The Immediate Effects of Instrument-Assisted Soft Tissue Mobilization on Pain and Function in Female Runners with Patellofemoral Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Sample Size Calculation
2.3. Study Protocol
2.4. Outcome Measures
2.4.1. Visual Analog Scale—Worst Pain (VAS-W)
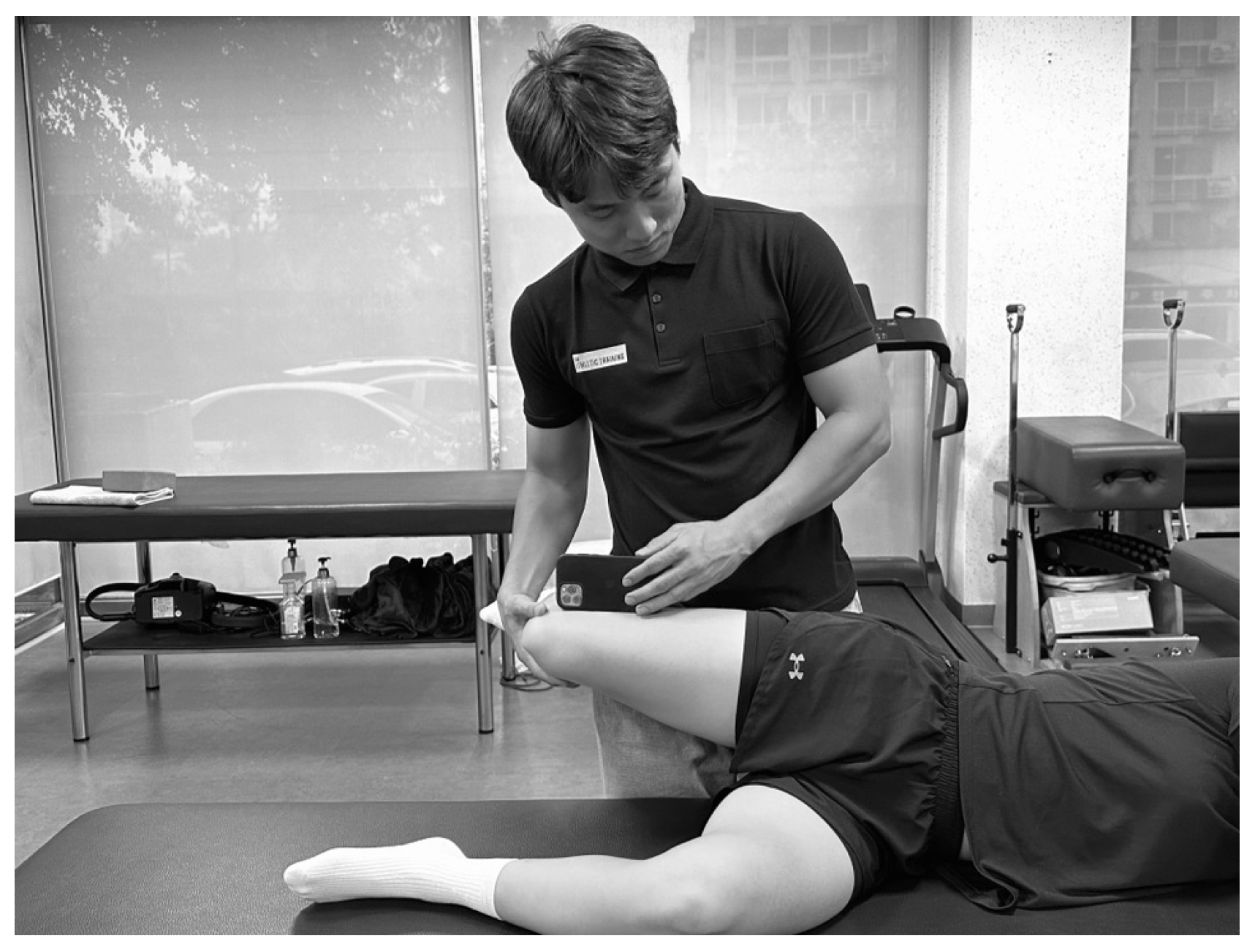
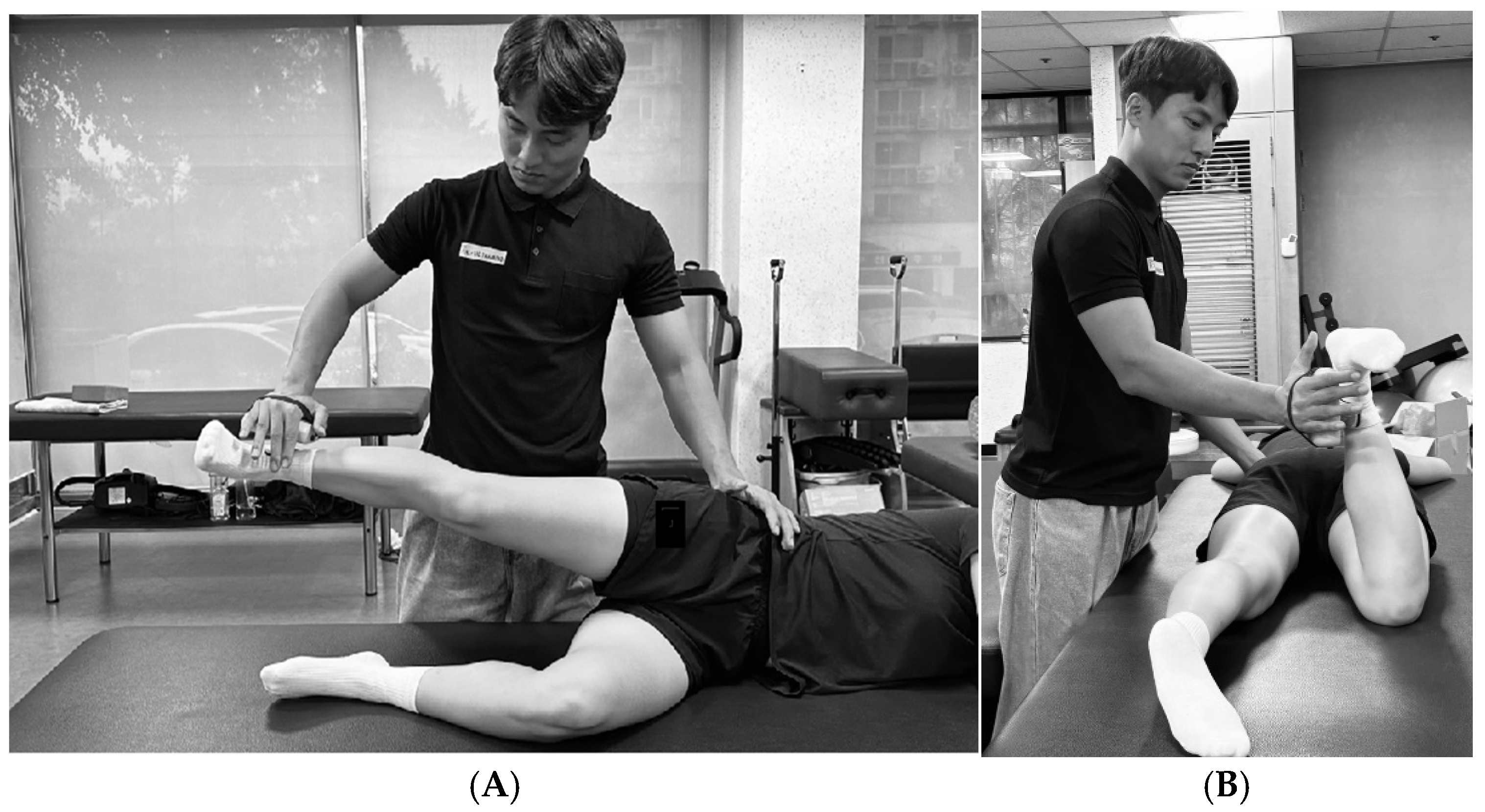
2.4.2. Hip Adduction ROM
2.4.3. Hip Strength
2.4.4. Hip Abduction
2.4.5. Hip External Rotation
2.5. Functional Performance and Pain
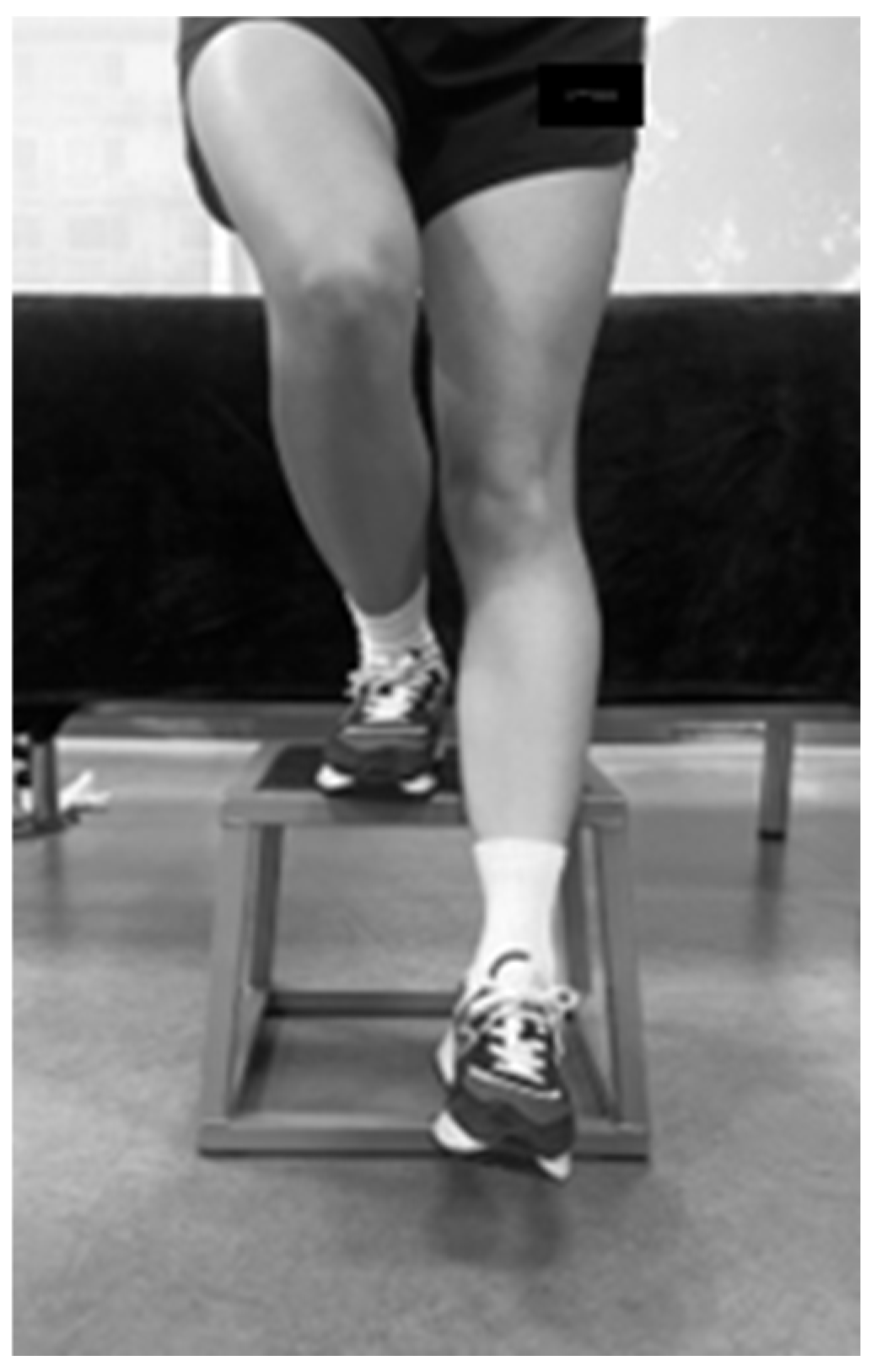
Step-Down Test
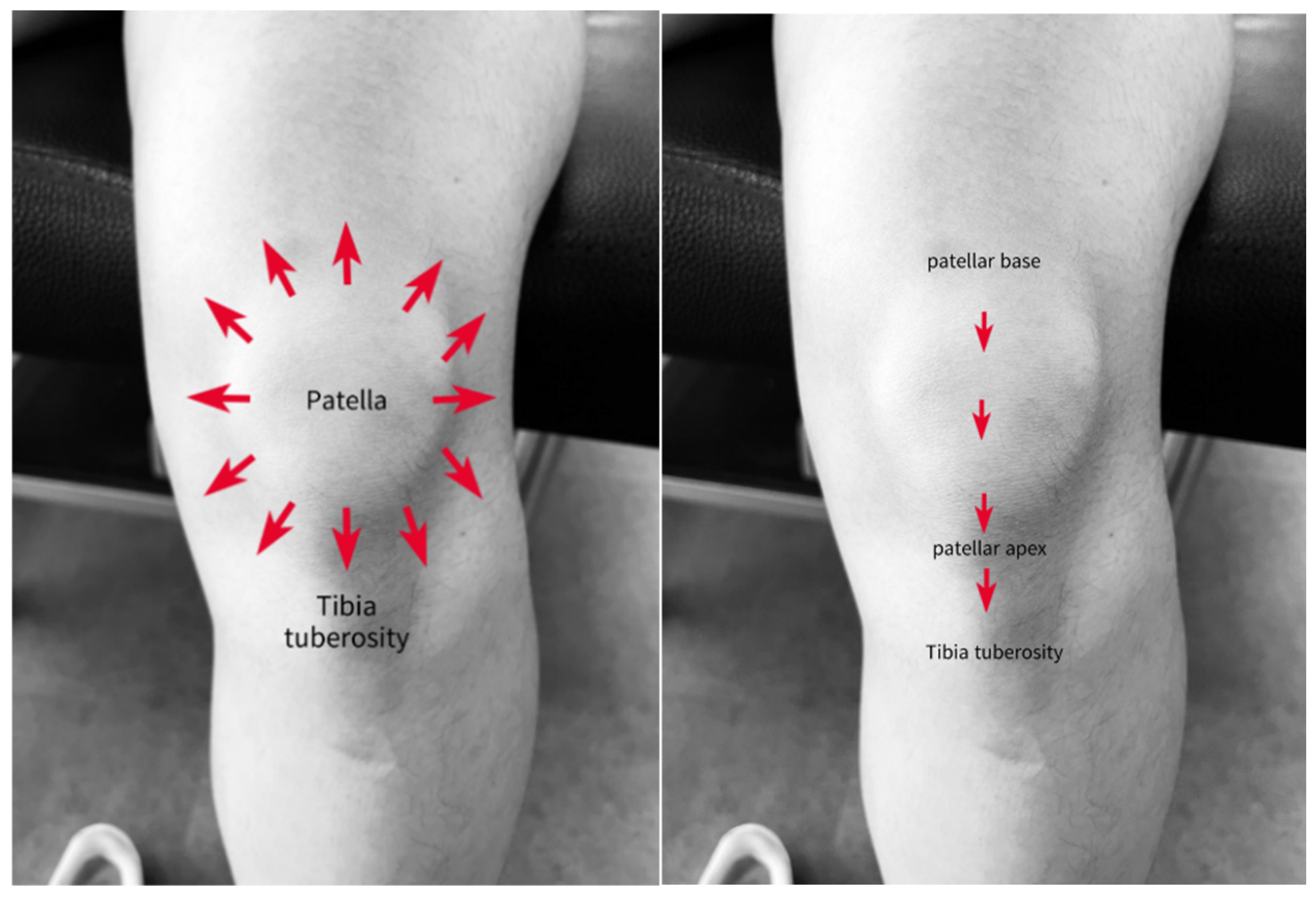
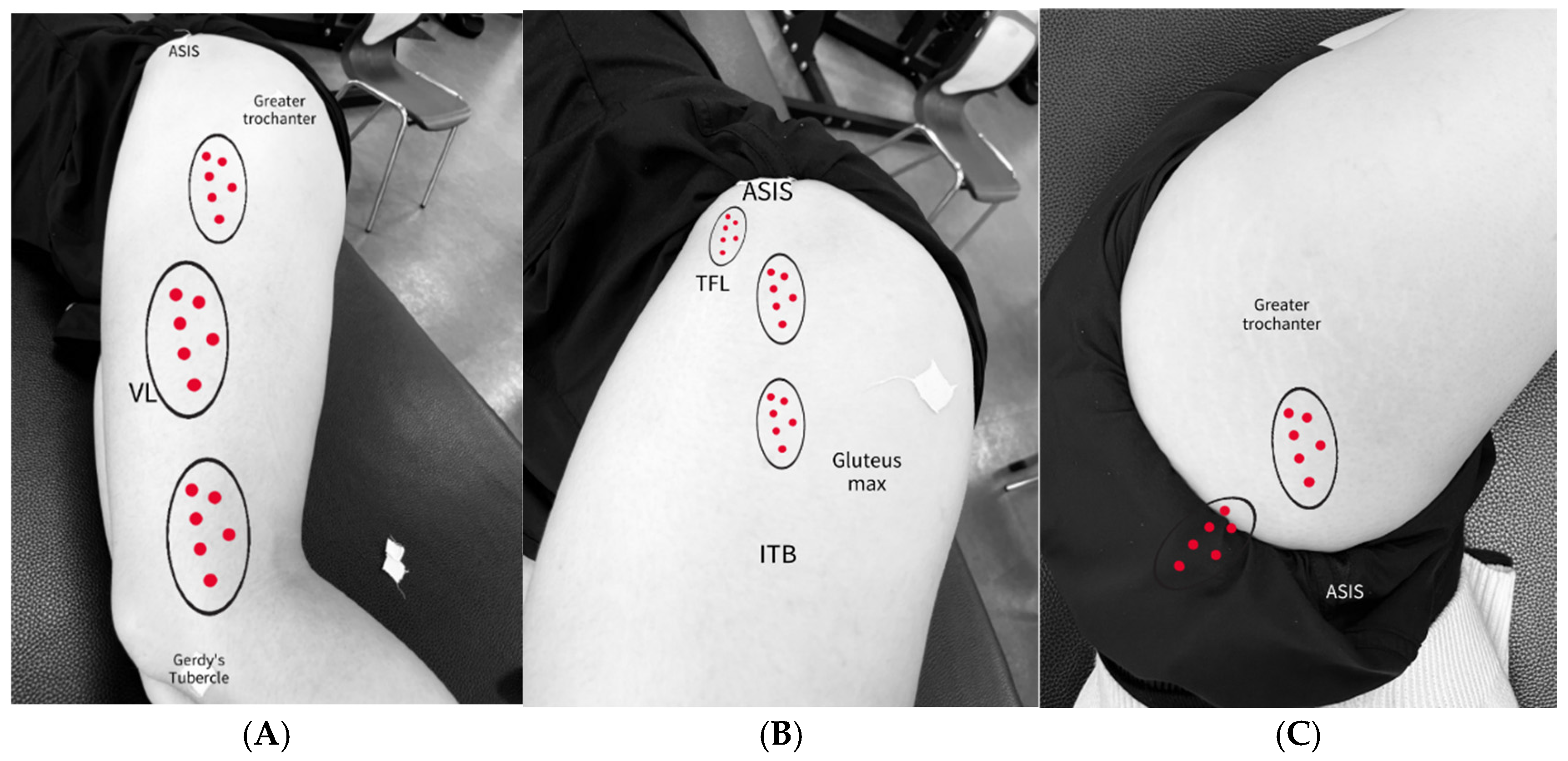
2.6. IASTM Application
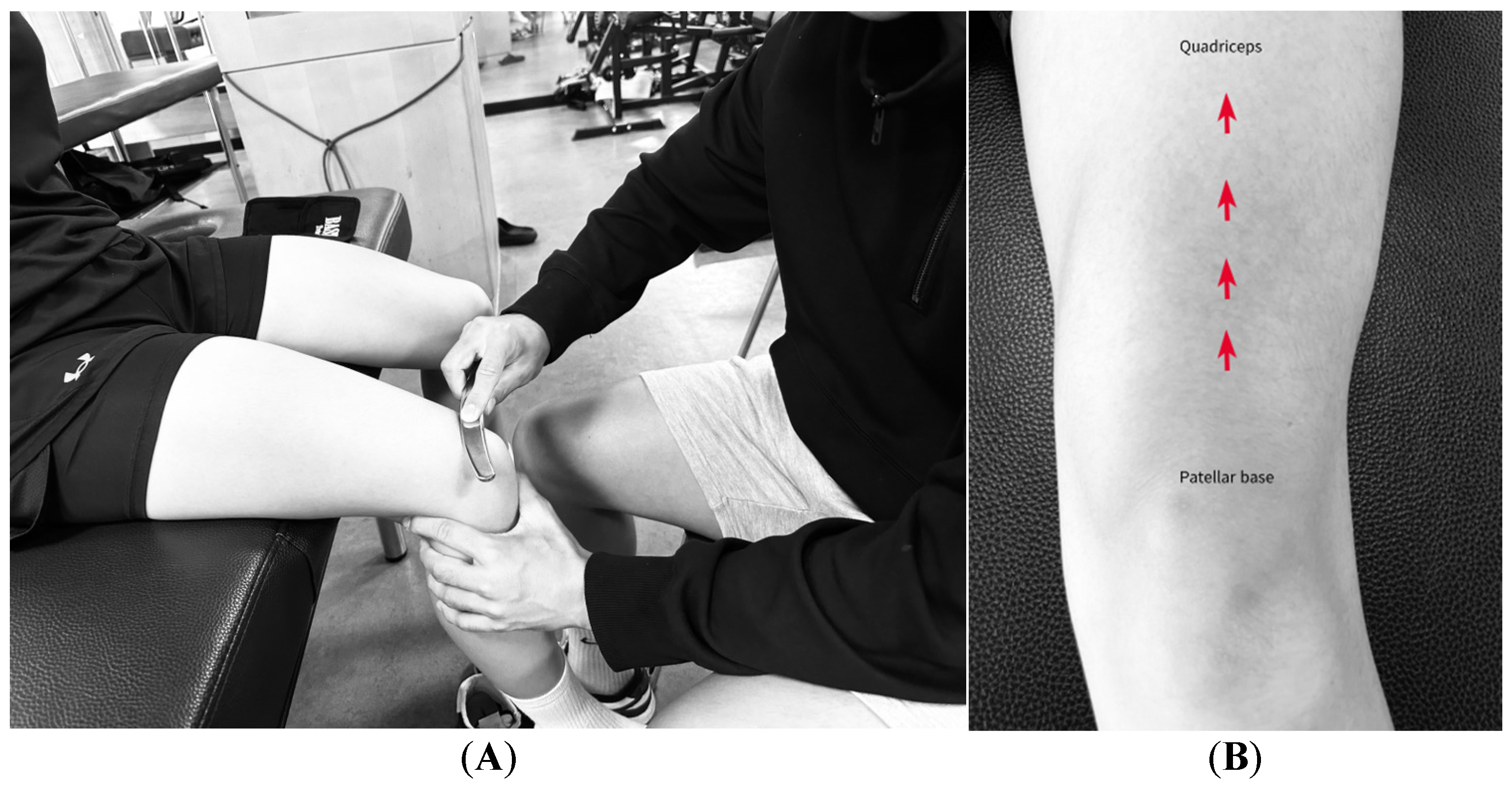
2.6.1. ITB
2.6.2. ITB and TFL/GMed Border
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFP | Patellofemoral Pain |
| VAS | Visual Analog Scale |
| IASTM | Instrument-assisted soft tissue mobilization |
| GMed | Gluteus Medius |
| ITB | Iliotibial band |
| MTrPs | Myofascial trigger points |
| ROM | Range of motion |
| TFL | Tensor fascia late |
| VL | Vastus lateralis |
| VM | Vastus medialis |
References
- Aderem, J.; Louw, Q.A. Biomechanical risk factors associated with iliotibial band syndrome in runners: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 356. [Google Scholar] [CrossRef]
- Akodu, A.; Nwakalor, N. Patellofemoral pain syndrome: Prevalence and coping strategies of amateur runners in Lagos state. Med. Sport. J. Rom. Sports Med. Soc. 2018, 14, 3059–3067. [Google Scholar]
- Aksan Sadikoglu, B.; Akbaba, Y.A.; Taskiran, H. Effects of ischemic compression and instrument-assisted soft tissue mobilization techniques in trigger point therapy in patients with rotator cuff pathology: Randomized controlled study. Somatosens. Mot. Res. 2022, 39, 70–80. [Google Scholar] [CrossRef]
- Anderson, G.; Herrington, L. A comparison of eccentric isokinetic torque production and velocity of knee flexion angle during step down in patellofemoral pain syndrome patients and unaffected subjects. Clin. Biomech. 2003, 18, 500–504. [Google Scholar] [CrossRef]
- Baker, R.T.; Nasypany, A.; Seegmiller, J.G.; Baker, J.G. Instrument-assisted soft tissue mobilization treatment for tissue extensibility dysfunction. Int. J. Athl. Ther. Train. 2013, 18, 16–21. [Google Scholar] [CrossRef]
- Bazett-Jones, D.M.; Cobb, S.C.; Huddleston, W.E.; O’cOnnor, K.M.; Armstrong, B.S.R.; Earl-Boehm, J.E. Effect of patellofemoral pain on strength and mechanics after an exhaustive run. Med. Sci. Sports Exerc. 2013, 45, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Bevilaqua-Grossi, D.; Monteiro-Pedro, V.; da Cunha Sousa, G.; Silva, Z.; Bérzin, F. Contribution to the anatomical study of the oblique portion of the vastus lateralis muscle. J. Morphol. Sci. 2004, 21, 47–52. [Google Scholar]
