Cardiac Surgery and Postoperative Atrial Fibrillation: The Role of Cancer
Abstract
1. Introduction
2. Epidemiology
3. Pathophysiology of AF
3.1. Atrial Myopathy as an AF Substrate
3.2. Role of Inflammation and Oxidative Stress
3.3. Impaired Proteostasis in AF
3.4. Neurocardiac Remodeling and Autonomic Dysfunction
3.5. Genetic Factors
3.6. Cancer and Specific Factors
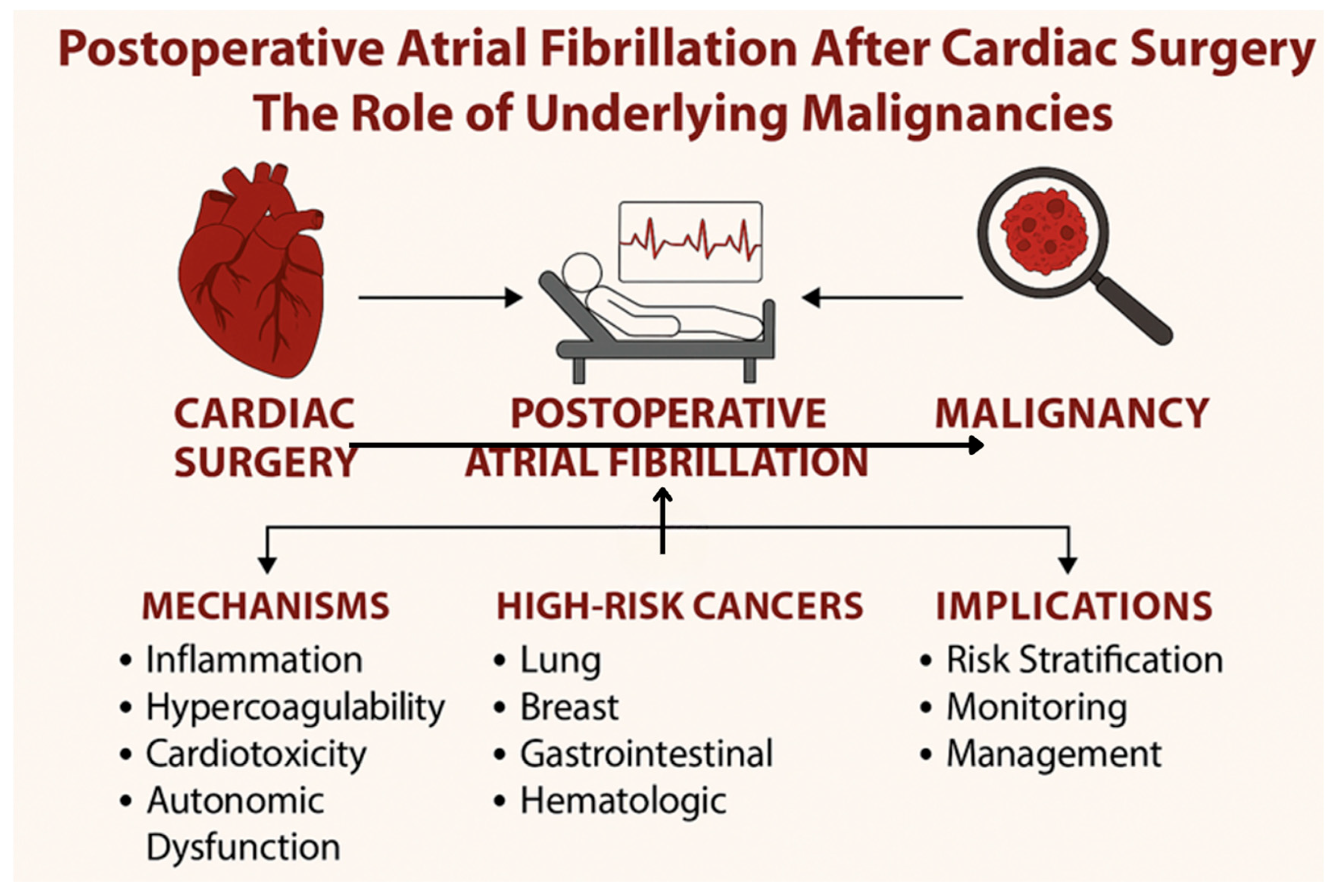
4. Clinical Implications and Risk Stratification
Temporal and Prognostic Interplay Between AF and Malignancy
5. Management in Cancer Patients
Medical Treatment
6. AF Triggers, Substrates, and Ablation Strategies
7. Durability and Recurrence of AF After PVI
8. Conclusions, Future Directions and Research Gap
- Hematologic malignancies, including multiple myeloma (adjusted subdistribution hazard ratio [aHR]: 3.34), leukemia (aHR: 2.64), and lymphoma (aHR: 2.29), have been consistently associated with markedly increased AF risk compared with non-cancer controls [53].
