SGLT2 Inhibitors in the Management of Cardio-Renal-Metabolic Syndrome: A New Therapeutic Era
Abstract
1. Introduction
2. Pathophysiology of Cardio-Renal-Metabolic Disease
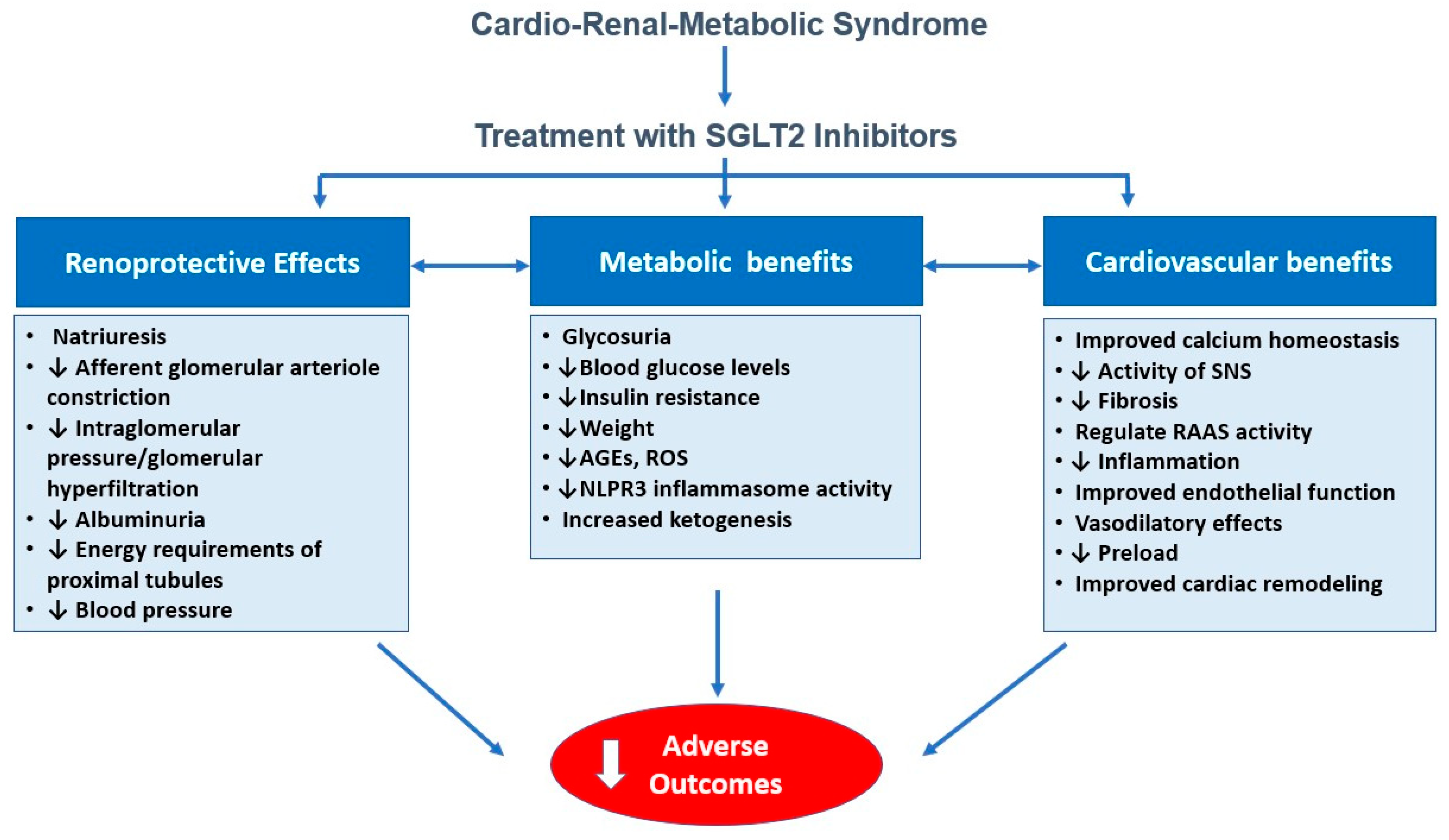
3. Mechanisms of Action of SGLT2 Inhibitors Beyond Glycemic Control
3.1. Hemodynamic Effect: Natriuresis, Osmotic Diuresis, Blood Pressure Reduction
3.2. Metabolic Modulation: Improved Mitochondrial Function and Substrate Utilization
3.3. Anti-Inflammatory and Antifibrotic Pathways
3.4. Impact on Arterial Stiffness, Endothelial Function, and Cardiac Remodeling
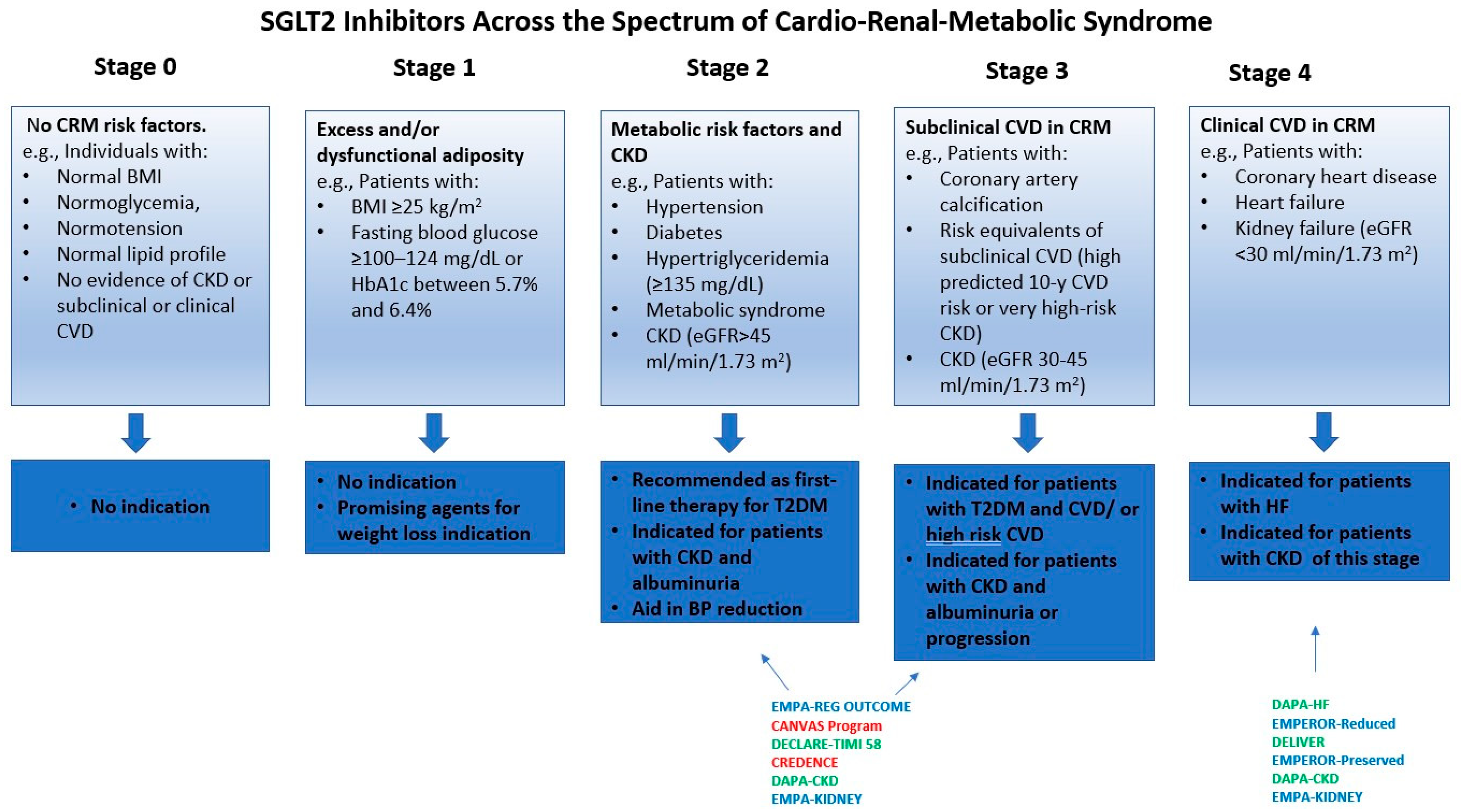
4. Clinical Evidence Across the CRM Spectrum
4.1. Diabetes Mellitus Type 2
4.2. Chronic Heart Failure
4.3. Acute Heart Failure
4.4. Chronic Kidney Disease
4.5. Acute Myocardial Infarction
4.6. Hypertension and Vascular Protection
4.7. Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis
4.8. Effects of SGLT2 Inhibitors in Patients Without Diabetes Mellitus
5. Real-World Data and Translational Insights
5.1. Observational Studies
5.2. Cardiac Imaging Studies
5.3. Biomarker-Based Evidence
6. Positioning SGLT2 Inhibitors in Contemporary Therapeutic Algorithms
6.1. Integration with RAAS Inhibitors, GLP-1 Receptor Agonists, and MRAs
6.2. Guidelines from ADA, ESC, KDIGO, and ACC
6.3. Safety of SGLT2 Inhibitors
6.4. Sex-Based Differences in SGLT2i Use
6.5. Multidisciplinary Implications for Primary Care, Cardiology, Nephrology, Endocrinology, and Geriatrics
7. Future Perspectives
8. Integrative Treatment Strategies for Cardio-Renal-Metabolic Syndrome: The Role of SGLT2 Inhibitors
- Obesity: SGLT2is provide modest weight loss when used alone. Greater reductions in absolute weight are achieved in combination with GLP-1 RAs. Given that SGLT2is and GLP-1 RAs are frequently co-administered for glycemic control and have a favorable safety profile, it is reasonable to consider adding an SGLT2i in obese patients with T2DM who are already on a GLP-1 RA.
- Arterial Hypertension: SGLT2is modestly lower blood pressure and are not indicated as first-line therapy for hypertension. They may be included as part of antidiabetic treatment in patients with T2DM and hypertension.
