Surgical Ovarian Suppression and Breast Cancer—What Do We Know About It?
Abstract
1. Introduction
2. Discussion
2.1. History of Surgical Ovarian Suppression
2.2. Current Standard in Ovarian Suppression
2.3. Effectiveness of Ovarian Suppression with Medications
2.3.1. Anti-Müllerian Hormone (AMH) as a Biomarker for Ovarian Reserve and Guidance in Ovarian Suppression Decisions
2.3.2. Global and Ethnic Perspectives on Ovarian Suppression and Biomarkers
2.4. Quality of Life in Menopausal Patients
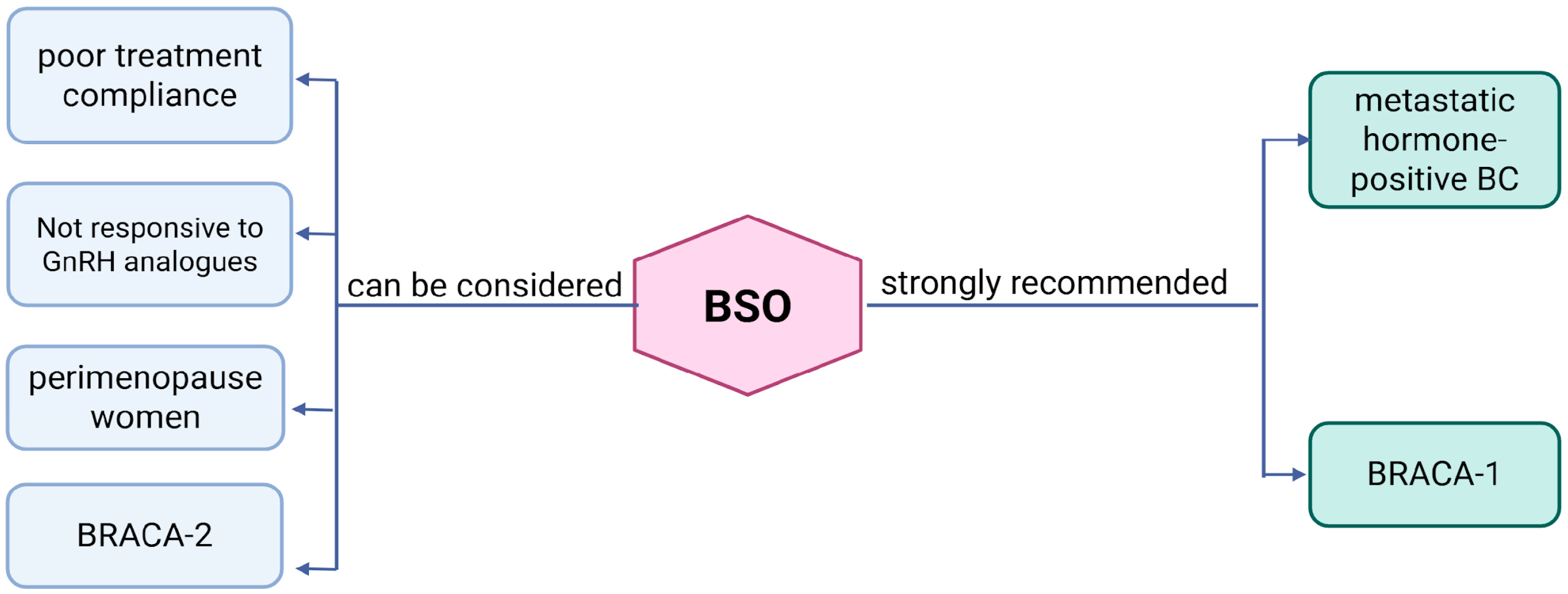
2.5. Ovarian Suppression in BRCA1/2 Carriers
3. Open Questions and Future Directions
- BSO is suitable for patients with hormone receptor-positive BC nearing natural menopause.