- Brantingham, J.W.; Globe, G.A.; Jensen, M.L.; Cassa, T.K.; Globe, D.R.; Price, J.L.; Mayer, S.N.; Lee, F.T. A feasibility study comparing two chiropractic protocols in the treatment of patellofemoral pain syndrome. J. Manip. Physiol. Ther. 2009, 32, 536–548. [Google Scholar] [CrossRef]
- Bron, C.; Dommerholt, J.D. Etiology of myofascial trigger points. Curr. Pain Headache Rep. 2012, 16, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Buist, I.; Bredeweg, S.W.; Bessem, B.; van Mechelen, W.; Lemmink, K.A.P.M.; Diercks, R.L. Incidence and risk factors of running-related injuries during preparation for a 4-mile recreational running event. Br. J. Sports Med. 2010, 44, 598–604. [Google Scholar] [CrossRef]
- Castanov, V.; Hassan, S.A.; Shakeri, S.; Vienneau, M.; Zabjek, K.; Richardson, D.; McKee, N.H.; Agur, A.M.R. Muscle architecture of vastus medialis obliquus and longus and its functional implications: A three-dimensional investigation. Clin. Anat. 2019, 32, 515–523. [Google Scholar] [CrossRef]
- Caylor, D.; Fites, R.; Worrell, T.W. The relationship between quadriceps angle and anterior knee pain syndrome. J. Orthop. Sports Phys. Ther. 1993, 17, 11–16. [Google Scholar] [CrossRef]
- Cheatham, S.; Martinez, R.; Montalvo, A.; Odai, M.; Echeverry, S.; Robinson, B.; Bailum, E.; Viecco, K. Myofascial compression interventions: Comparison of roller massage, instrument assisted soft-tissue mobilization, and floss band on passive knee motion among inexperienced individuals. Clin. Pract. Athl. Train. 2020, 3, 24–36. [Google Scholar] [CrossRef]
- Chen, G.; Gu, Z.; Wang, P.; Qi, Y.; Dai, J. Analysis of lower limb muscle strength characteristics of amateur runners with patellofemoral pain: A cross-sectional study. PLoS ONE 2024, 19, e0305141. [Google Scholar] [CrossRef]
- Choi, B.S.; Bin Kwon, S.; Jeon, S.; Kim, M.; Ku, Y.; Ro, D.H.; Han, H.-S. Relationship between muscle activation and sagittal knee joint biomechanics in patients with patellofemoral pain syndrome: A cross-sectional study. Knee Surg. Relat. Res. 2025, 37, 7. [Google Scholar] [CrossRef]
- Cichanowski, H.R.; Schmitt, J.S.; Johnson, R.J.; Niemuth, P.E. Hip strength in collegiate female athletes with patellofemoral pain. Med. Sci. Sports Exerc. 2007, 39, 1227–1232. [Google Scholar] [CrossRef]
- Willson, J.D.; Kernozek, T.W.; Arndt, R.L.; Reznichek, D.A.; Straker, J.S. Gluteal muscle activation during running in females with and without patellofemoral pain syndrome. Clin. Biomech. 2011, 26, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Z.; Darthuy, E. Iliotibial band tightness and patellofemoral pain syndrome: A case-control study. Man. Ther. 2009, 14, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Cowan, S.M.; Bennell, K.L.; Hodges, P.W.; Crossley, K.M.; McConnell, J. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch. Phys. Med. Rehabil. 2001, 82, 183–189. [Google Scholar] [CrossRef]