- High-risk T2DM: SGLT2is are indicated as first-line treatment.
- HFpEF/HFrEF: SGLT2is are strongly recommended for HF across the ejection-fraction spectrum, regardless of diabetes status. Patients with T2DM, BMI ≥ 27 kg/m2, and HFpEF benefit from combination with GLP-1 RAs.
- Acute HF: SGLT2is are recommended after clinical stabilization to enhance decongestion and improve clinical outcomes.
- Acute MI: SGLT2is may be considered prior to and after PCI in high-risk STEMI patients. It may also reduce the risk of contrast-induced acute kidney injury.
- ASCVD: SGLT2is are strongly recommended for patients with T2DM and ASCVD.
- Diabetic CKD: SGLT2is are strongly recommended for patients with T2DM and eGFR ≥ 20 mL/min/1.73 m2.
- NASH/NAFLD: SGLT2is improve hepatic steatosis. They may be reasonably added in patients with obesity who are already on GLP-1 RAs or GIP/GLP-1 RAs, particularly in cases of progressive liver disease.
- Non-Diabetic Patients: SGLT2is demonstrate cardio-renal benefits in this population. Evidence is currently insufficient for these agents to provide clear guidance for obesity, arterial hypertension, or ASCVD. Regarding CKD, SGLT2is are indicated for eGFR ≥ 20 mL/min/1.73 m2 with UACR ≥ 200 mg/g (≥20 mg/mmol), or in those with HF regardless of albuminuria. For adults with eGFR 20–45 mL/min/1.73 m2 and UACR < 200 mg/g (<20 mg/mmol), SGLT2is may be considered as a treatment option.
- Frail/Older Patients: SGLT2is should be continued when indicated, with enhanced monitoring for potential side effects.
- OSA: Evidence for the efficacy of SGLT2is is limited, and their use cannot be routinely recommended [216].
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2021. Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Masood, B.; Moorthy, M. Causes of Obesity: A Review. Clin. Med. 2023, 23, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, Regional, and National Burden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/Lifestyle Factors in the Pathogenesis and Prevention of Type 2 Diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic Kidney Disease and the Global Public Health Agenda: An International Consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Ostrominski, J.W.; Arnold, S.V.; Butler, J.; Fonarow, G.C.; Hirsch, J.S.; Palli, S.R.; Donato, B.M.K.; Parrinello, C.M.; O’connell, T.; Collins, E.B.; et al. Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999–2020. JAMA Cardiol. 2023, 8, 1050–1060. [Google Scholar] [CrossRef]
- Mutruc, V.; Bologa, C.; Șorodoc, V.; Ceasovschih, A.; Morărașu, B.C.; Șorodoc, L.; Catar, O.E.; Lionte, C. Cardiovascular–Kidney–Metabolic Syndrome: A New Paradigm in Clinical Medicine or Going Back to Basics? J. Clin. Med. 2025, 14, 2833. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-kidney-metabolic health: A presidential advisory from the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Minhas, A.M.K.; Mathew, R.O.; Sperling, L.S.; Nambi, V.; Virani, S.S.; Navaneethan, S.D.; Shapiro, M.D.; Abramov, D. Prevalence of the Cardiovascular-Kidney-Metabolic Syndrome in the United States. J. Am. Coll. Cardiol. 2024, 83, 1824–1826. [Google Scholar] [CrossRef]
- Akiyama, H.; Nishimura, A.; Morita, N.; Yajima, T. Evolution of Sodium-Glucose Co-Transporter 2 Inhibitors from a Glucose-Lowering Drug to a Pivotal Therapeutic Agent for Cardio-Renal-Metabolic Syndrome. Front. Endocrinol. 2023, 14, 1111984. [Google Scholar] [CrossRef]
- Gajjar, A.; Raju, A.K.; Gajjar, A.; Menon, M.; Shah, S.A.Y.; Dani, S.; Weinberg, A. SGLT2 Inhibitors and GLP-1 Receptor Agonists in Cardiovascular–Kidney–Metabolic Syndrome. Biomedicines 2025, 13, 1924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; He, X.; Li, X. Sodium-Glucose Transporter 2 Inhibitors and Cardiovascular-Kidney-Metabolic Syndrome: A Narrative Review. Front. Endocrinol. 2025, 16, 1554637. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Pamporis, K.; Stachteas, P.; Patoulias, D.; Bougioukas, K.I.; Fragakis, N. Efficacy and Safety of Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: An Overview of 36 Systematic Reviews. Heart Fail. Rev. 2023, 28, 1033–1051. [Google Scholar] [CrossRef] [PubMed]
- Stachteas, P.; Karakasis, P.; Patoulias, D.; Clemenza, F.; Fragakis, N.; Rizzo, M. The Effect of Sodium-Glucose Co-Transporter-2 Inhibitors on Markers of Subclinical Atherosclerosis. Ann. Med. 2023, 55, 2304667. [Google Scholar] [CrossRef]
- McLean, P.; Bennett, J.; Woods, E.T.; Chandrasekhar, S.; Newman, N.; Mohammad, Y.; Khawaja, M.; Rizwan, A.; Siddiqui, R.; Birnbaum, Y.; et al. SGLT2 Inhibitors Across Various Patient Populations in the Era of Precision Medicine: The Multidisciplinary Team Approach. npj Metab. Health Dis. 2025, 3, 29. [Google Scholar] [CrossRef]
- Theodorakis, N.; Nikolaou, M. From Cardiovascular-Kidney-Metabolic Syndrome to Cardiovascular-Renal-Hepatic-Metabolic Syndrome: Proposing an Expanded Framework. Biomolecules 2025, 15, 213. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A State-of-the-Art Review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.-P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef]
- Arneth, B. Mechanisms of Insulin Resistance in Patients with Obesity. Endocrines 2024, 5, 153–165. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin Resistance and Cardiovascular Disease. J. Int. Med. Res. 2023, 51, 3000605231164548. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.S.; Zhang, L.; Mitch, W.E. Molecular Mechanisms of Insulin Resistance in Chronic Kidney Disease. Kidney Int. 2015, 88, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.S.; Minanni, C.A.; de Araújo Lira, A.L.; Passarelli, M. Advanced Glycation End Products: A Sweet Flavor That Embitters Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 2404. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Lopez-Trevino, S.; Kankanamalage, H.R.; Jha, J.C. Diabetes and Renal Complications: An Overview on Pathophysiology, Biomarkers and Therapeutic Interventions. Biomedicines 2024, 12, 1098. [Google Scholar] [CrossRef]
- Jia, G.; Sowers, J.R. Hypertension in Diabetes: An Update of Basic Mechanisms and Clinical Disease. Hypertension 2021, 78, 1197–1205. [Google Scholar] [CrossRef]
- Cruickshank, K.; Riste, L.; Anderson, S.G.; Wright, J.S.; Dunn, G.; Gosling, R.G. Aortic Pulse-Wave Velocity and Its Relationship to Mortality in Diabetes and Glucose Intolerance: An Integrated Index of Vascular Function? Circulation 2002, 106, 2085–2090. [Google Scholar] [CrossRef]
- Costantino, V.V.; Gil Lorenzo, A.F.; Bocanegra, V.; Vallés, P.G. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan through Hsp70. Cells 2021, 10, 3146. [Google Scholar] [CrossRef]
- Long, A.N.; Dagogo-Jack, S. Comorbidities of Diabetes and Hypertension: Mechanisms and Approach to Target Organ Protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar] [CrossRef]
- Cohen, J.B.; Cohen, D.L. Cardiovascular and Renal Effects of Weight Reduction in Obesity and the Metabolic Syndrome. Curr. Hypertens. Rep. 2015, 17, 34–544. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.A.; Karthikeyan, K.; Abdullah, O.; Ghadban, R. Obesity and Cardiac Remodeling in Adults: Mechanisms and Clinical Implications. Prog. Cardiovasc. Dis. 2018, 61, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, F.F.; Schytz, P.A.; Heerspink, H.J.L.; von Scholten, B.J.; Idorn, T. Obesity-Related Kidney Disease: Current Understanding and Future Perspectives. Biomedicines 2023, 11, 2498. [Google Scholar] [CrossRef]
- Ahmed, N.; Dalmasso, C.; Turner, M.B.; Arthur, G.; Cincinelli, C.; Loria, A.S. From Fat to Filter: The Effect of Adipose Tissue-Derived Signals on Kidney Function. Nat. Rev. Nephrol. 2025, 21, 417–434. [Google Scholar] [CrossRef]
- Braam, B.; Joles, J.A.; Danishwar, A.H.; Gaillard, C.A. Cardiorenal Syndrome—Current Understanding and Future Perspectives. Nat. Rev. Nephrol. 2014, 10, 48–55. [Google Scholar] [CrossRef]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Rizvi, A.A.; Stoian, A.P.; Ciaccio, M.; Papanas, N.; Janez, A.; Sonmez, A.; Banach, M.; Sahebkar, A.; et al. Advances in the Pharmacological Management of Diabetic Nephropathy: A 2022 International Update. Biomedicines 2023, 11, 291. [Google Scholar] [CrossRef]
- O’Hara, D.V.; Lam, C.S.P.; McMurray, J.J.V.; Yi, T.W.; Hocking, S.; Dawson, J.; Raichand, S.; Januszewski, A.S.; Jardine, M.J. Applications of SGLT2 Inhibitors Beyond Glycaemic Control. Nat. Rev. Nephrol. 2024, 20, 513–529. [Google Scholar] [CrossRef]
