DeepCARS-Identified High-Risk Patients: Clinical Interventions and Outcomes During the Korean Healthcare Crisis
Abstract
1. Introduction
2. Materials and Methods
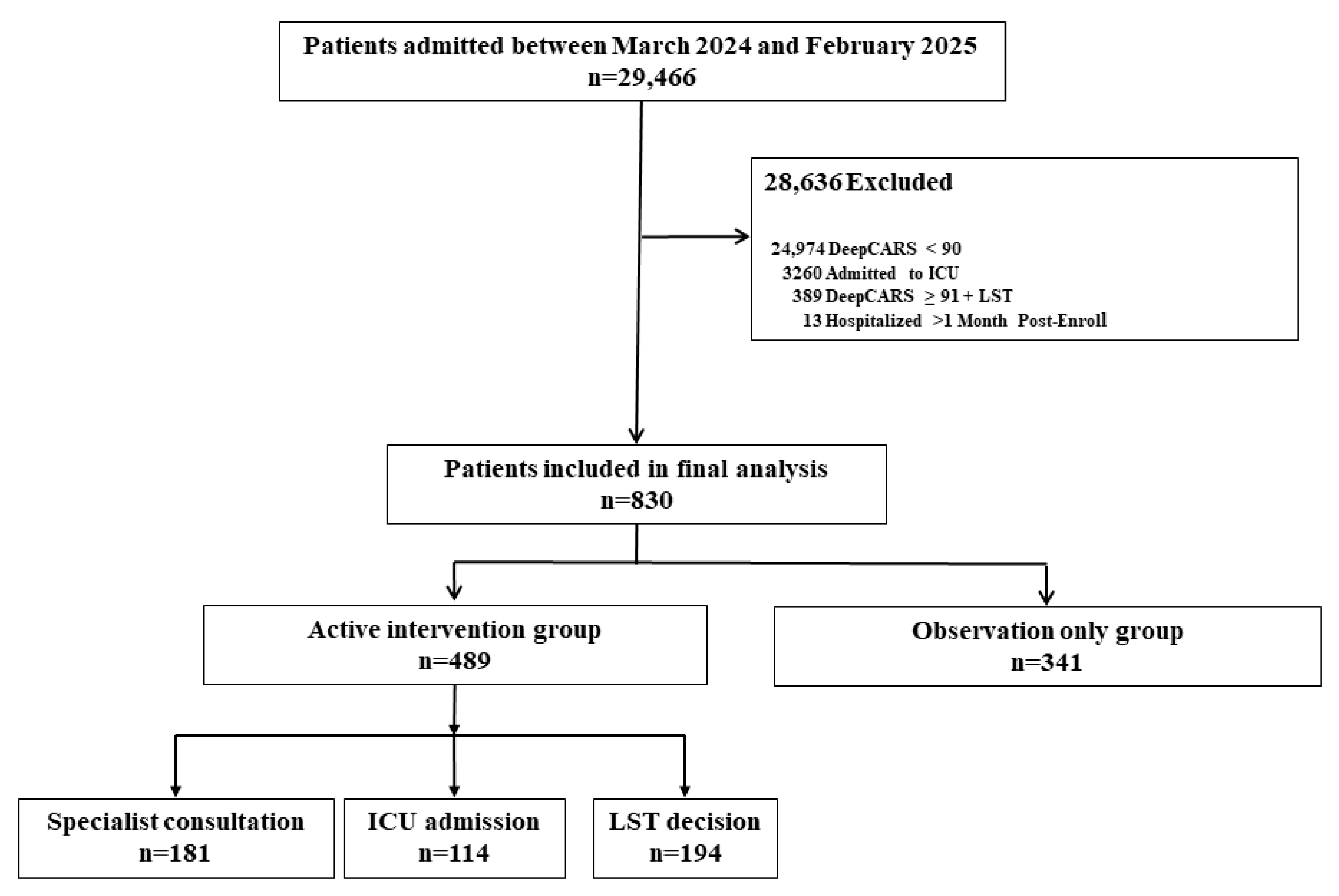
2.1. Study Design and Patient Selection
2.2. Ethics Statement
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparisons of Active Intervention and Observation Alone
3.3. Clinical Responses in Subgroups of the Active-Intervention Group
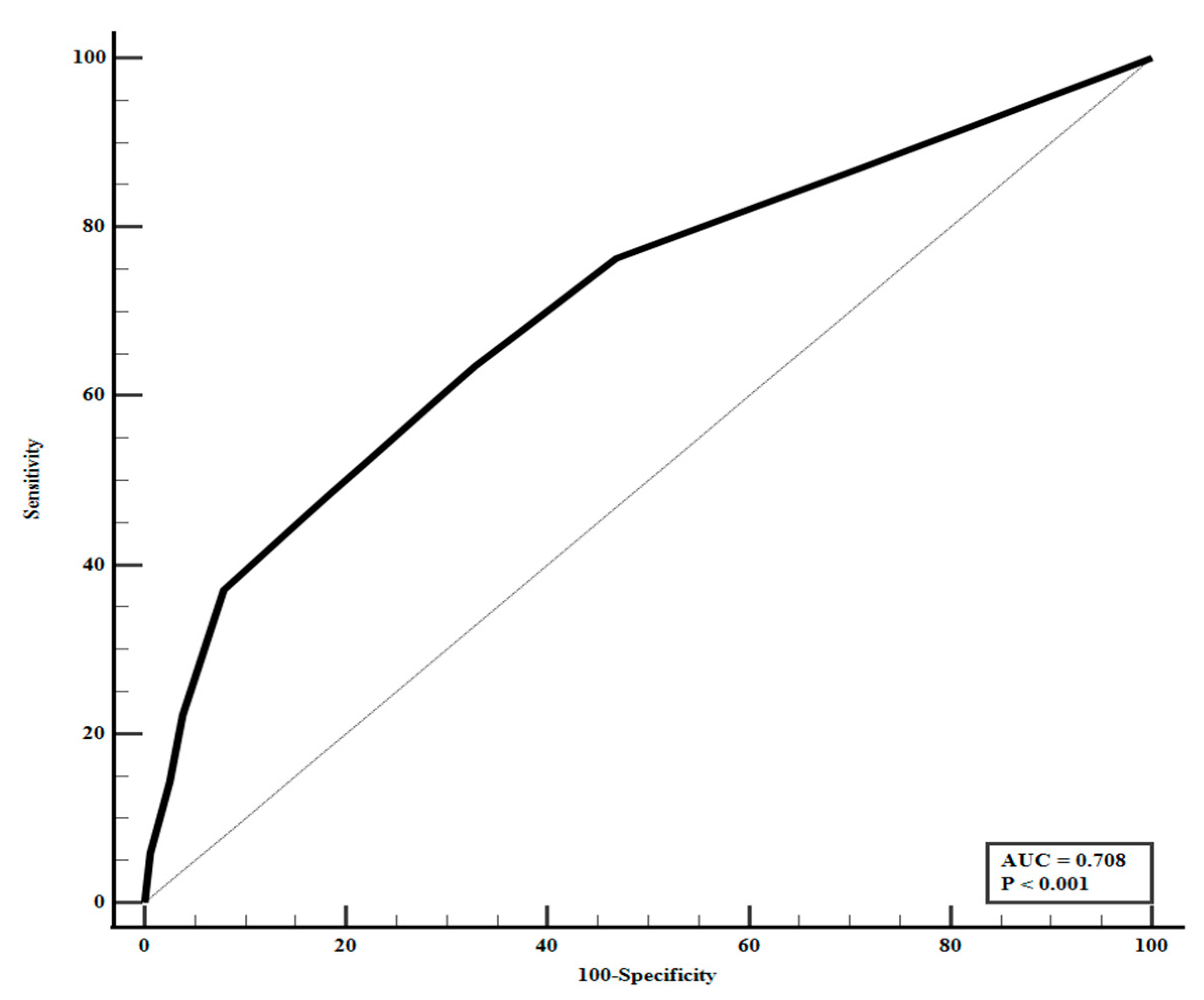
3.4. Predictors of Active Intervention in Total Patients
3.5. Predictors of Clinical Decisions Following DeepCARS Score ≥ 91
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DeepCARS | Deep learning–based Cardiac Arrest Risk Score |
| ICU | Intensive care unit |
| EWS | early warning systems |
| MEWS | Modified Early Warning Score |
| NEWS | National Early Warning Score |
| AI | artificial intelligence |
| LST | life-sustaining treatment |
| IRB | Institutional Review Board |
| ROC | receiver operating characteristic |
| OR | odds ratio |
| CI | confidence interval |
| AUC | area under the curve |
References
- Guan, G.; Lee, C.M.Y.; Begg, S.; Crombie, A.; Mnatzaganian, G. The use of early warning system scores in prehospital and emergency department settings to predict clinical deterioration: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0265559. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.; Nugawela, M.D.; Edwards, H.B.; Richards, A.; Le Roux, H.; Pullyblank, A.; Whiting, P. Can early warning scores identify deteriorating patients in pre-hospital settings? A systematic review. Resuscitation 2018, 132, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Tohira, H.; Finn, J.; Perkins, G.D.; Ho, K.M. The ability of early warning scores (EWS) to detect critical illness in the prehospital setting: A systematic review. Resuscitation 2016, 102, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Henry, K.E.; Giannini, H.M. Early warning systems for critical illness outside the intensive care unit. Crit. Care Clin. 2024, 40, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, L.A.; Dongelmans, D.A.; Elbers, P.W.G. Artificial intelligence to advance acute and intensive care medicine. Curr. Opin. Crit. Care 2024, 30, 246–250. [Google Scholar] [CrossRef] [PubMed]