- Crossley, K.M.; Bennell, K.L.; Cowan, S.M.; Green, S. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch. Phys. Med. Rehabil. 2004, 85, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; Stefanik, J.J.; Selfe, J.; Collins, N.J.; Davis, I.S.; Powers, C.M.; McConnell, J.; Vicenzino, B.; Bazett-Jones, D.M.; Esculier, J.-F.; et al. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br. J. Sports Med. 2016, 50, 839–843. [Google Scholar] [CrossRef]
- de Castro, M.P.; de Brito Fontana, H.; Fóes, M.C.; Santos, G.M.; Ruschel, C.; Roesler, H. Activation of the gluteus maximus, gluteus medius and tensor fascia lata muscles during hip internal and external rotation exercises at three hip flexion postures. J. Bodyw. Mov. Ther. 2021, 27, 487–492. [Google Scholar] [CrossRef]
- Dierks, T.A.; Manal, K.T.; Hamill, J.; Davis, I.S. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J. Orthop. Sports Phys. Ther. 2008, 38, 448–456. [Google Scholar] [CrossRef]
- Elmahdy, M.A.; Ayad, K.; Abdelrahman, A.M.; Abdelsalam, M.S. Correlation between contractile properties of quadriceps muscle and functional performance in runners with patellofemoral pain syndrome. Sport TK-Rev. Euroam. Cienc. Deporte 2023, 12, 32. [Google Scholar] [CrossRef]
- Eng, C.M.; Arnold, A.S.; Lieberman, D.E.; Biewener, A.A. The capacity of the human iliotibial band to store elastic energy during running. J. Biomech. 2015, 48, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Esculier, J.-F.; Roy, J.-S.; Bouyer, L.J. Lower limb control and strength in runners with and without patellofemoral pain syndrome. Gait Posture 2015, 41, 813–819. [Google Scholar] [CrossRef]
- Fairclough, J.; Hayashi, K.; Toumi, H.; Lyons, K.; Bydder, G.; Phillips, N.; Best, T.M.; Benjamin, M. The functional anatomy of the iliotibial band during flexion and extension of the knee: Implications for understanding iliotibial band syndrome. J. Anat. 2006, 208, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ferber, R.; Kendall, K.D.; McElroy, L. Normative and critical criteria for iliotibial band and iliopsoas muscle flexibility. J. Athl. Train. 2010, 45, 344–348. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Barton, G.; Borges, L.D.; Rabelo, N.D.d.A.; Politti, F.; Lucareli, P.R.G. Step down tests are the tasks that most differentiate the kinematics of women with patellofemoral pain compared to asymptomatic controls. Gait Posture 2019, 72, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Foch, E.; Brindle, R.A.; Milner, C.E. Weak associations between hip adduction angle and hip abductor muscle activity during running. J. Biomech. 2020, 110, 109965. [Google Scholar] [CrossRef]