- Sato, T.; Aizawa, Y.; Yuasa, S.; Kishi, S.; Fuse, K.; Fujita, S.; Ikeda, Y.; Kitazawa, H.; Takahashi, M.; Sato, M.; et al. The Effect of Dapagliflozin Treatment on Epicardial Adipose Tissue Volume. Cardiovasc. Diabetol. 2018, 17, 6. [Google Scholar] [CrossRef]
- Tang, J.; Ye, L.; Yan, Q.; Zhang, X.; Wang, L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Water and Sodium Metabolism. Front. Pharmacol. 2022, 13, 800490. [Google Scholar] [CrossRef]
- Packer, M.; Wilcox, C.S.; Testani, J.M. Critical Analysis of the Effects of SGLT2 Inhibitors on Renal Tubular Sodium, Water and Chloride Homeostasis and Their Role in Influencing Heart Failure Outcomes. Circulation 2023, 148, 354–372. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N. Renal Mechanisms of Diuretic Resistance in Congestive Heart Failure. Kidney Dial. 2023, 3, 56–72. [Google Scholar] [CrossRef]
- Stachteas, P.; Nasoufidou, A.; Patoulias, D.; Karakasis, P.; Karagiannidis, E.; Mourtzos, M.-A.; Samaras, A.; Apostolidou, X.; Fragakis, N. The Role of Sodium-Glucose Co-Transporter-2 Inhibitors on Diuretic Resistance in Heart Failure. Int. J. Mol. Sci. 2024, 25, 3122. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Gallardo, J.S.; Correa, A.; Contreras, J.P. Cardio-Renal Benefits of Sodium–Glucose Co-Transporter 2 Inhibitors in Heart Failure with Reduced Ejection Fraction: Mechanisms and Clinical Evidence. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- List, J.F.; Woo, V.; Morales, E.; Tang, W.; Fiedorek, F.T. Sodium-Glucose Cotransport Inhibition with Dapagliflozin in Type 2 Diabetes. Diabetes Care 2009, 32, 650–657. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; de Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin: A Glucose-Regulating Drug with Diuretic Properties in Subjects with Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Xiao, F.; Zimpelmann, J.; Woerle, H.-J.; Johansen, O.E.; Broedl, U.C.; von Eynatten, M.; Burns, K.D. Sodium Glucose Cotransport-2 Inhibition and Intrarenal RAS Activity in People with Type 1 Diabetes. Kidney Int. 2014, 86, 1057–1058. [Google Scholar] [CrossRef]
- Ansary, T.M.; Nakano, D.; Nishiyama, A. Diuretic Effects of Sodium Glucose Cotransporter 2 Inhibitors and Their Influence on the Renin-Angiotensin System. Int. J. Mol. Sci. 2019, 20, 629. [Google Scholar] [CrossRef]
- Manosroi, W.; Danpanichkul, P.; Atthakomol, P. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Aldosterone and Renin Levels in Diabetes Mellitus Type 2 Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 19603. [Google Scholar] [CrossRef]
- Adam, C.A.; Anghel, R.; Marcu, D.T.M.; Mitu, O.; Roca, M.; Mitu, F. Impact of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors on Arterial Stiffness and Vascular Aging—What Do We Know So Far? (A Narrative Review). Life 2022, 12, 803. [Google Scholar] [CrossRef]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. J. Am. Coll. Cardiol. Basic Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef]
- Pereira, M.J.; Eriksson, J.W. Emerging Role of SGLT-2 Inhibitors for the Treatment of Obesity. Drugs 2019, 79, 219–230. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and Safety of Sodium-Glucose Co-Transporter-2 Inhibitors in Type 2 Diabetes Mellitus: Systematic Review and Network Meta-Analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Zhang, N.; Chen, R.; Zhao, J.-G.; Yu, P. Efficacy and Safety of Sodium-Glucose Cotransporter 2 Inhibitors in Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials for 1 to 2 Years. J. Diabetes Complicat. 2015, 29, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Barbero, N.; Oller, J.; Sanz, A.B.; Ramos, A.M.; Ortiz, A.; Ruiz-Ortega, M.; Rayego-Mateos, S. Mitochondrial Dysfunction in the Cardio-Renal Axis. Int. J. Mol. Sci. 2023, 24, 8209. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Butler, A.E.; Jamialahmadi, T.; Sahebkar, A. Sodium-Glucose Cotransporter 2 Inhibitors and Mitochondrial Functions: State of the Art. EXCLI J. 2023, 22, 53–66. [Google Scholar] [CrossRef]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Xie, B.; Ramirez, W.; Mills, A.M.; Huckestein, B.R.; Anderson, M.; Pangburn, M.M.; Lang, E.Y.; Mullet, S.J.; Chuan, B.W.; Guo, L.; et al. Empagliflozin Restores Cardiac Metabolic Flexibility in Diet-Induced Obese C57BL6/J Mice. Curr. Res. Physiol. 2022, 5, 232–239. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef]
- Merovci, A.; Solis-Herrera, C.; Daniele, G.; Eldor, R.; Fiorentino, T.V.; Tripathy, D.; Xiong, J.; Perez, Z.; Norton, L.; Abdul-Ghani, M.A.; et al. Dapagliflozin Improves Muscle Insulin Sensitivity but Enhances Endogenous Glucose Production. J. Clin. Investig. 2014, 124, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Baartscheer, A.; Bleijlevens, B.; Schumacher, C.A.; Fiolet, J.W.T.; Koeman, A.; Jancev, M.; Hollmann, M.W.; Weber, N.C.; Coronel, R.; et al. Class Effects of SGLT2 Inhibitors in Mouse Cardiomyocytes and Hearts: Inhibition of Na+/H+ Exchanger, Lowering of Cytosolic Na+ and Vasodilation. Diabetologia 2018, 61, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Koyani, C.N.; Plastira, I.; Sourij, H.; Hallström, S.; Schmidt, A.; Rainer, P.P.; Bugger, H.; Frank, S.; Malle, E.; von Lewinski, D. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacol. Res. 2020, 158, 104870. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494. [Google Scholar] [CrossRef]
- Gao, Y.-M.; Feng, S.-T.; Wen, Y.; Tang, T.-T.; Wang, B.; Liu, B.-C. Cardiorenal protection of SGLT2 inhibitors-Perspectives from metabolic reprogramming. EBioMedicine 2022, 83, 104215. [Google Scholar] [CrossRef]
- Ren, C.; Sun, K.; Zhang, Y.; Hu, Y.; Hu, B.; Zhao, J.; He, Z.; Ding, R.; Wang, W.; Liang, C. Sodium-Glucose CoTransporter-2 Inhibitor Empagliflozin Ameliorates Sunitinib-Induced Cardiac Dysfunction via Regulation of AMPK-mTOR Signaling Pathway-Mediated Autophagy. Front. Pharmacol. 2021, 12, 664181. [Google Scholar] [CrossRef]
- Bozkurt, B.; Januzzi, J.L.; Bansal, S. Cardiovascular-Kidney-Metabolic Effects: Steroidal and Nonsteroidal Mineralocorticoid Receptor Antagonists. Rev. Cardiovasc. Med. 2025, 26, 38690. [Google Scholar] [CrossRef]
- Rykova, E.Y.; Klimontov, V.V.; Shmakova, E.; Korbut, A.I.; Merkulova, T.I.; Kzhyshkowska, J. Anti-Inflammatory Effects of SGLT2 Inhibitors: Focus on Macrophages. Int. J. Mol. Sci. 2025, 26, 1670. [Google Scholar] [CrossRef]
- Iannantuoni, F.; M de Marañon, A.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef]
- Gohari, S.; Ismail-Beigi, F.; Mahjani, M.; Ghobadi, S.; Jafari, A.; Ahangar, H.; Gohari, S. The effect of sodium-glucose co-transporter-2 (SGLT2) inhibitors on blood interleukin-6 concentration: A systematic review and meta-analysis of randomized controlled trials. BMC Endocr. Disord. 2023, 23, 257. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, S.-G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmann, M.W.; Preckel, B.; Koolwijk, P.; Van Hinsbergh, V.W.M.; et al. Empagliflozin and Dapagliflozin Reduce ROS Generation and Restore NO Bioavailability in Tumor Necrosis Factor α-Stimulated Human Coronary Arterial Endothelial Cells. Cell. Physiol. Biochem. 2019, 53, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Watanabe, M.; Yokota, T.; Tsuda, M.; Handa, H.; Koya, J.; Nishino, K.; Tatsuta, D.; Natsui, H.; Kadosaka, T.; et al. Empagliflozin suppresses mitochondrial reactive oxygen species generation and mitigates the inducibility of atrial fibrillation in diabetic rats. Front. Cardiovasc. Med. 2023, 10, 1005408. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J. Cell. Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef]
- Sukhanov, S.; Higashi, Y.; Yoshida, T.; Mummidi, S.; Aroor, A.R.; Jeffrey Russell, J.; Bender, S.B.; DeMarco, V.G.; Chandrasekar, B. The SGLT2 inhibitor Empagliflozin attenuates interleukin-17A-induced human aortic smooth muscle cell proliferation and migration by targeting TRAF3IP2/ROS/NLRP3/Caspase-1-dependent IL-1β and IL-18 secretion. Cell. Signal. 2021, 77, 109825. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Wang, Q. Effects and mechanisms of SGLT2 inhibitors on the NLRP3 inflammasome, with a focus on atherosclerosis. Front. Endocrinol. 2022, 13, 992937. [Google Scholar] [CrossRef]
- Dyck, J.R.B.; Sossalla, S.; Hamdani, N.; Coronel, R.; Weber, N.C.; Light, P.E.; Zuurbier, C.J. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: Evidence for potential off-target effects. J. Mol. Cell. Cardiol. 2022, 167, 17–31. [Google Scholar] [CrossRef]