- Integrated Predictive Models
- ○
- Develop and validate multivariable risk models that incorporate oncologic parameters (tumor type, stage, treatment history, systemic inflammation, prothrombotic state) alongside traditional cardiac variables such as age, left atrial enlargement, heart failure, and hypertension [83].
- ○
- Emulate efforts like the “POAF Score + biomarker panel” approach, which significantly improved prediction over clinical factors alone [84].
- Prospective Cohorts & Registries
- ○
- Launch multicenter, prospective studies with standardized definitions and detailed phenotyping (cancer type, timeline, treatment exposures).
- ○
- Build dedicated cardio-oncology surgical registries to generate real-world evidence and support TRIPOD-compliant model development [85].
- Biomarker Discovery & Validation
- ○
- Investigate serum and pericardial biomarkers—such as BNP, troponins, microRNAs, extracellular vesicles, circulating tumor DNA (ctDNA), systemic immune-inflammation index (SII), and others—for early identification of cardiac stress and arrhythmogenic remodeling.
- Mechanistic Translational Research
- ○
- Conduct preclinical, cellular, and electrophysiologic studies to explore cancer-mediated atrial vulnerabilities, including inflammation, fibrosis, connexin dysregulation, oxidative stress, and autonomic imbalance.
- Interventional Trials in High-Risk Patients
- Tailored Perioperative Protocols
- ○
- Create perioperative care algorithms specific to cancer patients, incorporating optimal surgical timing around chemotherapy, fluid/electrolyte balance, drainage strategies, and thromboprophylaxis.
- ○
- Employ multidisciplinary preoperative cardio-oncology evaluations to individualize management.
- Long-Term Outcomes & Survivorship Monitoring
- ○
- Study the long-term impact of POAF on recurrence-free survival, cardiovascular and thromboembolic events, and overall survival in cancer survivors. NOAF is associated with >3-fold increased risk of chronic AF [79].
- Digital Health & ML-Driven Risk Tools
- ○
- Incorporate wearable monitoring, ECG-based deep learning, and administrative data into real-time perioperative risk mitigation systems [80].
- Closer postoperative surveillance of patients with a history of cancer or active malignancy, particularly following high-risk procedures such as valve or combined surgeries.
- Consideration of targeted cancer screening in patients with new-onset POAF after cardiac surgery, especially if other risk factors are absent, as arrhythmia may occasionally be a marker of occult malignancy.
- Integration of POAF into oncologic risk assessment—clinicians should recognize POAF as a potential “red flag” event that warrants multidisciplinary discussion, including cardiology and oncology input.
- Individualized perioperative management, including optimization of inflammation and fluid balance, and careful adjustment of antiarrhythmic or anticoagulation strategies in patients with concurrent cancer.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georghiou, G.P.; Xanthopoulos, A.; Kanellopoulos, G.; Georghiou, P.; Georgiou, A.; Skoularigis, J.; Giamouzis, G.; Lampropoulos, K.; Patrikios, I.; Triposkiadis, F. Cancer Is a Major Determinant of Postoperative Atrial Fibrillation After Cardiac Surgery. J Clin. Med. 2025, 14, 2117. Available online: https://pubmed.ncbi.nlm.nih.gov/40142925/ (accessed on 25 June 2025). [CrossRef] [PubMed]
- Nardi, E.; Santoro, C.; Prastaro, M.; Canonico, M.E.; Paolillo, S.; Gargiulo, G.; Gargiulo, G.; Gargiulo, P.; Parlati, A.L.M.; Basile, C.; et al. Crosslink between atrial fibrillation and cancer: A therapeutic conundrum. Cardio Oncol. 2024, 10, 1–15. [Google Scholar] [CrossRef]
- Zhao, J.; Bhatnagar, V.; Ding, L.; Atay, S.M.; David, E.A.; Michael McFadden, P.; Stamnes, S.; Lechtholz-Zey, E.; Wightman, S.C.; Detterbeck, F.C.; et al. A systematic review of paraneoplastic syndromes associated with thymoma: Treatment modalities, recurrence, and outcomes in resected cases. Cardiovasc. Surg. 2020, 160, 306–320. [Google Scholar] [CrossRef]
- Hu, Y.F.; Chen, Y.J.; Lin, Y.J.; Chen, S.A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. Available online: https://pubmed.ncbi.nlm.nih.gov/25622848/ (accessed on 25 June 2025). [CrossRef] [PubMed]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. Available online: https://academic.oup.com/eurheartj/advance-article-abstract/doi/10.1093/eurheartj/ehy136/4942493 (accessed on 13 July 2025). [CrossRef]
- Erichsen, R.; Christiansen, C.F.; Mehnert, F.; Weiss, N.S.; Baron, J.A.; Sørensen, H.T. Colorectal cancer and risk of atrial fibrillation and flutter: A population-based case-control study. Intern. Emerg. Med. 2012, 7, 431–438. Available online: https://pubmed.ncbi.nlm.nih.gov/21968511/ (accessed on 13 July 2025). [CrossRef]
- Eikelboom, R.; Sanjanwala, R.; Le, M.L.; Yamashita, M.H.; Arora, R.C. Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 11, 544–554. Available online: https://pubmed.ncbi.nlm.nih.gov/32687821/ (accessed on 25 June 2025). [CrossRef] [PubMed]
- Boriani, G.; Mantovani, M.; Cherubini, B.; Tartaglia, E.; Bonini, N. Gestione e trattamento della fibrillazione atriale nel paziente con neoplasia: Uno snodo decisionale importante nell’ambito della cardio-oncologia. G Ital. Cardiol. 2024, 25, 346–349. Available online: https://pubmed.ncbi.nlm.nih.gov/38639125/ (accessed on 13 July 2025).