- De Corte, T.; Van Hoecke, S.; De Waele, J. Artificial intelligence in infection management in the ICU. Crit. Care 2022, 26, 79. [Google Scholar] [CrossRef] [PubMed]
- Lee, K. Transforming rapid response team through artificial intelligence. Acute Crit. Care 2025, 40, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Sendak, M.P.; Gao, M.; Brajer, N.; Balu, S. Presenting machine learning model information to clinical end users with model facts labels. npj Digit. Med. 2020, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Kim, K.H.; Choi, J.; Yoo, D.; Kim, J. External validation of deep learning-based cardiac arrest risk management system for predicting in-hospital cardiac arrest in patients admitted to general wards based on rapid response system operating and nonoperating periods: A single-center study. Crit. Care Med. 2024, 52, e110–e120. [Google Scholar] [CrossRef]
- Cho, K.J.; Kim, J.S.; Lee, D.H.; Lee, S.M.; Song, M.J.; Lim, S.Y.; Cho, Y.-J.; Jo, Y.H.; Shin, Y.; Lee, Y.J. Prospective, multicenter validation of the deep learning-based cardiac arrest risk management system for predicting in-hospital cardiac arrest or unplanned intensive care unit transfer in patients admitted to general wards. Crit. Care 2023, 27, 346. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Cho, K.J.; Kwon, O.; Park, H.; Lee, Y.; Kwon, J.M.; Park, J.; Kim, J.S.; Lee, M.-J.; Kim, A.J.; et al. A multicentre validation study of the deep learning-based early warning score for predicting in-hospital cardiac arrest in patients admitted to general wards. Resuscitation 2021, 163, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Shortliffe, E.H.; Sepulveda, M.J. Clinical decision support in the era of artificial intelligence. JAMA 2018, 320, 2199–2200. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.J.; Schisterman, E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (n = 830) | Hospital Mortality | p Value | |
|---|---|---|---|---|