- Packer, M. SGLT2 inhibitors: Role in protective reprogramming of cardiac nutrient transport and metabolism. Nat. Rev. Cardiol. 2023, 20, 443–462. [Google Scholar] [CrossRef]
- Søndergaard, E.; Lauritzen, E.S.; Lauritsen, K.M.; Åkerblom, A.; Nuutila, P.; Oldgren, J.; Gormsen, L.C. SGLT2 inhibition reduces myocardial oxygen consumption. Metab. Open 2022, 15, 100207. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef]
- Ashry, N.A.; Abdelaziz, R.R.; Suddek, G.M.; Saleh, M.A. Canagliflozin Ameliorates Aortic and Hepatic Dysfunction in Dietary-Induced Hypercholesterolemia in the Rabbit. Life Sci. 2021, 280, 119731. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin Acutely Improves Endothelial Dysfunction, Reduces Aortic Stiffness and Renal Resistive Index in Type 2 Diabetic Patients: A Pilot Study. Cardiovasc. Diabetol. 2017, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, Y.; Wang, C.; Niu, S.; Dong, X.; Guan, Y.; Chen, S. Empagliflozin Improves Aortic Injury in Obese Mice by Regulating Fatty Acid Metabolism. Open Med. 2024, 19, 20241012. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Vlachakis, P.K.; Pamporis, K.; Sagris, M.; Ktenopoulos, N.; Kassimis, G.; Antoniadis, A.P.; Fragakis, N. Sodium-Glucose Cotransporter 2 Inhibitors in Aortic Stenosis: Toward a Comprehensive Cardiometabolic Approach. Int. J. Mol. Sci. 2025, 26, 4494. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; Shah, S.J.; Lindholm, D.; et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: Rationale and design of the DELIVER trial. Eur. J. Heart Fail. 2021, 23, 1217–1225. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Mancia, G.; Cannon, C.P.; Tikkanen, I.; Zeller, C.; Ley, L.; Woerle, H.J.; Broedl, U.C.; Johansen, O.E. Impact of Empagliflozin on Blood Pressure in Patients with Type 2 Diabetes Mellitus and Hypertension by Background Antihypertensive Medication. Hypertension 2016, 68, 1355–1364. [Google Scholar] [CrossRef]
- Herrington, W.G.; Savarese, G.; Haynes, R.; Marx, N.; Mellbin, L.; Lund, L.H.; Dendale, P.; Seferovic, P.; Rosano, G.; Staplin, N.; et al. Cardiac, renal, and metabolic effects of sodium-glucose co-transporter 2 inhibitors: A position paper from the European Society of Cardiology ad-hoc task force on sodium-glucose co-transporter 2 inhibitors. Eur. J. Heart Fail. 2021, 23, 1260–1275. [Google Scholar] [CrossRef]
- Baigent, C.; Emberson, J.; Haynes, R.; Herrington, W.G.; Judge, P.; Landray, M.J.; Mayne, K.J.; Ng, S.Y.; Preiss, D.; Roddick, A.J.; et al. Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef]
- Peikert, A.; Solomon, S.D. Contemporary treatment options in heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1517–1524. [Google Scholar] [CrossRef]
- Nanayakkara, S.; Kaye, D.M. Management of heart failure with preserved ejection fraction: A review. Clin. Ther. 2015, 37, 2186–2198. [Google Scholar] [CrossRef]
- Pérez, M.S.; Rodríguez-Capitán, J.; Requena-Ibáñez, J.A.; Santos-Gallego, C.G.; Urooj Zafar, M.; Escolar, G.; Mancini, D.; Mitter, S.; Lam, D.; Contreras, J.P.; et al. Rationale and Design of the SOTA-P-CARDIA Trial (ATRU-V): Sotagliflozin in HFpEF Patients Without Diabetes. Cardiovasc. Drugs Ther. 2025, 39, 155–164. [Google Scholar] [CrossRef]
- Butler, J.; Packer, M.; Filippatos, G.; Ferreira, J.P.; Zeller, C.; Schnee, J.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Anker, S.D. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur. Heart J. 2022, 43, 416–426. [Google Scholar] [CrossRef]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; Vaduganathan, M.; Gasparyan, S.B.; Bengtsson, O.; Lindholm, D.; et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: A patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat. Med. 2022, 28, 1956–1964. [Google Scholar] [CrossRef]
- Karakasis, P.; Theofilis, P.; Patoulias, D.; Schuermans, A.; Vlachakis, P.K.; Klisic, A.; Rizzo, M.; Fragakis, N. Sodium-glucose cotransporter 2 inhibitors and outcomes in transthyretin amyloid cardiomyopathy: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2025, 55, e14392. [Google Scholar] [CrossRef]
- Biegus, J.; Voors, A.A.; Collins, S.P.; Kosiborod, M.N.; Teerlink, J.R.; Angermann, C.E.; Tromp, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; et al. Impact of empagliflozin on decongestion in acute heart failure: The EMPULSE trial. Eur. Heart J. 2023, 44, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Longo, M.; Caruso, P.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Sodium-glucose co-transporter-2 inhibitors for the prevention of cardiorenal outcomes in type 2 diabetes: An updated meta-analysis. Diabetes Obes. Metab. 2021, 23, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; Abdulah, D.M.; et al. Global Burden of Cardiovascular Diseases and Risks Collaborators. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. J. Am. Coll. Cardiol. Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef]
- James, S.; Erlinge, D.; Storey, R.F.; McGuire, D.K.; de Belder, M.; Eriksson, N.; Andersen, K.; Austin, D.; Arefalk, G.; Carrick, D.; et al. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Évid. 2024, 3, EVIDoa2300286. [Google Scholar] [CrossRef]
- Butler, J.; Jones, W.S.; Udell, J.A.; Anker, S.D.; Petrie, M.C.; Harrington, J.; Mattheus, M.; Zwiener, I.; Amir, O.; Bahit, M.C.; et al. Empagliflozin after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1455–1466. [Google Scholar] [CrossRef]
- Khani, E.; Aslanabadi, N.; Mehravani, K.; Rezaei, H.; Afsharirad, H.; Entezari-Maleki, T. Empagliflozin Effects in Patients with ST-Elevation Myocardial Infarction Undergoing Primary PCI: The EMI-STEMI Randomized Clinical Trial. Am. J. Cardiovasc. Drugs 2024, 24, 673–684. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Kassimis, G.; Koufakis, T.; Klisic, A.; Doumas, M.; Fragakis, N.; Rizzo, M. Therapeutic Potential of Sodium-glucose Co-transporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists for Patients with Acute Coronary Syndrome: A Review of Clinical Evidence. Curr. Pharm. Des. 2024, 30, 2109–2119. [Google Scholar] [CrossRef]
- Kario, K.; Okada, K.; Kato, M.; Nishizawa, M.; Yoshida, T.; Asano, T.; Uchiyama, K.; Niijima, Y.; Katsuya, T.; Urata, H.; et al. Twenty-Four-Hour Blood Pressure-Lowering Effect of a Sodium-Glucose Cotransporter 2 Inhibitor in Patients With Diabetes and Uncontrolled Nocturnal Hypertension: Results From the Randomized, Placebo-Controlled SACRA Study. Circulation 2019, 139, 2089–2097. [Google Scholar] [CrossRef]
- Teo, Y.H.; Chia, A.Z.Q.; Teo, Y.N.; Chong, E.Y.; Syn, N.L.; Cheong, J.Y.A.; Ong, H.T.; Wee, C.F.; Ting, A.Z.H.; Tan, J.T.A.; et al. The Impact of Sodium-Glucose Cotransporter Inhibitors on Blood Pressure: A Meta-Analysis and Metaregression of 111 Randomized-Controlled Trials. J. Hypertens. 2022, 40, 2353–2372. [Google Scholar] [CrossRef]
- Wei, R.; Wang, W.; Pan, Q.; Guo, L. Effects of SGLT-2 Inhibitors on Vascular Endothelial Function and Arterial Stiffness in Subjects with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 826604. [Google Scholar] [CrossRef]
- Alshnbari, A.S.; Millar, S.A.; O’Sullivan, S.E.; Idris, I. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Endothelial Function: A Systematic Review of Preclinical Studies. Diabetes Ther. 2020, 11, 1947–1963. [Google Scholar] [CrossRef]
- Genua, I.; Cusi, K. Pharmacological Approaches to Nonalcoholic Fatty Liver Disease: Current and Future Therapies. Diabetes Spectr. 2024, 37, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Latva-Rasku, A.; Honka, M.-J.; Kullberg, J.; Mononen, N.; Lehtimäki, T.; Saltevo, J.; Kirjavainen, A.K.; Saunavaara, V.; Iozzo, P.; Johansson, L.; et al. The SGLT2 Inhibitor Dapagliflozin Reduces Liver Fat but Does Not Affect Tissue Insulin Sensitivity: A Randomized, Double-Blind, Placebo-Controlled Study With 8-Week Treatment in Type 2 Diabetes Patients. Diabetes Care 2019, 42, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Kahl, S.; Gancheva, S.; Straßburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized, Double-Blind, Phase 4, Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, B.; Ancel, P.; Abdullah, A.E.; Maurice, F.; Abdesselam, I.; Calen, A.; Soghomonian, A.; Houssays, M.; Varlet, I.; Eisinger, M.; et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: The EMPACEF study. Cardiovasc. Diabetol. 2021, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.H.; Teo, Y.N.; Syn, N.L.; Kow, C.S.; Yoong, C.S.Y.; Tan, B.Y.Q.; Yeo, T.C.; Lee, C.H.; Lin, W.; Sia, C.H. Effects of Sodium/Glucose Cotransporter 2 (SGLT2) Inhibitors on Cardiovascular and Metabolic Outcomes in Patients Without Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. J. Am. Heart Assoc. 2021, 10, e019463. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P. SGLT2 Inhibitors in CKD: Are They Really Effective in All Patients? Nephrol. Dial. Transplant. 2025, 7, gfaf051. [Google Scholar] [CrossRef]