- BSO is appropriate for patients with metastatic hormone receptor-positive BC.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 30 July 2025).[Green Version]
- Kim, J.; Harper, A.; McCormack, V.; Sung, H.; Houssami, N.; Morgan, E.; Mutebi, M.; Garvey, G.; Soerjomataram, I.; Fidler-Benaoudia, M.M. Global patterns and trends in breast cancer incidence and mortality across 185 countries. Nat. Med. 2025, 31, 1154–1162. [Google Scholar] [CrossRef]
- Liao, L. Inequality in breast cancer: Global statistics from 2022 to 2050. Breast 2024, 79, 103851. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. eClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.; Bergh, J.; Burstein, H.; Cardoso, M.; Carey, L.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 35, 159–182. [Google Scholar] [CrossRef]
- Kelly, C.M.; Bennett, K.E.; Cahir, C.; Eisen, A.; Pusztai, L. Clinical Management of Ovarian Function Suppression in Premenopausal Women with Breast Cancer: A Survey of Members of ASCO. JCO Oncol. Pract. 2025, 21, 654–662. [Google Scholar] [CrossRef]
- On Cancer of the Breast/by Thomas William Nunn.|Wellcome Collection. Available online: https://wellcomecollection.org/works/jduf3za3 (accessed on 30 July 2025).
- Love, R.R.; Philips, J. Oophorectomy for Breast Cancer: History Revisited. J. Natl. Cancer Inst. 2002, 94, 1433–1434. [Google Scholar] [CrossRef]
- On the Treatment of Inoperable Cases of Carcinoma of the Mamma: Suggestions for a New Method of Treatment, with Illustrative Cases—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/29584099/ (accessed on 30 July 2025).
- Boyd, S. On ouphorectomy in the treatment of cancer. Br. Med. J. 1897, 2, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.; Jaffe, H.L.; Rabwin, M.H.; Rosenblum, D.H.; Simkin, B. Adrenalectomy for control of cancer of the breast. Calif. Med. 1956, 85, 213–219. [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Systemic Treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet 1992, 339, 1–15. [Google Scholar]
- Ejlertsen, B.; Mouridsen, H.T.; Jensen, M.-B.; Bengtsson, N.-O.; Bergh, J.; Cold, S.; Edlund, P.; Ewertz, M.; de Graaf, P.W.; Kamby, C.; et al. Similar Efficacy for Ovarian Ablation Compared with Cyclophosphamide, Methotrexate, and Fluorouracil: From a Randomized Comparison of Premenopausal Patients with Node-Positive, Hormone Receptor–Positive Breast Cancer. J. Clin. Oncol. 2006, 24, 4956–4962. [Google Scholar] [CrossRef]
- Jakesz, R.; Hausmaninger, H.; Samonigg, H.; Kubista, E.; Depisch, D.; Fridrik, M.; Stierer, M.; Gnant, M.; Steger, G.; Kolb, R.; et al. Comparison of adjuvant therapy with tamoxifen and goserelin vs. CMF in premenopausal stage I and II hormone-responsive breast cancer patients: Four-year results of Austrian Breast Cancer Study Group (ABCSG) trial 5. Eur. J. Cancer 1999, 35, S83. [Google Scholar] [CrossRef]
- Scottish Cancer Trials Breast Group. Adjuvant ovarian ablation versus CMF chemotherapy in premenopausal women with pathological stage II breast carcinoma: The Scottish trial. Lancet 1993, 341, 1293–1298. [Google Scholar] [CrossRef]
- Bastiaannet, E.; Charman, J.; Johannesen, T.B.; Schrodi, S.; Siesling, S.; van Eycken, L.; Walsh, P.M.; Audisio, R.A.; Boelens, P.G.; Rubio, I.T.; et al. A European, Observational Study of Endocrine Therapy Administration in Patients with an Initial Diagnosis of Hormone Receptor-Positive Advanced Breast Cancer. Clin. Breast Cancer 2018, 18, e613–e619. [Google Scholar] [CrossRef] [PubMed]
- Wei, S. Hormone receptors in breast cancer: An update on the uncommon subtypes. Pathol. Res. Pract. 2023, 250, 154791. [Google Scholar] [CrossRef]
- van der Meer, D.J.; Kramer, I.; van Maaren, M.C.; van Diest, P.J.; Linn, S.C.; Maduro, J.H.; Strobbe, L.J.; Siesling, S.; Schmidt, M.K.; Voogd, A.C. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017. Int. J. Cancer 2020, 148, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A.; Bianchi-Micheli, G.; Cardoso, M.J.; Curigliano, G.; Gelmon, K.A.; Gentilini, O.; et al. ESO–ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann. Oncol. 2022, 33, 1097–1118. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; for the Straw +10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop +10: Addressing the unfinished agenda of staging reproductive aging. Climacteric 2012, 15, 105–114. [Google Scholar] [CrossRef]
- Menopause. Available online: https://www.who.int/news-room/fact-sheets/detail/menopause (accessed on 17 October 2025).