| Survivors (n = 637) | Non-Survivors (n = 193) | |||
| Demographics | ||||
| Age, years, mean ± SD | 69.8 ± 12.2 | 69.6 ± 12.2 | 70.4 ± 12.7 | 0.442 |
| Male sex, n (%) | 510 (61.4) | 390 (61.2) | 120 (62.2) | 0.866 |
| Admission department, n (%) | ||||
| Medical | 583 (70.2) | 448 (70.3) | 135 (69.9) | 0.929 |
| Surgical | 247 (29.8) | 189 (29.7) | 58 (30.1) | |
| Hospital length of stay, days, median (IQR) | 16 (8–29) | 15 (8–29) | 18 (7–30) | 0.507 |
| Underlying comorbidities before admission, n (%) | ||||
| Cardiovascular diseases | 534 (64.3) | 411 (64.5) | 123 (63.7) | 0.864 |
| Hemato-oncologic diseases | 513 (61.8) | 368 (57.8) | 145 (75.1) | <0.001 |
| Diabetes mellitus | 292 (35.2) | 231 (36.3) | 61 (31.6) | 0.263 |
| Chronic lung diseases | 140 (16.9) | 110 (17.3) | 30 (15.5) | 0.661 |
| Chronic kidney diseases | 113 (13.6) | 89 (14.0) | 24 (12.4) | 0.633 |
| Chronic liver diseases | 83 (10.0) | 59 (9.3) | 24 (12.4) | 0.218 |
| Neurologic diseases | 21 (2.5) | 20 (3.1) | 1 (0.5) | 0.038 |
| Immunosuppressive state | 18 (2.2) | 16 (2.5) | 2 (1.0) | 0.272 |
| Rheumatologic | 17 (2.0) | 15 (2.4) | 2 (1.0) | 0.386 |
| DeepCARS score, mean ± SD | 93.9 ± 2.3 | 93.5 ± 2.1 | 95.2 ± 2.5 | <0.001 |
| DeepCARS score ≥ 91 during after-hours duty, n (%) | 567 (68.3) | 436 (68.4) | 131 (67.9) | 0.930 |
| Characteristic | Intervention Group (n = 489) | Observation Only Group (n = 341) | p Value |
|---|---|---|---|
| Demographics | |||
| Age, years, mean ± SD | 70.3 ± 12.3 | 69.1 ± 12.1 | 0.163 |
| Male sex, n (%) | 313 (64.0) | 197 (57.8) | 0.071 |
| Admission department, n (%) | |||
| Medical | 336 (68.7) | 247 (72.4) | 0.280 |
| Surgical | 153 (31.3) | 94 (27.6) | |
| DeepCARS score ≥ 91 during after-hours duty, n (%) | 327 (66.9) | 240 (70.4) | 0.290 |
| Hospital length of stay, days, median (IQR) | 18 (9–32) | 13 (7–24) | <0.001 |
| Underlying comorbidities before admission, n (%) | |||
| Cardiovascular diseases | 312 (63.8) | 222 (65.1) | 0.713 |
| Hemato-oncologic diseases | 316 (64.6) | 197 (57.8) | 0.046 |
| Diabetes mellitus | 164 (33.5) | 128 (37.5) | 0.238 |