- Nyström, T. Key Results from Observational Studies and Real-World Evidence of Sodium-Glucose Cotransporter-2 Inhibitor Effectiveness and Safety in Reducing Cardio-Renal Risk. Diabetes Obes. Metab. 2024, 26 (Suppl. S5), 35–57. [Google Scholar] [CrossRef]
- Patorno, E.; Goldfine, A.B.; Schneeweiss, S.; Everett, B.M.; Glynn, R.J.; Liu, J.; Kim, S.C. Cardiovascular Outcomes Associated with Canagliflozin versus Other Non-Gliflozin Antidiabetic Drugs: Population Based Cohort Study. BMJ 2018, 360, k119. [Google Scholar] [CrossRef]
- Udell, J.A.; Yuan, Z.; Rush, T.; Sicignano, N.M.; Galitz, M.; Rosenthal, N. Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World. Circulation 2018, 137, 1450–1459. [Google Scholar] [CrossRef]
- Ryan, P.B.; Buse, J.B.; Schuemie, M.J.; DeFalco, F.; Yuan, Z.; Stang, P.E.; Berlin, J.A.; Rosenthal, N. Comparative Effectiveness of Canagliflozin, SGLT2 Inhibitors and Non-SGLT2 Inhibitors on the Risk of Hospitalization for Heart Failure and Amputation in Patients With Type 2 Diabetes Mellitus: A Real-World Meta-Analysis of 4 Observational Databases (OBSERVE-4D. Diabetes, Obes. Metab. 2018, 20, 2585–2597. [Google Scholar] [CrossRef]
- Suissa, S.; Henry, D.; Caetano, P.; Dormuth, C.R.; Ernst, P.; Hemmelgarn, B.; Lelorier, J.; Levy, A.; Martens, P.J.; Paterson, J.M.; et al. CNODES: The Canadian Network for Observational Drug Effect Studies. Open Med. 2012, 6, e134-40. [Google Scholar]
- Pasternak, B.; Ueda, P.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Wintzell, V.; Melbye, M.; et al. Use of Sodium Glucose Cotransporter 2 Inhibitors and Risk of Major Cardiovascular Events and Heart Failure: Scandinavian Register Based Cohort Study. BMJ 2019, 366, l4772. [Google Scholar] [CrossRef]
- Pasternak, B.; Wintzell, V.; Melbye, M.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Svanström, H.; et al. Use of Sodium-Glucose Co-Transporter 2 Inhibitors and Risk of Serious Renal Events: Scandinavian Cohort Study. BMJ 2020, 369, m1186. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Cavender, M.A.; Fu, A.Z.; Wilding, J.P.; Khunti, K.; Holl, R.W.; Norhammar, A.; Birkeland, K.I.; Jørgensen, M.E.; Thuresson, M.; et al. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017, 136, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Lam, C.S.P.; Kohsaka, S.; Kim, D.J.; Karasik, A.; Shaw, J.; Tangri, N.; Goh, S.-Y.; Thuresson, M.; Chen, H.; et al. Cardiovascular Events Associated With SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL 2 Study. J. Am. Coll. Cardiol. 2018, 71, 2628–2639. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Karasik, A.; Thuresson, M.; Melzer-Cohen, C.; Chodick, G.; Khunti, K.; Wilding, J.P.H.; Garcia Rodriguez, L.A.; Cea-Soriano, L.; Kohsaka, S.; et al. Kidney Outcomes Associated with Use of SGLT2 Inhibitors in Real-World Clinical Practice (CVD-REAL 3): A Multinational Observational Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 27–35. [Google Scholar] [CrossRef]
- Patorno, E.; Pawar, A.; Franklin, J.M.; Najafzadeh, M.; Déruaz-Luyet, A.; Brodovicz, K.G.; Sambevski, S.; Bessette, L.G.; Santiago Ortiz, A.J.; Kulldorff, M.; et al. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care. Circulation 2019, 139, 2822–2830. [Google Scholar] [CrossRef]
- Karasik, A.; Lanzinger, S.; Chia-Hui Tan, E.; Yabe, D.; Kim, D.J.; Sheu, W.H.-H.; Melzer-Cohen, C.; Holl, R.W.; Ha, K.H.; Khunti, K.; et al. Empagliflozin Cardiovascular and Renal Effectiveness and Safety Compared to Dipeptidyl Peptidase-4 Inhibitors Across 11 Countries in Europe and Asia: Results from the EMPagliflozin CompaRative EffectIveness and SafEty (EMPRISE) Study. Diabetes Metab. 2023, 49, 101418. [Google Scholar] [CrossRef]
- Vistisen, D.; Carstensen, B.; Elisabetta, P.; Lanzinger, S.; Tan, E.C.; Yabe, D.; Kim, D.J.; Sheu, W.-H.; Melzer-Cohen, C.; Holl, R.W.; et al. Empagliflozin Is Associated with Lower Cardiovascular Risk Compared with Dipeptidyl Peptidase-4 Inhibitors in Adults With and Without Cardiovascular Disease: EMPagliflozin CompaRative EffectIveness and SafEty (EMPRISE) Study Results from Europe and Asia. Cardiovasc. Diabetol. 2023, 22, 233. [Google Scholar] [CrossRef]
- Colombo, G.; Biering-Sorensen, T.; Ferreira, J.P.; Lombardi, C.M.; Bonelli, A.; Garascia, A.; Metra, M.; Inciardi, R.M. Cardiac Remodelling in the Era of the Recommended Four Pillars Heart Failure Medical Therapy. ESC Heart Fail. 2025, 12, 1029–1044. [Google Scholar] [CrossRef]
- Leiner, T.; Bogaert, J.; Friedrich, M.G.; Mohiaddin, R.; Muthurangu, V.; Myerson, S.; Powell, A.J.; Raman, S.V.; Pennell, D.J. SCMR Position Paper (2020) on Clinical Indications for Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2020, 22, 76. [Google Scholar] [CrossRef]
- Leo, I.; Salerno, N.; Figliozzi, S.; Cersosimo, A.; Ielapi, J.; Stankowski, K.; Bisaccia, G.; Dellegrottaglie, S.; Canino, G.; De Rosa, S.; et al. Effect of SGLT2 inhibitors on cardiac structure and function assessed by cardiac magnetic resonance: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2025, 24, 345. [Google Scholar] [CrossRef]
- Brown, A.J.M.; Gandy, S.; McCrimmon, R.; Houston, J.G.; Struthers, A.D.; Lang, C.C. A Randomized Controlled Trial of Dapagliflozin on Left Ventricular Hypertrophy in People with Type Two Diabetes: The DAPA-LVH Trial. Eur. Heart J. 2020, 41, 3421–3432. [Google Scholar] [CrossRef]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; et al. Effect of Empagliflozin on Left Ventricular Mass in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Martuszewski, A.; Paluszkiewicz, P.; Poręba, R.; Gać, P. Clinical Significance of Extracellular Volume of Myocardium (ECV) Assessed by Computed Tomography: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2066. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.; Coelho-Filho, O.R.; Verma, S.; Chowdhury, B.; Zuo, F.; Quan, A.; Thorpe, K.E.; Bonneau, C.; Teoh, H.; Gilbert, R.E.; et al. Empagliflozin Reduces Myocardial Extracellular Volume in Patients with Type 2 Diabetes and Coronary Artery Disease. J. Am. Coll. Cardiol. Cardiovasc. Imaging 2021, 14, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Albulushi, A.; Askari, K.M.; Al-Abedi, A.M.; Al-Kulaibi, M.A.; Hasan, M.S.; Hosseini, Z.; Al-Rahman, M.T.; Tanoh, D.B.; Hasan, A.S.; Al-Helli, Y.; et al. Impact of SGLT2 Inhibitors on Myocardial Fibrosis in Diabetic HFpEF: A Longitudinal Study. Eur. J. Med Res. 2025, 30, 592. [Google Scholar] [CrossRef]
- Morawiec, B.; Fournier, S.; Tapponnier, M.; Prior, J.O.; Monney, P.; Dunet, V.; Lauriers, N.; Recordon, F.; Trana, C.; Iglesias, J.-F.; et al. Performance of Highly Sensitive Cardiac Troponin T Assay to Detect Ischaemia at PET-CT in Low-Risk Patients with Acute Coronary Syndrome: A Prospective Observational Study. BMJ Open 2017, 7, e014655. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Sattar, N.; Xu, J.; Butler, J.; Mahaffey, K.W.; Neal, B.; Shaw, W.; Rosenthal, N.; Pfeifer, M.; Hansen, M.K.; et al. Stress Cardiac Biomarkers, Cardiovascular and Renal Outcomes, and Response to Canagliflozin. J. Am. Coll. Cardiol. 2022, 79, 432–444. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Butler, J.; Jarolim, P.; Sattar, N.; Vijapurkar, U.; Desai, M.; Davies, M.J. Effects of Canagliflozin on Cardiovascular Biomarkers in Older Adults with Type 2 Diabetes. J. Am. Coll. Cardiol. 2017, 70, 704–712. [Google Scholar] [CrossRef]
- Phrommintikul, A.; Wongcharoen, W.; Kumfu, S.; Jaiwongkam, T.; Gunaparn, S.; Chattipakorn, S.; Chattipakorn, N. Effects of Dapagliflozin vs Vildagliptin on Cardiometabolic Parameters in Diabetic Patients with Coronary Artery Disease: A Randomised Study. Br. J. Clin. Pharmacol. 2019, 85, 1337–1347. [Google Scholar] [CrossRef]
- Griffin, M.; Rao, V.S.; Ivey-Miranda, J.; Fleming, J.; Mahoney, D.; Maulion, C.; Suda, N.; Siwakoti, K.; Ahmad, T.; Jacoby, D.; et al. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation 2020, 142, 1028–1039. [Google Scholar] [CrossRef]