- Francis, P.A.; Fleming, G.F.; Pagani, O.; Walley, B.; Loi, S.; Colleoni, M.; Regan, M.M. 15-year outcomes for women with premenopausal hormone receptor-positive early breast cancer (BC) in the SOFT and TEXT trials assessing benefits from adjuvant exemestane (E) + ovarian function suppression (OFS) or tamoxifen (T)+OFS. J. Clin. Oncol. 2025, 43, 505. [Google Scholar] [CrossRef]
- Tevaarwerk, A.J.; Wang, M.; Zhao, F.; Fetting, J.H.; Cella, D.; Wagner, L.I.; Martino, S.; Ingle, J.N.; Sparano, J.A.; Solin, L.J.; et al. Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E-3193, INT-0142): A trial of the eastern cooperative oncology group. J. Clin. Oncol. 2014, 32, 3948–3958. [Google Scholar] [CrossRef]
- Jakesz, R.; Hausmaninger, H.; Kubista, E.; Gnant, M.; Menzel, C.; Bauernhofer, T.; Seifert, M.; Haider, K.; Mlineritsch, B.; Steindorfer, P.; et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: Evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer—Austrian Breast and Colorectal Cancer Study Group Trial 5. J. Clin. Oncol. 2002, 20, 4621–4627. [Google Scholar] [CrossRef]
- Oseledchyk, A.; Gemignani, M.L.; Zhou, Q.C.; Iasonos, A.; Elahjji, R.; Adamou, Z.; Feit, N.; Goldfarb, S.B.; Roche, K.L.; Sonoda, Y.; et al. Surgical Ovarian Suppression for Adjuvant Treatment in Hormone Receptor Positive Breast Cancer in Premenopausal Patients. Int. J. Gynecol. Cancer 2020, 31, 222–231. [Google Scholar] [CrossRef]
- Gray, R.G.; Bradley, R.; Braybrooke, J.; Clarke, M.; Hills, R.K.; Peto, R.; Bergh, J.C.S.; Swain, S.M.; Davidson, N.E.; Francis, P.A.; et al. Effects of ovarian ablation or suppression on breast cancer recurrence and survival: Patient-level meta-analysis of 14,993 pre-menopausal women in 25 randomized trials. J. Clin. Oncol. 2023, 41, 503. [Google Scholar] [CrossRef]
- Nomura, Y.; Tashiro, H.; Hisamatsu, K.; Shinozuka, K. A randomized trial of adjuvant endocrine therapy, chemotherapy, and chemoendocrine therapy for operable breast cancer stratified by estrogen receptors. Cancer 1988, 61, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Shirouzu, M.; Takayama, T. Direct comparisons of adjuvant endocrine therapy, chemotherapy, and chemoendocrine therapy for operable breast cancer patients stratified by estrogen receptor and menopausal status. Breast Cancer Res. Treat. 1998, 49, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ferrandina, G.; Amadio, G.; Marcellusi, A.; Azzolini, E.; Puggina, A.; Pastorino, R.; Ricciardi, W.; Scambia, G. Bilateral Salpingo-Oophorectomy Versus GnRH Analogue in the Adjuvant Treatment of Premenopausal Breast Cancer Patients: Cost-Effectiveness Evaluation of Breast Cancer Outcome, Ovarian Cancer Prevention and Treatment. Clin. Drug Investig. 2017, 37, 1093–1102. [Google Scholar] [CrossRef]