| Chronic lung diseases | 80 (16.4) | 60 (17.6) | 0.639 |
| Chronic kidney diseases | 62 (12.7) | 51 (15.0) | 0.356 |
| Chronic liver diseases | 59 (12.1) | 24 (7.0) | 0.019 |
| Neurologic diseases | 11 (2.2) | 10 (2.9) | 0.654 |
| Immunosuppressive state | 10 (2.0) | 8 (1.0) | 0.811 |
| Rheumatologic | 10 (2.0) | 7 (2.1) | >0.999 |
| DeepCARS score, mean ± SD | 94.6 ± 2.4 | 92.9 ± 1.8 | <0.001 |
| Vital signs at DeepCARS score ≥ 91, median (IQR) (a) | |||
| Systolic blood pressure, mmHg (n = 464/n = 310) | 107 (90–127) | 100 (90–120) | 0.183 |
| Diastolic blood pressure, mmHg (n = 464/n = 309) | 61 (54–80) | 60 (52–72) | 0.338 |
| Pulse rate, /min (n = 461/n = 307) | 114 (101–128) | 110 (98–121) | <0.001 |
| Respiratory rate, /min (n = 454/n = 280) | 24 (20–28) | 20 (20–22) | <0.001 |
| Body temperature (axilla), °C (n = 451/n = 287) | 36.5 (36.3–37.2) | 36.5 (36.3–37.1) | 0.725 |
| SpO2, % (n = 453/n = 272) | 96 (95–98) | 97 (95–98) | 0.929 |
| In-hospital mortality, n (%) | 187 (38.2) | 6 (1.8) | <0.001 |
| Characteristic | Consultation Only (n = 181) | ICU Transfer (n = 114) | Life-Sustaining Treatment Decision (n = 194) |
|---|---|---|---|
| Demographics | |||
| Age, years, mean ± SD | 69.9 ± 12.8 | 68.6 ± 13.9 | 71.5 ± 10.8 * |
| Male sex, n (%) | 113 (62.4) | 79 (69.3) | 121 (62.4) |
| Admission department: medical, n (%) | 118 (65.2) | 60 (52.6) | 158 (81.4) * |
| Hospital length of stay, median (IQR) | 17 (10–35) | 24 (14–35) * | 14 (7–28) |
| Underlying comorbidities before admission, n (%) | |||
| Cardiovascular diseases | 116 (64.1) | 81 (71.1) | 115 (59.3) |
| Hemato-oncologic diseases | 99 (54.7) | 66 (57.9) | 151 (77.8) * |
| Diabetes mellitus | 65 (35.9) | 35 (30.7) | 64 (33.0) |
| Chronic lung diseases | 24 (13.3) | 20 (17.5) | 36 (18.6) |
| Chronic kidney diseases | 19 (10.6) | 21 (18.4) | 22 (11.3) |
| Chronic liver diseases | 23 (12.7) | 16 (14.0) | 20 (10.3) |
| Neurologic diseases | 3 (1.7) | 4 (3.5) | 4 (2.1) |
| Rheumatologic | 3 (1.7) | 4 (3.5) | 3 (1.5) |
| Immunosuppressive state | 7 (3.9) | 2 (0.4) | 1 (0.2) |
| DeepCARS score, mean ± SD | 94.0 ± 2.2 | 95.1 ± 2.5 * | 94.8 ± 1.4 * |