- Sakuma, M.; Nakamura, M.; Tanaka, F.; Onoda, T.; Itai, K.; Tanno, K.; Ohsawa, M.; Sakata, K.; Yoshida, Y.; Kawamura, K.; et al. Plasma B-Type Natriuretic Peptide Level and Cardiovascular Events in Chronic Kidney Disease in a Community-Based Population. Circ. J. 2010, 74, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Spanaus, K.-S.; Kronenberg, F.; Ritz, E.; Schlapbach, R.; Fliser, D.; Hersberger, M.; Kollerits, B.; König, P.; von Eckardstein, A.; for the Mild-to-Moderate Kidney Disease Study Group. B-Type Natriuretic Peptide Concentrations Predict the Progression of Nondiabetic Chronic Kidney Disease: The Mild-to-Moderate Kidney Disease Study. Clin. Chem. 2007, 53, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L., Jr.; Zannad, F.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Ferreira, J.P.; Sattar, N.; Verma, S.; Vedin, O.; et al. Prognostic Importance of NT-proBNP and Effect of Empagliflozin in the EMPEROR-Reduced Trial. J. Am. Coll. Cardiol. 2021, 78, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Brooksbank, K.J.M.; Wetherall, K.; Mangion, K.; Roditi, G.; Campbell, R.T.; Berry, C.; Chong, V.; Coyle, L.; Docherty, K.F.; et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients with Type 2 Diabetes, or Prediabetes, and Heart Failure with Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021, 143, 516–525. [Google Scholar] [CrossRef]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; et al. Empagliflozin in Acute Myocardial Infarction: The EMMY Trial. Eur. Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Xu, J.; Li, J.; Shaw, W.; Oh, R.; Pfeifer, M.; Butler, J.; Sattar, N.; Mahaffey, K.W.; Neal, B.; et al. Effects of Canagliflozin on Amino-Terminal Pro-B-Type Natriuretic Peptide: Implications for Cardiovascular Risk Reduction. J. Am. Coll. Cardiol. 2020, 76, 2076–2085. [Google Scholar] [CrossRef]
- Martín, E.; López-Aguilera, J.; González-Manzanares, R.; Anguita, M.; Gutiérrez, G.; Luque, A.; Paredes, N.; Oneto, J.; Perea, J.; Castillo, J.C. Impact of Canagliflozin in Patients with Type 2 Diabetes after Hospitalization for Acute Heart Failure: A Cohort Study. J. Clin. Med. 2021, 10, 505. [Google Scholar] [CrossRef]
- Kusunose, K.; Imai, T.; Tanaka, A.; Dohi, K.; Shiina, K.; Yamada, T.; Kida, K.; Eguchi, K.; Teragawa, H.; Takeishi, Y.; et al. Effects of Canagliflozin on NT-proBNP Stratified by Left Ventricular Diastolic Function in Patients with Type 2 Diabetes and Chronic Heart Failure: A Sub Analysis of the CANDLE Trial. Cardiovasc. Diabetol. 2021, 20, 186. [Google Scholar] [CrossRef]
- Jensen, J.; Omar, M.; Kistorp, C.; Poulsen, M.K.; Tuxen, C.; Gustafsson, I.; Køber, L.; Gustafsson, F.; Faber, J.; Fosbøl, E.L.; et al. Twelve Weeks of Treatment with Empagliflozin in Patients with Heart Failure and Reduced Ejection Fraction: A Double-Blinded, Randomized, and Placebo-Controlled Trial. Am. Heart J. 2020, 228, 47–56. [Google Scholar] [CrossRef]
- Tanaka, A.; Hisauchi, I.; Taguchi, I.; Sezai, A.; Toyoda, S.; Tomiyama, H.; Sata, M.; Ueda, S.; Oyama, J.I.; Kitakaze, M.; et al. Effects of Canagliflozin in Patients with Type 2 Diabetes and Chronic Heart Failure: A Randomized Trial (CANDLE). ESC Heart Fail. 2020, 7, 1585–1594. [Google Scholar] [CrossRef]
- Ejiri, K.; Miyoshi, T.; Kihara, H.; Hata, Y.; Nagano, T.; Takaishi, A.; Toda, H.; Nanba, S.; Nakamura, Y.; Akagi, S.; et al. Effect of Luseogliflozin on Heart Failure With Preserved Ejection Fraction in Patients With Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e015103. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Manta, A.; Mavroeidi, I.; Asimakopoulou, A.; Syrigos, A.; Nastos, C.; Pikoulis, E.; Kollias, A. Changes in Cardiovascular and Renal Biomarkers Associated with SGLT2 Inhibitors Treatment in Patients with Type 2 Diabetes Mellitus. Pharmaceutics 2023, 15, 2526. [Google Scholar] [CrossRef] [PubMed]
- van Kimmenade, R.R.; Januzzi, J.L.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of Amino-Terminal Pro-Brain Natriuretic Peptide, Galectin-3, and Apelin for the Evaluation of Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef]
- van der Velde, A.R.; Gullestad, L.; Ueland, T.; Aukrust, P.; Guo, Y.; Adourian, A.; Muntendam, P.; van Veldhuisen, D.J.; de Boer, R.A. Prognostic Value of Changes in Galectin-3 Levels Over Time in Patients with Heart Failure: Data from CORONA and COACH. Circ. Heart Fail. 2013, 6, 219–226. [Google Scholar] [CrossRef]
- Haller, P.M.; Wiviott, S.D.; Berg, D.D.; Jarolim, P.; Goodrich, E.L.; Bhatt, D.L.; Gause-Nilsson, I.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. Galectin-3 and kidney function in type 2 diabetes treated with dapagliflozin: Analysis from DECLARE-TIMI 58. ESC Heart Fail. 2025. submitted. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an Interleukin-1-Like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Januzzi, J.L. The Biology of ST2: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115 (Suppl. S7), 3B–7B. [Google Scholar] [CrossRef]
- Tang, W.W.; Wu, Y.; Grodin, J.L.; Hsu, A.P.; Hernandez, A.F.; Butler, J.; Metra, M.; Voors, A.A.; Felker, G.M.; Troughton, R.W.; et al. Prognostic Value of Baseline and Changes in Circulating Soluble ST2 Levels and the Effects of Nesiritide in Acute Decompensated Heart Failure. J. Am. Coll. Cardiol. Heart Fail. 2016, 4, 68–77. [Google Scholar] [CrossRef]
- Anand, I.S.; Rector, T.S.; Kuskowski, M.; Snider, J.; Cohn, J.N. Prognostic Value of Soluble ST2 in the Valsartan Heart Failure Trial. Circ. Heart Fail. 2014, 7, 418–426. [Google Scholar] [CrossRef]
- Dekkers, C.C.J.; Petrykiv, S.; Laverman, G.D.; Cherney, D.Z.; Gansevoort, R.T.; Heerspink, H.J.L. Effects of the SGLT-2 Inhibitor Dapagliflozin on Glomerular and Tubular Injury Markers. Diabetes, Obes. Metab. 2018, 20, 1988–1993. [Google Scholar] [CrossRef]
- Darawshi, S.; Yaseen, H.; Gorelik, Y.; Faor, C.; Szalat, A.; Abassi, Z.; Heyman, S.N.; Khamaisi, M. Biomarker Evidence for Distal Tubular Damage but Cortical Sparing in Hospitalized Diabetic Patients with Acute Kidney Injury (AKI) While on SGLT2 Inhibitors. Ren. Fail. 2020, 42, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003, 289, 2560–2572, Erratum in JAMA 2003, 290, 197. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, X.; Xu, G. Combination Therapy with SGLT2 Inhibitors for Diabetic Kidney Disease. Biomed. Pharmacother. 2020, 127, 110192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-M.; Zhang, M.; Zhan, Z.-L.; Qiu, M. Does Combination Therapy with SGLT2 Inhibitors and Renin–Angiotensin System Blockers Lead to Greater Reduction in Cardiorenal Events among Patients with Type 2 Diabetes? Front. Cardiovasc. Med. 2021, 8, 679124. [Google Scholar] [CrossRef]
- Marx, N.; Husain, M.; Lehrke, M.; Verma, S.; Sattar, N. GLP-1 Receptor Agonists for the Reduction of Atherosclerotic Cardiovascular Risk in Patients with Type 2 Diabetes. Circulation 2022, 146, 1882–1894. [Google Scholar] [CrossRef]
- Ferhatbegović, L.; Mršić, D.; Macić-Džanković, A. The Benefits of GLP1 Receptors in Cardiovascular Diseases. Front. Clin. Diabetes Healthc. 2023, 4, 1293926. [Google Scholar] [CrossRef]
- Waqas, S.A.; Sohail, M.U.; Saad, M.; Minhas, A.M.K.; Greene, S.J.; Fudim, M.; Fonarow, G.C.; Abramov, D.; Khan, M.S.; Ahmed, R. Efficacy of GLP-1 Receptor Agonists in Patients with Heart Failure and Mildly Reduced or Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Card. Fail. 2025, 31, 1076–1080. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Halpern, B.; Wharton, S.; Wilding, J.P.H.; Perreault, L.; Zhang, S.; Battula, R.; Bunck, M.C.; Ahmad, N.N.; Jouravskaya, I. SURMOUNT-1 Investigators. Tirzepatide for Obesity Treatment and Diabetes Prevention. N Engl J Med. 2025, 392, 958–971. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-Inflammatory Role of Glucagon-Like Peptide 1 Receptor Agonists and Its Clinical Implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, S.F.; Pusapati, S.; Anwar, M.S.; Lohana, D.; Kumar, P.; Nandula, S.A.; Nawaz, F.K.; Tracey, K.; Yang, H.; LeRoith, D.; et al. Glucagon-Like Peptide-1: A Multi-Faceted Anti-Inflammatory Agent. Front. Immunol. 2023, 14, 1148209. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lin, Y.; Wang, S.; Zhang, L.; Guo, L. GLP-1 Inhibits High-Glucose-Induced Oxidative Injury of Vascular Endothelial Cells. Sci. Rep. 2017, 7, 8008. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Wadid, M.; Makwana, B.; Kumar, A.; Khadke, S.; Bhatti, A.; Banker, A.; Husami, Z.; Labib, S.; Venesy, D.; et al. GLP-1 Receptor Agonists Among Patients with Overweight or Obesity, Diabetes, and HFpEF on SGLT2 Inhibitors. J. Am. Coll. Cardiol. Heart Fail. 2024, 12, 1814–1826. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Rossing, P.; Bakris, G.; Belmar, N.; Bosch-Traberg, H.; Busch, R.; Charytan, D.M.; Hadjadj, S.; Gillard, P.; Górriz, J.L.; et al. Effects of Semaglutide with and without Concomitant SGLT2 Inhibitor Use in Participants with Type 2 Diabetes and Chronic Kidney Disease in the FLOW Trial. Nat. Med. 2024, 30, 2849–2856. [Google Scholar] [CrossRef]