- Fulkerson, J.P.; Arendt, E.A. Anterior knee pain in females. Clin. Orthop. Relat. Res. 2000, 372, 69–73. [Google Scholar] [CrossRef]
- Gawda, P.; Ginszt, M.; Zawadka, M.; Skublewska-Paszkowska, M.; Smołka, J.; Łukasik, E.; Majcher, P. Bioelectrical Activity of Vastus Medialis and Rectus Femoris Muscles in Recreational Runners with Anterior Knee Pain. J. Hum. Kinet. 2019, 66, 81–88. [Google Scholar] [CrossRef]
- Glaviano, N.R.; Saliba, S. Differences in gluteal and quadriceps muscle activation during weight-bearing exercises between female subjects with and without patellofemoral pain. J. Strength Cond. Res. 2022, 36, 55–62. [Google Scholar] [CrossRef]
- Gottschalk, F.R.; Kourosh, S.O.; Leveau, B. The functional anatomy of tensor fasciae latae and gluteus medius and minimus. J. Anat. 1989, 166, 179–189. [Google Scholar]
- Gulick, D.T. Instrument-assisted soft tissue mobilization increases myofascial trigger point pain threshold. J. Bodyw. Mov. Ther. 2018, 22, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Hains, G.; Hains, F. Patellofemoral pain syndrome managed by ischemic compression to the trigger points located in the peri-patellar and retro-patellar areas: A randomized clinical trial. Clin. Chiropr. 2010, 13, 201–209. [Google Scholar] [CrossRef]
- Hammer, W.I. The effect of mechanical load on degenerated soft tissue. J. Bodyw. Mov. Ther. 2008, 12, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Heinert, B.L.; Kernozek, T.W.; Greany, J.F.; Fater, D.C. Hip abductor weakness and lower extremity kinematics during running. J. Sport Rehabil. 2008, 17, 243–256. [Google Scholar] [CrossRef]
- Hollander, K.; Rahlf, A.L.; Wilke, J.; Edler, C.; Steib, S.; Junge, A.; Zech, A. Sex-Specific Differences in Running Injuries: A Systematic Review with Meta-Analysis and Meta-Regression. Sports Med. 2021, 51, 1011–1039. [Google Scholar] [CrossRef]
- Hutchinson, L.A.; Lichtwark, G.A.; Willy, R.W.; Kelly, L.A. The Iliotibial Band: A Complex Structure with Versatile Functions. Sports Med. 2022, 52, 995–1008. [Google Scholar] [CrossRef]
- Kakouris, N.; Yener, N.; Fong, D.T. A systematic review of running-related musculoskeletal injuries in runners. J. Sport Health Sci. 2021, 10, 513–522. [Google Scholar] [CrossRef]
- Kim, J.; Sung, D.J.; Lee, J. Therapeutic effectiveness of instrument-assisted soft tissue mobilization for soft tissue injury: Mechanisms and practical application. J. Exerc. Rehabil. 2017, 13, 12–22. [Google Scholar] [CrossRef]
- Krause, D.A.; Neuger, M.D.; Lambert, K.A.; Johnson, A.E.; DeVinny, H.A.; Hollman, J.H. Effects of examiner strength on reliability of hip-strength testing using a handheld dynamometer. J. Sport Rehabil. 2014, 23, 56–64. [Google Scholar] [CrossRef]