- Van Gelder, I.C.; Kotecha, D.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart. J. 2024, 45, 3314–3414. Available online: https://pubmed.ncbi.nlm.nih.gov/39210723/ (accessed on 25 September 2025). [CrossRef]
- Gillinov, A.M.; Bagiella, E.; Moskowitz, A.J.; Raiten, J.M.; Groh, M.A.; Bowdish, M.E.; Ailawadi, G.; Kirkwood, K.A.; Perrault, L.P.; Parides, M.K.; et al. Rate Control versus Rhythm Control for Atrial Fibrillation after Cardiac Surgery. N. Engl. J. Med. 2016, 374, 1911–1921. Available online: https://pubmed.ncbi.nlm.nih.gov/27043047/ (accessed on 25 September 2025). [CrossRef]
- Zhou, M.; Wang, H.; Chen, J.; Zhao, L. Epicardial adipose tissue and atrial fibrillation: Possible mechanisms, potential therapies, and future directions. PACE Pacing Clin. Electrophysiol. 2020, 43, 133–145. Available online: https://pubmed.ncbi.nlm.nih.gov/31682014/ (accessed on 13 July 2025). [CrossRef] [PubMed]
- Chen, G.; Zhou, H.; Wang, C.; Qin, R.; Yang, Q.; Hou, Y.; Zhang, M.; Zhang, C.; Wang, N.; Feng, Y. Chemotherapy cardiotoxicity research in cancer patients: a bibliometric and visual analysis (1994–2024). Front. Oncol. 2025, 15, 1502361. [Google Scholar] [CrossRef]
- Scheggi, V.; Menale, S.; Marcucci, R.; Dematté, A.; Giovacchini, J.; Cenni, N.; Vitale, G.; Alterini, B.; Salvicchi, A.; Tamburini, M.; et al. Postoperative atrial fibrillation after thoracic surgery (PoAF): Risk factors and outcome. Cardiothorac. Surg. 2023, 31, 1–7. Available online: https://cts.springeropen.com/articles/10.1186/s43057-023-00109-7 (accessed on 13 July 2025). [CrossRef]
- Murtaza, M.; Baig, M.M.A.; Ahmed, J.; Serbanoiu, L.I.; Busnatu, S.S. Higher Mortality Associated with New-Onset Atrial Fibrillation in Cancer Patients: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 867002. [Google Scholar] [CrossRef]
- Xing, Y.; Yan, L.; Li, X.; Xu, Z.; Wu, X.; Gao, H.; Chen, Y.; Ma, X.; Liu, J.; Zhang, J. The relationship between atrial fibrillation and NLRP3 inflammasome: A gut microbiota perspective. Front. Immunol. 2023, 14, 1273524. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10703043/ (accessed on 13 July 2025). [CrossRef]
- Osranek, M.; Fatema, K.; Qaddoura, F.; Al-Saileek, A.; Barnes, M.E.; Bailey, K.R.; Gersh, B.J.; Tsang, T.S.; Zehr, K.J.; Seward, J.B. Left Atrial Volume Predicts the Risk of Atrial Fibrillation After Cardiac Surgery. A Prospective Study. J. Am. Coll. Cardiol. 2006, 48, 779–786. Available online: https://pubmed.ncbi.nlm.nih.gov/16904549/ (accessed on 26 June 2025). [CrossRef]
- Tufano, A.; Coppola, A. How to manage anticoagulation for cancer-associated thrombosis and atrial fibrillation in cancer. Thromb. Update 2024, 15, 100169. Available online: https://www.sciencedirect.com/science/article/pii/S2666572724000117 (accessed on 13 July 2025). [CrossRef]
- Yuan, M.; Zhang, Z.; Tse, G.; Feng, X.; Korantzopoulos, P.; Letsas, K.P.; Yan, B.P.; Wu, W.K.K.; Zhang, H.; Li, G.; et al. Association of Cancer and the Risk of Developing Atrial Fibrillation: A Systematic Review and Meta-Analysis. Cardiol. Res. Pract. 2019, 2019, 8985273. Available online: https://pubmed.ncbi.nlm.nih.gov/31110819/ (accessed on 25 June 2025). [CrossRef]