- Simms-Williams, N.; Treves, N.; Yin, H.; Lu, S.; Yu, O.; Pradhan, R.; Renoux, C.; Suissa, S.; Azoulay, L. Effect of Combination Treatment with Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors on Incidence of Cardiovascular and Serious Renal Events: Population Based Cohort Study. BMJ 2024, 385, e078242, Erratum in BMJ 2024, 385, q1094; Erratum in BMJ 2024, 385, q1237. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Vaduganathan, M.; Claggett, B.; Jhund, P.S.; Desai, A.S.; Henderson, A.D.; Lam, C.S.; Pitt, B.; Senni, M.; et al. Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2024, 391, 1475–1485. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Oliveira, A.C.; Vasques-Novoa, F.; Leite, A.R.; Mendonça, L.; Zannad, F.; Butler, J.; Leite-Moreira, A.; Saraiva, F.; Neves, J.S. Mineralocorticoid Receptor Antagonist Combined with SGLT2 Inhibitor versus SGLT2 Inhibitor Alone in Chronic Kidney Disease: A Meta-Analysis of Randomized Trials. Am. J. Nephrol. 2025, 56, 236–242. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Vart, P.; Jongs, N.; Neuen, B.L.; Bakris, G.; Claggett, B.; Vaduganathan, M.; McCausland, F.; Docherty, K.F.; Jhund, P.S.; et al. Estimated Lifetime Benefit of Novel Pharmacological Therapies in Patients with Type 2 Diabetes and Chronic Kidney Disease: A Joint Analysis of Randomized Controlled Clinical Trials. Diabetes Obes. Metab. 2023, 25, 3327–3336. [Google Scholar] [CrossRef]
- Neuen, B.L.; Heerspink, H.J.L.; Vart, P.; Claggett, B.L.; Fletcher, R.A.; Arnott, C.; de Oliveira Costa, J.; Falster, M.O.; Pearson, S.-A.; Mahaffey, K.W.; et al. Estimated Lifetime Cardiovascular, Kidney, and Mortality Benefits of Combination Treatment with SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Nonsteroidal MRA Compared with Conventional Care in Patients with Type 2 Diabetes and Albuminuria. Circulation 2024, 149, 450–462. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S158–S178, Erratum in Diabetes Care 2024, 47, 1238. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; A Ajjan, R.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. ESC Guidelines for the Management of Cardiovascular Disease in Patients with Diabetes. Eur. Heart J. 2023, 44, 4043–4140, Erratum in Eur. Heart J. 2023, 44, 5060; Erratum in Eur. Heart J. 2024, 45, 518. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- A McDonagh, T.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639, Erratum in Eur. Heart J. 2024, 45, 53. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. ESC Guidelines for the Management of Chronic Coronary Syndromes. Eur. Heart J. 2024, 45, 3415–3537, Erratum in Eur. Heart J. 2025, 46, 1565. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156, Erratum in Circulation 2024, 149, e167; Erratum in Circulation 2024, 149, e936; Erratum in Circulation 2024, 149, e1413. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032, Erratum in Circulation 2022, 145, e1033; Erratum in Circulation 2022, 146, e185; Erratum in Circulation 2023, 147, e674. [Google Scholar] [CrossRef]
- Younes, A.M.; Salem, M.; Maraey, A.; Nomigolzar, S.; Sewell, K.; Khalil, M.; Elzanaty, A.; Saeyeldin, A.; Dar, M. Safety outcomes of SGLT2i in the heart failure trials: A systematic review and Meta-analysis. Int. J. Cardiol. 2022, 366, 51–56. [Google Scholar] [CrossRef]
- Kaze, A.D.; Zhuo, M.; Kim, S.C.; Patorno, E.; Paik, J.M. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: A meta-analysis. Cardiovasc. Diabetol. 2022, 21, 47. [Google Scholar] [CrossRef]
- Mohan, P.; Saini, S.; Kairi, J.K. Greater concern about hypoglycemia in Type 2 diabetics is the need of the hour-findings from a prospective, single-center, observational study. J. Family Med. Prim. Care. 2019, 8, 493–497. [Google Scholar] [CrossRef]
- Morace, C.; Lorello, G.; Bellone, F.; Quartarone, C.; Ruggeri, D.; Giandalia, A.; Mandraffino, G.; Minutoli, L.; Squadrito, G.; Russo, G.T.; et al. Ketoacidosis and SGLT2 Inhibitors: A Narrative Review. Metabolites 2024, 14, 264. [Google Scholar] [CrossRef]
- Li, C.X.; Liu, L.Y.; Zhang, C.X.; Geng, X.H.; Gu, S.M.; Wang, Y.Q.; Liu, H.; Xie, Q.; Liang, S. Comparative safety of different sodium-glucose transporter 2 inhibitors in patients with type 2 diabetes: A systematic review and network meta-analysis of randomized controlled trials. Front. Endocrinol. 2023, 14, 1238399. [Google Scholar] [CrossRef]
- Yau, K.; Dharia, A.; Alrowiyti, I.; Cherney, D.Z. Prescribing SGLT2 Inhibitors in Patients With CKD: Expanding Indications and Practical Considerations. Kidney Int. Rep. 2022, 7, 1463–1476. [Google Scholar] [CrossRef]
- Liu, H. Case literature analysis of Fournier’s gangrene caused by sodium-glucose protein-2 inhibitors. Front. Med. 2024, 11, 1301105. [Google Scholar] [CrossRef] [PubMed]
- Rivera, F.B.; Tang, V.A.S.; De Luna, D.V.; Lerma, E.V.; Vijayaraghavan, K.; Kazory, A.; Shah, N.S.; Volgman, A.S. Sex differences in cardiovascular outcomes of SGLT-2 inhibitors in heart failure randomized controlled trials: A systematic review and meta-analysis. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 26, 100261. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: A systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Swartling, O.; Yang, Y.; Clase, C.M.; Fu, E.L.; Hecking, M.; Hödlmoser, S.; Trolle-Lagerros, Y.; Evans, M.; Carrero, J.J. Sex Differences in the Recognition, Monitoring, and Management of CKD in Health Care: An Observational Cohort Study. J. Am. Soc. Nephrol. 2022, 33, 1903–1914. [Google Scholar] [CrossRef]
- Androutsakos, T.; Nasiri-Ansari, N.; Bakasis, A.-D.; Kyrou, I.; Efstathopoulos, E.; Randeva, H.S.; Kassi, E. SGLT-2 Inhibitors in NAFLD: Expanding Their Role beyond Diabetes and Cardioprotection. Int. J. Mol. Sci. 2022, 23, 3107. [Google Scholar] [CrossRef]
- Huang, B.; Yen, C.-L.; Wu, C.-Y.; Tsai, C.-Y.; Chen, J.-J.; Hsiao, C.-C.; Chen, Y.-C.; Hsieh, I.-C.; Yang, H.-Y. SGLT2 inhibitors reduce the risk of renal failure in CKD stage 5 patients with Type 2 DM. Sci. Rep. 2025, 15, 5872, Erratum in Sci. Rep. 2025, 15, 12440. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Ruža, I.; Marra, A.M.; Gómez-Huelgas, R. Comparative safety and efficacy analysis of GLP-1 receptor agonists and SGLT-2 inhibitors among frail individuals with type 2 diabetes in the era of continuous population ageing. Eur. J. Intern. Med. 2025, 131, 162–165. [Google Scholar] [CrossRef]
- Aldafas, R.; Crabtree, T.; Alkharaiji, M.; Vinogradova, Y.; Idris, I. Sodium-glucose cotransporter-2 inhibitors (SGLT2) in frail or older people with type 2 diabetes and heart failure: A systematic review and meta-analysis. Age Ageing 2024, 53, afad254. [Google Scholar] [CrossRef] [PubMed]
- Bellary, S.; Barnett, A.H. SGLT2 inhibitors in older adults: Overcoming the age barrier. Lancet Healthy Longev. 2023, 4, e127–e128. [Google Scholar] [CrossRef]
- Agarwal, R.; Green, J.B.; Heerspink, H.J.L.; E Mann, J.F.; McGill, J.B.; Mottl, A.K.; Rosenstock, J.; Rossing, P.; Vaduganathan, M.; Brinker, M.; et al. COmbinatioN effect of FInerenone anD EmpaglifloziN in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint (CONFIDENCE) trial: Baseline clinical characteristics. Nephrol. Dial. Transplant. 2025, 40, 1559–1569. [Google Scholar] [CrossRef]
- Scheen, A.J. GLP-1 Receptor Agonists and SGLT2 Inhibitors in Type 2 Diabetes: Pleiotropic Cardiometabolic Effects and Add-on Value of a Combined Therapy. Drugs 2024, 84, 1347–1364. [Google Scholar] [CrossRef]
- Karakasis, P.; Sagris, M.; Patoulias, D.; Koufakis, T.; Theofilis, P.; Klisic, A.; Fragakis, N.; El Tanani, M.; Rizzo, M. Mitigating Increased Cardiovascular Risk in Patients with Obstructive Sleep Apnea Using GLP-1 Receptor Agonists and SGLT2 Inhibitors: Hype or Hope? Biomedicines 2024, 12, 2503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Trial/Author | SGLT2is | Primary Endpoint | Population | Follow-Up (Months) | Main Outcomes |
|---|---|---|---|---|---|
| HFrEF | |||||