- Lee, J.; Young, A.; Erb, N.J.; Herzog, V.W. Acute and residual effects of IASTM and roller massage stick on hamstring range of motion. J. Allied Health 2020, 49, e51–e55. [Google Scholar]
- Lee, T.Q.; Morris, G.; Csintalan, R.P. The influence of tibial and femoral rotation on patellofemoral contact area and pressure. J. Orthop. Sports Phys. Ther. 2003, 33, 686–693. [Google Scholar] [CrossRef]
- Liao, T.-C.; Yang, N.; Ho, K.-Y.; Farrokhi, S.; Powers, C.M. Femur Rotation Increases Patella Cartilage Stress in Females with Patellofemoral Pain. Med. Sci. Sports Exerc. 2015, 47, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y. A comparative study of the efficacy of instrument-assisted soft tissue mobilization and massage techniques in patients with patellofemoral joint pain. Front. Med. 2023, 10, 1305733. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L. Effect of instrument-assisted soft tissue mobilization combined with blood flow restriction training on function, pain and strength of patients with patellofemoral joint pain. BMC Musculoskelet. Disord. 2023, 24, 698. [Google Scholar] [CrossRef] [PubMed]
- Loghmani, M.T.; Warden, S.J. Instrument-assisted cross-fiber massage accelerates knee ligament healing. J. Orthop. Sports Phys. Ther. 2009, 39, 506–514. [Google Scholar] [CrossRef]
- Loghmani, M.T.; Warden, S.J. Instrument-assisted cross fiber massage increases tissue perfusion and alters microvascular morphology in the vicinity of healing knee ligaments. BMC Complement. Altern. Med. 2013, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Loudon, J.K.; Wiesner, D.; Goist-Foley, H.L.; Asjes, C.; Loudon, K.L. Intrarater Reliability of Functional Performance Tests for Subjects With Patellofemoral Pain Syndrome. J. Athl. Train. 2020, 37, 256–261. [Google Scholar]
- Malinzak, R.A.; Colby, S.M.; Kirkendall, D.T.; Yu, B.; Garrett, W.E. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin. Biomech. 2001, 16, 438–445. [Google Scholar] [CrossRef]
- Markovic, G. Acute effects of instrument assisted soft tissue mobilization vs. foam rolling on knee and hip range of motion in soccer players. J. Bodyw. Mov. Ther. 2015, 19, 690–696. [Google Scholar] [CrossRef]
- Meira, E.P.; Brumitt, J. Influence of the hip on patients with patellofemoral pain syndrome: A systematic review. Sports Health 2011, 3, 455–465. [Google Scholar] [CrossRef]
- Mellinger, S.; Neurohr, G.A. Evidence based treatment options for common knee injuries in runners. Ann. Transl. Med. 2019, 7 (Suppl. S7), S249. [Google Scholar] [CrossRef]
- Motealleh, A.; Gheysari, E.; Shokri, E.; Sobhani, S. The immediate effect of lumbopelvic manipulation on EMG of vasti and gluteus medius in athletes with patellofemoral pain syndrome: A randomized controlled trial. Man. Ther. 2016, 22, 16–21. [Google Scholar] [CrossRef]