- Hagemann, A.R.; Zighelboim, I.; Odibo, A.O.; Rader, J.S.; Mutch, D.G.; Powell, M.A. Cost-benefit of laparoscopic versus medical ovarian suppression in premenopausal breast cancer. Breast J. 2010, 17, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.J.; Kim, S.H.; Lee, K.-H.; Kim, T.-Y.; Kim, Y.J.; Han, S.-W.; Kang, E.; Kim, E.-K.; Kim, K.; No, J.H.; et al. Bilateral Salpingo-oophorectomy compared to gonadotropin-releasing hormone agonists in premenopausal hormone receptor–positive metastatic breast cancer patients treated with aromatase inhibitors. Cancer Res. Treat. 2017, 49, 1153–1163. [Google Scholar] [CrossRef]
- Khan, F.; Rojas, K.; Schlumbrecht, M.; Jeudin, P. Oophorectomy in Premenopausal Patients with Estrogen Receptor-Positive Breast Cancer: New Insights into Long-Term Effects. Curr. Oncol. 2023, 30, 1794–1804. [Google Scholar] [CrossRef]
- Dowsett, M.; Lønning, P.E.; Davidson, N.E. Incomplete estrogen suppression with gonadotropin-releasing hormone agonists may reduce clinical efficacy in premenopausal women with early breast cancer. J. Clin. Oncol. 2016, 34, 1580–1583. [Google Scholar] [CrossRef]
- Tesch, M.E.; Zheng, Y.; Rosenberg, S.M.; Poorvu, P.D.; Ruddy, K.J.; Tamimi, R.; Schapira, L.; Peppercorn, J.; Borges, V.; Come, S.E.; et al. Estrogen levels in young women with hormone receptor-positive breast cancer on ovarian function suppression therapy. npj Breast Cancer 2024, 10, 67. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Zhang, L.; Yang, S.; Zhou, T.; Luo, K.; Liu, S. Ovarian escape in premenopausal breast cancer: Challenges and strategies for optimizing hormone suppression. Cancer Treat. Rev. 2025, 139, 102970. [Google Scholar] [CrossRef]
- McCann, K.E.; Goldfarb, S.B.; Traina, T.A.; Regan, M.M.; Vidula, N.; Kaklamani, V. Selection of appropriate biomarkers to monitor effectiveness of ovarian function suppression in pre-menopausal patients with ER+ breast cancer. npj Breast Cancer 2024, 10, 8. [Google Scholar] [CrossRef]
- La Marca, A.; Volpe, A. Anti-Müllerian hormone (AMH) in female reproduction: Is measurement of circulating AMH a useful tool? Clin. Endocrinol. 2006, 64, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-C.I.; Haunschild, C.; Chung, K.; Komrokian, S.; Boles, S.; Sammel, M.D.; DeMichele, A. Prechemotherapy antimullerian hormone, age, and body size predict timing of return of ovarian function in young breast cancer patients. Cancer 2014, 120, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Choi, J.; Park, C.S.; Seong, M.K.; Hong, S.E.; Kim, J.S.; Park, I.C.; Lee, J.K.; Noh, W.C.; The ASTRRA trial investigators. Post-chemotherapy serum anti-Müllerian hormone level predicts ovarian function recovery. Endocr. Connect. 2018, 7, 949–956. [Google Scholar] [CrossRef]
- Weidlinger, S.; Weidlinger, M.; Schamm, R.M.; Vidal, A.; Pape, J.; Karrer, T.; Rabaglio, M.; von Wolff, M. High impact of chemotherapy on ovarian reserve in breast cancer survivors of reproductive age: A systematic review and meta-analysis. Breast 2025, 82, 104514. [Google Scholar] [CrossRef] [PubMed]
- Van Zwol-Jannssen, C.; Van Rosmalen, M.M.; Oomen-De Hoop, E.; Drooger, J.C.; van der Padt-Pruijsten, A.; Zuetenhorst, H.J.M.; Louwers, J.U.; Visser, J.A.; Laven, J.S.E.; Jager, A. Early prediction of menopausal status after chemotherapy in women with early breast cancer in order to optimize adjuvant endocrine therapy. Breast 2025, 83, 104562. [Google Scholar] [CrossRef]