| DAPA-HF [86] | Dapagliflozin | Composite of worsening HF (hospitalization or urgent visit requiring IV therapy) or CV death | 4744 adults (≥18 years) with LVEF ≤ 40% and NYHA class II–IV symptoms | 18 | Dapagliflozin was associated with a reduction in the primary composite outcome (HR: 0.74; 95% CI: 0.65–0.85), total HHF (HR: 0.70; 95% CI: 0.59–0.83), and CV death (HR: 0.82; 95% CI: 0.69–0.98). |
| EMPEROR-Reduced [87] | Empagliflozin | Primary composite outcome of CV death or HHF (including first and recurrent events) | 3730 adults (≥18 years) with LVEF ≤ 40% and NYHA class II–IV symptoms | 16 | Empagliflozin treatment resulted in a reduction in the primary composite outcome (HR: 0.75; 95% CI: 0.65–0.86) and a decreased risk of first HHF (HR: 0.69; 95% CI: 0.59–0.81), with no significant difference observed in CV death |
| HFpEF | |||||
| EMPEROR-Preserved [88] | Empagliflozin | Primary composite outcome of CV death or HHF (including first and recurrent events) | 5988 adults (≥18 years) with LVEF > 40% and NYHA class II–IV symptoms | 26 | Empagliflozin reduced the risk of the primary composite outcome (HR: 0.79; 95% CI: 0.69–0.90) and total HHF (HR: 0.71; 95% CI: 0.60–0.83), with no significant effect observed on CV death (HR: 0.91; 95% CI: 0.76–1.09). |
| DELIVER [89] | Dapagliflozin | Composite of worsening HF (hospitalization or urgent visit requiring IV therapy) or CV death | 10,584 adults (≥18 years) with LVEF > 40% and NYHA class II–IV symptoms, including those with improved LVEF | 28 | Dapagliflozin lowered the risk of the primary composite outcome (HR: 0.82; 95% CI: 0.73–0.92) and reduced the incidence of worsening HF events (HR: 0.79; 95% CI: 0.69–0.91), with no significant difference observed in CV death between groups (HR: 0.88; 95% CI: 0.74–1.05). |
| SOLOIST-WHF [90] | Sotagliflozin | Total number of CV deaths, hospitalizations, and urgent HF visits (first and recurrent events) | 1222 adults (18–85 years) with T2DM hospitalized for worsening HF and treated with intravenous diuretics | 9 | Sotagliflozin significantly reduced primary endpoint events (HR: 0.67; 95% CI: 0.52–0.85) |
| CKD | |||||
| DAPA-CKD [91] | Dapagliflozin | Time-to-event analysis of first occurrence of ≥50% decline in eGFR, end-stage kidney disease (dialysis ≥ 28 days, transplant, or eGFR < 15 mL/min/1.73 m2 for ≥28 days), or death from renal or CV causes | 4304 adults (≥18 years) with eGFR 25 to <75 mL/min/1.73 m2, UACR 200 to <5000 mg/g, and stable RAS inhibitor therapy for ≥4 weeks before randomization | 29 | Dapagliflozin reduced the risk of the primary composite outcome (HR: 0.61; 95% CI: 0.51–0.72), lowered the incidence of the composite of sustained ≥50% eGFR decline, end-stage kidney disease, or renal death (HR: 0.56; 95% CI: 0.45–0.68), decreased the risk of CV death or HHF (HR: 0.71; 95% CI: 0.55–0.92), and reduced all-cause mortality (HR: 0.69; 95% CI: 0.53–0.88). |
| EMPA-KIDNEY [92] | Empagliflozin | First occurrence of kidney disease progression (end-stage kidney disease defined as initiation of maintenance dialysis or kidney transplantation, sustained eGFR < 10 mL/min/1.73 m2, sustained ≥ 40% decline from baseline eGFR, or death due to renal causes) or CV death | 6609 adults (≥18 years) with either eGFR ≥ 20 to <45 mL/min/1.73 m2 (regardless of UACR) or eGFR ≥ 45 to <90 mL/min/1.73 m2 with UACR ≥ 200 mg/g; on stable dose of a single RAS inhibitor | 24 | Empagliflozin lowered the risk of progression of kidney disease or CV death (HR: 0.72; 95% CI: 0.64–0.82) and reduced all-cause hospitalization (HR: 0.86; 95% CI: 0.78–0.95), while no significant differences were observed between groups in HHF, CV death (4.0% vs. 4.6%), or all-cause mortality (4.5% vs. 5.1%). |
| CREDENCE [93] | Canagliflozin | Composite outcome of end-stage kidney disease (dialysis ≥ 30 days, kidney transplant, or eGFR < 15 mL/min/1.73 m2 for ≥30 days), sustained doubling of serum creatinine from baseline (≥30 days), or death due to renal or cardiovascular causes | 4401 adults (≥30 years) with T2DM, eGFR 30 to <90 mL/min/1.73 m2, UACR 300 to <5000 mg/g, and stable RAS inhibitor therapy for ≥4 weeks prior to randomization | 31 | Canagliflozin reduced the risk of the primary outcome by 30% compared to placebo (HR: 0.70; 95% CI: 0.59–0.82), lowered the renal-specific composite outcome by 34% (HR: 0.66; 95% CI: 0.53–0.81), decreased the risk of end-stage kidney disease by 32% (HR: 0.68; 95% CI: 0.54–0.86), reduced the risk of CV death, MI, or stroke (HR: 0.80; 95% CI: 0.67–0.95), and significantly lowered HHF (HR: 0.61; 95% CI: 0.47–0.80), with no significant differences observed in rates of amputation or fracture. |
| T2DM | |||||
| EMPA-REG OUTCOME [94] | Empagliflozin | Primary composite outcome: CV death, nonfatal MI, or nonfatal stroke | 7020 adults (≥18 years) with T2DM at high risk for CV events | 37 | Empagliflozin reduced the primary composite outcome compared to placebo (10.5% vs. 12.1%; HR 0.86; 95% CI 0.74–0.99; p = 0.04). No significant differences were seen in MI or stroke rates. However, empagliflozin significantly lowered CV death (3.7% vs. 5.9%; 38% RRR), HHF (2.7% vs. 4.1%; 35% RRR), and all-cause mortality (5.7% vs. 8.3%; 32% RRR). |
| DECLARE–TIMI 58 [95] | Dapagliflozin | Primary composite outcome of CV death, nonfatal MI, or nonfatal stroke | 17,160 adults (≥18 years) with T2DM who had or were at risk for ASCVD | 50 | Dapagliflozin demonstrated noninferiority for MACE (HR 0.93; 95% CI 0.84–1.03; p = 0.17) without a significant reduction in MACE rates. It significantly reduced CV death or HHF (HR 0.83; 95% CI 0.73–0.95; p = 0.005), driven by fewer HHF (HR 0.73; 95% CI 0.61–0.88). Renal events were also reduced (HR 0.76; 95% CI 0.67–0.87), with no significant difference in all-cause mortality (HR 0.93; 95% CI 0.82–1.04). |
| CANVAS Program [96] | Canagliflozin | Primary composite outcome of CV death, nonfatal MI, or nonfatal stroke | 10,142 adults (≥18 years) with T2DM and an elevated risk of CVD | 47 | Canagliflozin lowered the primary outcome rate compared to placebo (26.9 vs. 31.5 events per 1000 patient-years; HR 0.86; 95% CI 0.75–0.97; p < 0.001 for noninferiority; p = 0.02 for superiority). While renal outcomes did not meet formal statistical significance per the prespecified testing sequence, canagliflozin showed potential benefits in slowing albuminuria progression (HR 0.73; 95% CI 0.67–0.79) and reducing a composite of sustained ≥40% eGFR decline, renal-replacement therapy, or renal death (HR 0.60; 95% CI 0.47–0.77). |
| Hypertension | |||||
| EMPA-REG BP [97] | Empagliflozin | Reduction in BP, tolerance, safety | 825 adults with T2DM and hypertension | 3 | Empagliflozin significantly lowered blood pressure in patients with T2DM and hypertension, demonstrating good tolerability |
| Obesity | |||||
| Liu XY/Meta-analysis [54] | Empagliflozin, Dapagliflozin, Canagliflozin | Efficacy and safety. Evaluation of glucose lowering, BP, weight loss. | 11,162 adults with T2DM | 19 | SGLT2is significantly reduced body weight (for 1-year result, WMD: −2.477; 95% CI: −2.568 to −2.385; I2 = 0.0%; for 2 years result, WMD: −2.990; 95% CI: −3.642 to −2.337; I2 = 0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriou, K.; Karakasis, P.; Nasoufidou, A.; Stachteas, P.; Klisic, A.; Karagiannidis, E.; Fyntanidou, B.; Popovic, D.S.; Patoulias, D.; Antoniadis, A.P.; et al. SGLT2 Inhibitors in the Management of Cardio-Renal-Metabolic Syndrome: A New Therapeutic Era. Medicina 2025, 61, 1903. https://doi.org/10.3390/medicina61111903
Grigoriou K, Karakasis P, Nasoufidou A, Stachteas P, Klisic A, Karagiannidis E, Fyntanidou B, Popovic DS, Patoulias D, Antoniadis AP, et al. SGLT2 Inhibitors in the Management of Cardio-Renal-Metabolic Syndrome: A New Therapeutic Era. Medicina. 2025; 61(11):1903. https://doi.org/10.3390/medicina61111903
Chicago/Turabian StyleGrigoriou, Konstantinos, Paschalis Karakasis, Athina Nasoufidou, Panagiotis Stachteas, Aleksandra Klisic, Efstratios Karagiannidis, Barbara Fyntanidou, Djordje S. Popovic, Dimitrios Patoulias, Antonios P. Antoniadis, and et al. 2025. "SGLT2 Inhibitors in the Management of Cardio-Renal-Metabolic Syndrome: A New Therapeutic Era" Medicina 61, no. 11: 1903. https://doi.org/10.3390/medicina61111903
APA StyleGrigoriou, K., Karakasis, P., Nasoufidou, A., Stachteas, P., Klisic, A., Karagiannidis, E., Fyntanidou, B., Popovic, D. S., Patoulias, D., Antoniadis, A. P., & Fragakis, N. (2025). SGLT2 Inhibitors in the Management of Cardio-Renal-Metabolic Syndrome: A New Therapeutic Era. Medicina, 61(11), 1903. https://doi.org/10.3390/medicina61111903