- Mubashar, H.; Hassan, D.; Bushra, M.; Zaryyab; Rahman, A.; Manahil, A. Effects of Instrumented Assisted Soft Tissue Mobilization (IASTM) Technique Versus Stretching on Ililotibal Band in Patients with Anterior Knee Pain. Pak. J. Med. Health Sci. 2022, 16, 353–357. [Google Scholar] [CrossRef]
- Noehren, B.; Scholz, J.; Davis, I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br. J. Sports Med. 2011, 45, 691–696. [Google Scholar] [CrossRef]
- Osailan, A.; Jamaan, A.; Talha, K.; Alhndi, M. Instrument assisted soft tissue mobilization (IASTM) versus stretching: A comparison in effectiveness on hip active range of motion, muscle torque and power in people with hamstring tightness. J. Bodyw. Mov. Ther. 2021, 27, 200–206. [Google Scholar] [CrossRef]
- Park, K.-M.; Cynn, H.-S.; Choung, S.-D. Musculoskeletal predictors of movement quality for the forward step-down test in asymptomatic women. J. Orthop. Sports Phys. Ther. 2013, 43, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Pişirici, P.; Şakul, B.U. Investigation of the functional and biomechanical effect of instrument-assisted soft tissue mobilization technique in individuals with asymptomatic dynamic knee valgus-Randomized controlled trial. J. Bodyw. Mov. Ther. 2024, 39, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Piva, S.R.; Goodnite, E.A.; Childs, J.D. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2005, 35, 793–801. [Google Scholar] [CrossRef]
- Willett, G.M.; Keim, S.A.; Shostrom, V.K.; Lomneth, C.S. An anatomic investigation of the ober test. Am. J. Sports Med. 2016, 44, 696–701. [Google Scholar] [CrossRef]
- Reese, N.B.; Bandy, W.D. Use of an inclinometer to measure flexibility of the iliotibial band using the Ober test and the modified Ober test: Differences in magnitude and reliability of measurements. J. Orthop. Sports Phys. Ther. 2003, 33, 326–330. [Google Scholar] [CrossRef]
- Roach, S.; Sorenson, E.; Headley, B.; Juan, J.G.S. Prevalence of myofascial trigger points in the hip in patellofemoral pain. Arch. Phys. Med. Rehabil. 2013, 94, 522–526. [Google Scholar] [CrossRef]
- Rozenfeld, E.; Finestone, A.S.; Moran, U.; Damri, E.; Kalichman, L. The prevalence of myofascial trigger points in hip and thigh areas in anterior knee pain patients. J. Bodyw. Mov. Ther. 2020, 24, 31–38. [Google Scholar] [CrossRef]
- Souza, R.B.; Draper, C.E.; Fredericson, M.; Powers, C.M. Femur rotation and patellofemoral joint kinematics: A weight-bearing magnetic resonance imaging analysis. J. Orthop. Sports Phys. Ther. 2010, 40, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Stow, R. Instrument-Assisted Soft Tissue Mobilization. Int. J. Athl. Ther. Train. 2011, 16, 5. [Google Scholar] [CrossRef]
- Sulowska-Daszyk, I.; Skiba, A. The Influence of Self-Myofascial Release on Muscle Flexibility in Long-Distance Runners. Int. J. Environ. Res. Public Health 2022, 19, 457. [Google Scholar] [CrossRef]