- Lambertini, M.; Allegranza, D.; Laubender, R.P.; Harbeck, N.; Swain, S.M.; Geyer, C.E.; Slamon, D.J.; Bobba, G.; Lambertini, C.; de Haas, S.; et al. Predicting ovarian function loss after chemotherapy and anti-HER2 therapy in young breast cancer patients. J. Natl. Cancer Inst. 2025, djaf198. [Google Scholar] [CrossRef]
- Anderson, R.A.; Cameron, D.; Clatot, F.; Demeestere, I.; Lambertini, M.; Nelson, S.M.; Peccatori, F. Anti-Müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: A systematic review. Hum. Reprod. Updat. 2022, 28, 417–434. [Google Scholar] [CrossRef]
- Andeson, R.A. What does Anti-Mullerian hormone tell you about ovarian function? Clin. Endocrinol. 2012, 77, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Del Mastro, L.; Viglietti, G.; Pondé, N.F.; Solinas, C.; de Azambuja, E. Ovarian Function Suppression in Premenopausal Women with Early-Stage Breast Cancer. Curr. Treat Options Oncol. 2017, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O. NCCN Harmonized Guidelines for Sub-Saharan Africa: A Collaborative Methodology for Translating Resource-Adapted Guidelines Into Actionable In-Country Cancer Control Plans. JCO Glob. Oncol. 2020, 6, 1419–1421. [Google Scholar] [CrossRef]
- Farrell, E. Premature menopause. “I feel like an alien”. Aust. Fam. Physician 2002, 31, 419–421. [Google Scholar]
- Carpenter, J.S.; Andrykowski, M.A. Menopausal symptoms in breast cancer survivors. Oncol. Nurs. Forum 1999, 26, 1311–1317. [Google Scholar] [PubMed]
- Knobf, M.T. Reproductive and hormonal sequelae of chemotherapy in women. Premature menopause and impaired fertility can result, effects that are especially disturbing to young women. Am. J. Nurs. 2006, 106 (Suppl. 3), 60–65. [Google Scholar] [CrossRef]
- Hamoda, H.; Panay, N.; Pedder, H.; Arya, R.; Savvas, M.; on behalf of the Medical Advisory Council of the British Menopause Society. The British Menopause Society & Women’s Health Concern 2020 recommendations on hormone replacement therapy in menopausal women. Post Reprod. Health 2020, 26, 181–209. [Google Scholar] [CrossRef]
- Demir, O.; Ozalp, M.; Sal, H.; Aran, T.; Osmanağaoğlu, M. The relationship of menopausal symptoms with the type of menopause and lipid levels. Menopausal Rev. 2020, 19, 6–10. [Google Scholar] [CrossRef]
- Secoșan, C.; Balint, O.; Pirtea, L.; Grigoraș, D.; Bălulescu, L.; Ilina, R. Surgically Induced Menopause—A Practical Review of Literature. Medicina 2019, 55, 482. [Google Scholar] [CrossRef] [PubMed]
- Price, M.A.B.; Alvarado, B.E.; Rosendaal, N.T.M.; Câmara, S.M.; Pirkle, C.M.; Velez, M.P. Early and surgical menopause associated with higher Framingham Risk Scores for cardiovascular disease in the Canadian Longitudinal Study on Aging. Menopause 2021, 28, 484–490. [Google Scholar] [CrossRef]
- Tan, S.; Yang, J.; Guo, G.; Hong, S.; Guo, L.; Situ, H. The risk of dementia in breast cancer survivors: A meta-analysis of observational studies. Ann. Med. 2025, 57, 2529579. [Google Scholar] [CrossRef]
