0.36, 0.79) | 0.93 (0.91, 0.94) | 0.17 (0.14, 0.18) | 0.65 (0.51, 0.78) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavcheva, S.E.; Shefket, S.A.; Bocheva, Y.; Angelov, A. High-Sensitivity Troponin T as a Prognostic Factor of Conventional Echocardiographic Parameters in Cancer Patients: A Prospective Observational Study. Medicina 2025, 61, 1911. https://doi.org/10.3390/medicina61111911
Slavcheva SE, Shefket SA, Bocheva Y, Angelov A. High-Sensitivity Troponin T as a Prognostic Factor of Conventional Echocardiographic Parameters in Cancer Patients: A Prospective Observational Study. Medicina. 2025; 61(11):1911. https://doi.org/10.3390/medicina61111911
Chicago/Turabian StyleSlavcheva, Svetoslava Elefterova, Sevim Ahmed Shefket, Yana Bocheva, and Atanas Angelov. 2025. "High-Sensitivity Troponin T as a Prognostic Factor of Conventional Echocardiographic Parameters in Cancer Patients: A Prospective Observational Study" Medicina 61, no. 11: 1911. https://doi.org/10.3390/medicina61111911
APA StyleSlavcheva, S. E., Shefket, S. A., Bocheva, Y., & Angelov, A. (2025). High-Sensitivity Troponin T as a Prognostic Factor of Conventional Echocardiographic Parameters in Cancer Patients: A Prospective Observational Study. Medicina, 61(11), 1911. https://doi.org/10.3390/medicina61111911