- Taunton, J.E.; Ryan, M.B.; Clement, D.B.; McKenzie, D.C.; Lloyd-Smith, D.R.; Zumbo, B.D. A retrospective case-control analysis of 2002 running injuries. Br. J. Sports Med. 2002, 36, 95–101. [Google Scholar] [CrossRef]
- Tumia, N.; Maffulli, N. Patellofemoral pain in female athletes. Sports Med. Arthrosc. Rev. 2002, 10, 69–75. [Google Scholar] [CrossRef]
- Unuvar, B.S.; Demirdel, E.; Gercek, H. The Effects of Different Myofascial Release Techniques on Pain, Range of Motion, and Muscle Strength in Athletes With Iliotibial Band Tightness: A Randomized Controlled Study. J. Sport Rehabil. 2024, 33, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, R.N.; Siem, D.; Van Middelkoop, M.; Van Os, A.G.; Bierma-Zeinstra, S.M.; Koes, B.W. Incidence and determinants of lower extremity running injuries in long distance runners: A systematic review. Br. J. Sports Med. 2007, 41, 469–480; discussion 480. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Jaideep, A.; Khankal, R.; Gazbare, P.; Abraham, B. Effectiveness of compressive myofascial release vs instrument assisted soft tissue mobilization in subjects with active trigger points of the calf muscle limiting ankle dorsiflexion. Int. J. Health Sci. Res. 2019, 9, 98–106. [Google Scholar]
- Wellmon, R.H.; Gulick, D.T.; Paterson, M.L.; Gulick, C.N. Validity and Reliability of 2 Goniometric Mobile Apps: Device, Application, and Examiner Factors. J. Sport Rehabil. 2016, 25, 371–379. [Google Scholar] [CrossRef] [PubMed]






| HK (n = 14) | K (n = 14) | t | p | |
|---|---|---|---|---|
| Age (years) | 23.39 ± 2.55 | 25.75 ± 4.16 | 0.360 | 0.722 |
| Height (m) | 1.62 ± 0.05 | 165.67 ± 4.66 | 0.176 | 0.861 |
| Weight (kg) | 55.9 ± 5.19 | 58.50 ± 5.68 | 1.764 | 0.089 |
| BMI | 21.4 ± 1.43 | 20.4 ± 1.43 | 1.869 | 0.073 |
| Running distance (km) (week) | 16.6 ± 6.62 | 16.9 ± 7.73 | −0.131 | 0.868 |
| Variable | Group | Baseline (95% CI) | Immediate (95% CI) | Week 1 (95% CI) |
|---|---|---|---|---|
| Running VAS | HK | 5.49 ± 2.14 (4.28–6.68) | - | 1.30 ± 1.08 * (0.69–1.90) |
| K | 5.30 ± 1.45 (4.46–6.13) | - | 1.57 ± 1.20 * (0.87–2.26) | |
| Hip AD ROM | HK | 11.28 ± 3.91 (9.01–13.53) | 15.41 ± 4.06 * (13.07–17.75) | 14.37 ± 5.04 *† (11.45–17.26) |
| K | 11.10 ± 3.51 (9.07–13.12) | 14.51 ± 4.44 * (11.94–17.08) | 14.83 ± 5.01 *† (11.93–17.71) | |
| Hip AB Strength | HK | 14.11 ± 2.34 (12.75–15.46) | 15.45 ± 1.87 * (14.37–16.53) | 15.41 ± 2.39 *† (14.02–16.78) |
| K | 15.79 ± 3.02 (14.04–17.53) | 16.01 ± 2.94 * (14.30–17.70) | 15.79 ± 3.43 *† (13.81–17.77) | |
| Hip ER Strength | HK | 17.91 ± 3.24 (16.04–19.78) | 18.43 ± 2.55 (16.95–19.89) | 17.44 ± 5.3 (14.35–20.53) |
| K | 18.03 ± 2.02 (16.85–19.19) | 18.11 ± 1.96 (16.98–19.24) | 18.47 ± 2.52 (17.01–19.92) | |