- Vriens, I.J.; De Bie, A.J.; Aarts, M.J.; de Boer, M.; van Hellemond, I.E.; Roijen, J.H.; van Golde, R.J.; Voogd, A.C.; Tjan-Heijnen, V.C. The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget 2017, 8, 11372–11379. [Google Scholar] [CrossRef]
- Ma, L.; Yang, B.; Wu, J. Revisiting ovarian function suppression with GnRH agonists for premenopausal women with breast cancer: Who should use and the impact on survival outcomes. Cancer Treat. Rev. 2024, 129, 102770. [Google Scholar] [CrossRef]
- Boccardo, F.; Rubagotti, A.; Perrotta, A.; Amoroso, D.; Balestrero, M.; De Matteis, A.; Zola, P.; Sismondi, P.; Francini, G.; Petrioli, R.; et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: Results of a multicentric Italian study. Ann. Oncol. 1994, 5, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.P.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Huzarski, T.; Gronwald, J.; Singer, C.F.; Moller, P.; Lynch, H.T.; Armel, S.; Karlan, B.; Foulkes, W.D.; Neuhausen, S.L.; et al. Bilateral oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers. J. Natl. Cancer Inst. 2017, 109, djw177. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.; Seynaeve, C.; van Asperen, C.J.; Ausems, M.G.; Collee, J.M.; van Doorn, H.C.; Gomez Garcia, E.B.; Kets, C.M.; van Leeuwen, F.E.; Meijers-Heijboer, H.E.; et al. Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: Revisiting the evidence for risk reduction. J. Natl. Cancer Inst. 2015, 107, djv033. [Google Scholar] [CrossRef]
- Metcalfe, K.; Lynch, H.T.; Foulkes, W.D.; Tung, N.; Kim-Sing, C.; Olopade, O.I.; Eisen, A.; Rosen, B.; Snyder, C.; Gershman, S.; et al. Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncol. 2015, 1, 306–313. [Google Scholar] [CrossRef]
- The Hereditary Breast Cancer Clinical Study Group; Valentini, A.; Lubinski, J.; Byrski, T.; Ghadirian, P.; Moller, P.; Lynch, H.T.; Ainsworth, P.; Neuhausen, S.L.; Weitzel, J.; et al. The impact of pregnancy on breast cancer survival in women who carry a BRCA1 or BRCA2 mutation. Breast Cancer Res. Treat. 2013, 142, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Huzarski, T.; Byrski, T.; Gronwald, J.; Górski, B.; Domagała, P.; Cybulski, C.; Oszurek, O.; Szwiec, M.; Gugała, K.; Stawicka, M.; et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 2013, 31, 3191–3196. [Google Scholar] [CrossRef] [PubMed]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.; Narod, S.A. Does oophorectomy reduce breast cancer mortality for BRCA mutation carriers after breast cancer? Expert Rev. Anticancer. Ther. 2018, 18, 305–306. [Google Scholar] [CrossRef] [PubMed]
| Study | Overall Survival | Disease-Free Survival Rate | ||||
|---|---|---|---|---|---|---|
| Tamoxifen | Tamoxifen Plus Ovarian Suppression | AIs Plus Ovarian Suppression | Tamoxifen | Tamoxifen Plus Ovarian Suppression | AIs Plus Ovarian Suppression | |
| SOFT (8 years) | 91.5% | 93.3% | 92.1% | 78.9% | 83.2% | 85.9% |
| TEXT + SOFT (8 years) | 93.3% | 93.4% | 82.8% | 86.8% | ||
| Study (Year) | Population | Intervention | Main Findings |