| Vital signs at DeepCARS score ≥ 91, median (IQR) (a) | |||
| Systolic blood pressure, mmHg (n = 464/n = 310) | 110 (94–122) | 100 (90–130) | 109 (90–127) |
| Diastolic blood pressure, mmHg (n = 464/n = 309) | 63 (57–80) | 61 (53–80) | 60 (52–77) |
| Pulse rate, /min (n = 461/n = 307) | 113 (98–127) | 116 (102–132) | 115 (102–125) |
| Respiratory rate, /min (n = 454/n = 280) | 22 (20–25) | 26 (22–30) * | 24 (20–28) |
| Body temperature (axilla), °C (n = 451/n = 287) | 36.5 (36.3–37.4) | 36.3 (36.3–37.0) | 36.5 (36.3–37.1) |
| SpO2, % (n = 453/n = 272) | 97 (95–99) | 96 (95–99) | 96 (95–98) |
| DeepCARS score ≥ 91 during after-hours duty, n (%) | 120 (66.3) | 72 (63.2) | 135 (69.6) |
| Hospital mortality, n (%) | 6 (3.3) | 54 (47.4) | 127 (65.5) * |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| DeepCARS Score ≥ 94 | 3.572 (2.669–4.782) | <0.001 | 3.517 (2.623–4.716) | <0.001 |
| Chronic liver diseases | 1.812 (1.103–2.977) | 0.019 | 1.782 (1.061–2.994) | 0.029 |
| Hemato-oncologic diseases | 1.335 (1.005–1.773) | 0.046 | ||
| Variables | ICU Transfer vs. Consultation Only (Ref = Consultation Only) | ICU Transfer vs. LST Decision (Ref = LST Decision) | LST Decision vs. Consultation Only (Ref = Consultation Only) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Admission department: Medical | 0.479 (0.272–0.844) | 0.011 | 0.237 (0.132–0.424) | <0.001 | 1.996 (1.161–3.433) | 0.012 |
| Male sex | 1.310 (0.730–2.352) | 0.366 | 1.770 (0.990–3.165) | 0.054 | 0.736 (0.453–1.195) | 0.215 |
| Comorbidity: Cardiovascular diseases | 2.215 (1.140–4.302) | 0.019 | 1.723 (0.910–3.262) | 0.095 | 1.268 (0.743–2.165) | 0.383 |
| Comorbidity: Hemato-oncologic diseases | 1.929 (1.061–3.508) | 0.031 | 0.538 (0.289–1.003) | 0.051 | 3.576 (2.071–6.174) | <0.001 |
| Comorbidity: Diabetes mellitus | 0.696 (0.383–1.263) | 0.233 | 0.794 (0.439–1.439) | 0.447 | 0.878 (0.530–1.456) | 0.614 |
| Comorbidity: Chronic lung diseases | 1.065 (0.489–2.318) | 0.875 | 0.813 (0.388–1.704) | 0.583 | 1.254 (0.649–2.422) | 0.501 |
| Comorbidity: Chronic kidney diseases | 2.200 (0.981–4.936) | 0.056 | 1.780 (0.801–3.955) | 0.157 | 1.240 (0.575–2.673) | 0.583 |