| Step-down (number) | HK | 8.79 ± 4.41 (6.24–11.32) | 11.57 ± 4.13 * (9.18–13.95) | 12.36 ± 4.67 *† (9.66–15.05) |
| K | 11.00 ± 2.88 (9.33–12.66) | 13.36 ± 3.67 * (11.23–15.47) | 13.93 ± 4.68 *† (11.22–16.63) | |
| Step-down VAS | HK | 3.44 ± 1.29 (2.69–4.18) | 0.51 ± 0.66 * (0.12–0.88) | 0.56 ± 1.02 *† (0–1.15) |
| K | 3.26 ± 1.41 (2.45–4.07) | 1.44 ± 1.39 * (0.64–2.24) | 0.47 ± 0.59 *† (0.23–1.15) |
| Effect | df | F | p | η2 |
|---|---|---|---|---|
| Time | 1, 26 | 144.400 | <0.001 ** | 0.847 |
| Time * Group | 1, 26 | 0.482 | 0.494 | 0.018 |
| Variable | Effect | df | F | p | η2 | Post-Hoc (Bonferroni) |
|---|---|---|---|---|---|---|
| Hip AD ROM | Time | 2, 52 | 19.508 | <0.001 ** | 0.139 | B vs. I (p < 0.001 **) |
| Time * Group | 2, 52 | 0.519 | 0.598 | 0.020 | B vs. W (p < 0.001 **) | |
| I vs. W (p = 1.000) | ||||||
| Hip AB Strength | Time | 2, 52 | 4.199 | 0.020 * | 0.139 | B vs. I (p =.009 *) |
| Time * Group | 2, 52 | 2.992 | 0.059 | 0.103 | B vs. W (p = 0.113) | |
| I vs. W (p = 1.000) | ||||||
| Hip ER Strength | Time | 2, 52 | 0.188 | 0.829 | 0.007 | B vs. I (p = 1.000) |
| Time * Group | 2, 52 | 0.702 | 0.500 | 0.026 | B vs. W (p = 1.000) | |
| I vs. W (p = 1.000) | ||||||
| Step-down (number) | Time | 2, 52 | 47.250 | <0.001 ** | 0.645 | B vs. I (p < 0.001 **) B vs. W (p < 0.001 **) |
| Time * Group | 2, 52 | 0.431 | 0.652 | 0.016 | I vs. W (p = 0.218) |
| Effect | Values | F | df | df for Error | p |
|---|---|---|---|---|---|
| Time | 0.000 | 0.000 | 2.000 | 25.000 | 1.000 |
| Time * Group | 0.163 | 2.430 | 2.000 | 25.000 | 0.109 |
| Comparison | Z | p | Effect Size (r) |
|---|---|---|---|
| Baseline—Immediate | −4.524 | <0.001 ** | 0.855 |
| Baseline—Week1 | −4.624 | <0.001 ** | 0.874 |
| Immediate—Week1 | −2.015 | 0.044 * | 0.381 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.C.; Kim, Y.K. The Immediate Effects of Instrument-Assisted Soft Tissue Mobilization on Pain and Function in Female Runners with Patellofemoral Pain. Medicina 2025, 61, 1912. https://doi.org/10.3390/medicina61111912
Cho SC, Kim YK. The Immediate Effects of Instrument-Assisted Soft Tissue Mobilization on Pain and Function in Female Runners with Patellofemoral Pain. Medicina. 2025; 61(11):1912. https://doi.org/10.3390/medicina61111912
Chicago/Turabian StyleCho, Seong Chan, and Young Kyun Kim. 2025. "The Immediate Effects of Instrument-Assisted Soft Tissue Mobilization on Pain and Function in Female Runners with Patellofemoral Pain" Medicina 61, no. 11: 1912. https://doi.org/10.3390/medicina61111912
APA StyleCho, S. C., & Kim, Y. K. (2025). The Immediate Effects of Instrument-Assisted Soft Tissue Mobilization on Pain and Function in Female Runners with Patellofemoral Pain. Medicina, 61(11), 1912. https://doi.org/10.3390/medicina61111912