|---|---|---|---|
| Tesch et al., 2024 [35] | Premenopausal women <40 years on GnRHa | Estradiol suppression thresholds | ~5% had inadequate E2 suppression (escape phenomenon) |
| Luo et al., 2025 [36] | Premenopausal BC on GnRH | Review/analysis | Described “ovarian escape” and need for standardized monitoring |
| McCann et al., 2024 [37] | ER+ BC, on GnRHa | Biomarker assessment | Highlighted variability in E2 suppression by BMI and drug kinetics |
| Dowsett et al., 2016 [34] | Early BC, premenopausal | Clinical commentary | Incomplete estrogen suppression may reduce efficacy |
| Study (Year) | Type of Menopause | Key Outcomes | Main Conclusion |
|---|---|---|---|
| Demir et al., 2020 [52] | Surgical vs. natural | Higher physical/psychological symptoms in surgical group | Surgical menopause significantly worsens QoL |
| Secoșan et al., 2019 [53] | Surgical | Review | Increased risk of CVD and bone loss after oophorectomy |
| Price et al., 2021 [54] | Early/surgical vs. natural | Framingham Risk Score | Early/surgical menopause doubles CVD risk |
| Boccardo et al., 1994 [58] | GnRHas vs. oophorectomy | Side effects | Slightly more side effects with medical suppression |
| Tan et al., 2025 [55] | Post-BC survivors | Dementia risk | Endocrine therapy not linked to dementia risk |
| Study (Year) | Population | Key Findings | Effect on Breast Cancer Mortality |
|---|---|---|---|
| Metcalfe et al., 2015 [62] | 676 BRCA1/2 carriers | Oophorectomy within 2 yrs ↓ mortality (HR 0.27) in BRCA1 | Significant reduction (60%) in BRCA1; NS in BRCA2 |
| Valentini et al., 2013 [63] | 397 BRCA1+ | Oophorectomy ↓ mortality by 80% | Strong benefit for BRCA1 |
| Huzarski et al., 2013 [64] | BRCA1+ | 70% decrease in BC mortality | Confirmed survival benefit |
| Domchek et al., 2010 [65] | 2482 BRCA1/2 | ↓ BC-specific mortality 73% (BRCA1 only) | No effect in BRCA2 |
| Kotsopoulos et al., 2017 [60] | Healthy BRCA1/2 | No decrease in BC risk after oophorectomy | Reevaluation needed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yordanov, A.; Hasan, I.; Vasileva-Slaveva, M.; Tsoneva, E.; Kostov, S.; Yanachkova, V. Surgical Ovarian Suppression and Breast Cancer—What Do We Know About It? Medicina 2025, 61, 1905. https://doi.org/10.3390/medicina61111905
Yordanov A, Hasan I, Vasileva-Slaveva M, Tsoneva E, Kostov S, Yanachkova V. Surgical Ovarian Suppression and Breast Cancer—What Do We Know About It? Medicina. 2025; 61(11):1905. https://doi.org/10.3390/medicina61111905
Chicago/Turabian StyleYordanov, Angel, Ihsan Hasan, Mariela Vasileva-Slaveva, Eva Tsoneva, Stoyan Kostov, and Vesselina Yanachkova. 2025. "Surgical Ovarian Suppression and Breast Cancer—What Do We Know About It?" Medicina 61, no. 11: 1905. https://doi.org/10.3390/medicina61111905
APA StyleYordanov, A., Hasan, I., Vasileva-Slaveva, M., Tsoneva, E., Kostov, S., & Yanachkova, V. (2025). Surgical Ovarian Suppression and Breast Cancer—What Do We Know About It? Medicina, 61(11), 1905. https://doi.org/10.3390/medicina61111905