| Comorbidity: Chronic liver diseases | 1.223 (0.486–3.079) | 0.669 | 1.629 (0.665–3.990) | 0.285 | 0.765 (0.357–1.640) | 0.583 |
| Comorbidity: Neurologic diseases | 3.003 (0.368–24.522) | 0.305 | 1.275 (0.183–8.866) | 0.806 | 2.508 (0.375–16.773) | 0.343 |
| Comorbidity: Immunosuppressive state | 0.740 (0.124–4.430) | 0.742 | 3.094 (0.232–41.237) | 0.393 | 0.246 (0.028–2.166) | 0.206 |
| Comorbidity: Rheumatologic diseases | 3.140 (0.451–21.885) | 0.248 | 1.695 (0.299–9.610) | 0.551 | 1.832 (0.276–12.163) | 0.531 |
| Age, year | 0.990 (0.967–1.013) | 0.393 | 0.978 (0.954–1.001) | 0.065 | 1.013 (0.992–1.035) | 0.235 |
| Mean blood pressure, mmHg | 0.984 (0.968–1.000) | 0.055 | 1.000 (0.984–1.016) | 0.997 | 0.984 (0.970–0.988) | 0.030 |
| Pulse rate, /min | 1.016 (1.002–1.148) | 0.022 | 1.009 (0.996–1.022) | 0.178 | 1.006 (0.994–1.019) | 0.313 |
| Respiratory rate, /min | 1.098 (1.051–1.148) | <0.001 | 1.033 (0.997–1.070) | 0.075 | 1.064 (1.021–1.109) | 0.003 |
| Body temperature (axilla), °C | 1.003 (0.836–1.203) | 0.974 | 0.996 (0.825–1.202) | 0.968 | 1.003 (0.894–1.125) | 0.961 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Yoo, W.; Hwang, S.; Lee, K. DeepCARS-Identified High-Risk Patients: Clinical Interventions and Outcomes During the Korean Healthcare Crisis. Medicina 2025, 61, 1896. https://doi.org/10.3390/medicina61111896
Jang H, Yoo W, Hwang S, Lee K. DeepCARS-Identified High-Risk Patients: Clinical Interventions and Outcomes During the Korean Healthcare Crisis. Medicina. 2025; 61(11):1896. https://doi.org/10.3390/medicina61111896
Chicago/Turabian StyleJang, Hyojin, Wanho Yoo, Sora Hwang, and Kwangha Lee. 2025. "DeepCARS-Identified High-Risk Patients: Clinical Interventions and Outcomes During the Korean Healthcare Crisis" Medicina 61, no. 11: 1896. https://doi.org/10.3390/medicina61111896
APA StyleJang, H., Yoo, W., Hwang, S., & Lee, K. (2025). DeepCARS-Identified High-Risk Patients: Clinical Interventions and Outcomes During the Korean Healthcare Crisis. Medicina, 61(11), 1896. https://doi.org/10.3390/medicina61111896