- Menichelli, D.; Gazzaniga, G.; Pannunzio, A.; Palumbo, I.M.; Pani, A.; Pignatelli, P.; Valeriani, E.; Pastori, D. Risk of New-Onset Atrial Fibrillation in Opioid Users: A Systematic Review and Meta-Analysis on 24,006,367 Participants. Drug Saf. 2025, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zuo, Z.; Wang, X.; Sun, Y.; Xu, D.; Liu, G.; Tong, Y.; Zhang, Z. Epidemiology, risk factors and mechanism of breast cancer and atrial fibrillation. Cardio Oncol. 2024, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gulizia, M.M.; Turazza, F.M.; Ameri, P.; Alings, M.; Collins, R.; De Luca, L.; Di Nisio, M.; Lucci, D.; Gabrielli, D.; Janssens, S.; et al. Characteristics and Management of Patients with Cancer and Atrial Fibrillation: The BLITZ-AF Cancer Registry. JACC Adv. 2024, 3, 100991. [Google Scholar] [CrossRef] [PubMed]
- Vinter, N.; Christesen, A.M.S.; Fenger-Grøn, M.; Tjønneland, A.; Frost, L. Atrial Fibrillation and Risk of Cancer: A Danish Population-Based Cohort Study. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2018, 7, e009543. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6201425/ (accessed on 13 July 2025). [CrossRef] [PubMed]
- Gjerstorff, M.L. The Danish cancer registry. Scand. J. Public Health. 2011, 39, 42–45. [Google Scholar] [CrossRef]
- Werring, M.; Patel, D.; Moores, L. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. Available online: https://doi.org/10.1016/j.chest.2018.07.040 (accessed on 14 July 2025).
- Yun, J.P.; Choi, E.K.; Han, K.-D.; Park, S.H.; Jung, J.H.; Park, S.H.; Ahn, H.-J.; Lim, J.-H.; Lee, S.-R.; Oh, S. Risk of Atrial Fibrillation According to Cancer Type: A Nationwide Population-Based Study. JACC CardioOncol. 2021, 3, 221–232. Available online: https://pubmed.ncbi.nlm.nih.gov/34396327/ (accessed on 27 June 2025). [CrossRef]
- Conen, D.; Wong, J.A.; Sandhu, R.K.; Cook, N.R.; Lee, I.M.; Buring, J.E.; Albert, C.M. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016, 1, 389–396. Available online: https://pubmed.ncbi.nlm.nih.gov/27438314/ (accessed on 27 June 2025). [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 847012. [Google Scholar] [CrossRef]
- McMullen, J.R.; Boey, E.J.H.; Ooi, J.Y.Y.; Seymour, J.F.; Keating, M.J.; Tam, C.S. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 2014, 124, 3829–3830. Available online: https://pubmed.ncbi.nlm.nih.gov/25498454/ (accessed on 13 July 2025). [CrossRef]
- Ding, W.Y.; Harrison, S.; Gupta, D.; Lip, G.Y.H.; Lane, D.A. Stroke and Bleeding Risk Assessments in Patients with Atrial Fibrillation: Concepts and Controversies. Front. Med. 2020, 7, 54. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017, 14, e275–e444. Available online: https://pubmed.ncbi.nlm.nih.gov/28506916/ (accessed on 14 July 2025). [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805. Available online: https://pubmed.ncbi.nlm.nih.gov/23525094/ (accessed on 14 July 2025). [CrossRef]
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef]
- Olesen, M.S.; Bentzen, B.H.; Nielsen, J.B.; Steffensen, A.B.; David, J.P.; Jabbari, J.; Jensen, H.K.; Haunsø, S.; Svendsen, J.H.; Schmitt, N. Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation. BMC Med. Genet. 2012, 13, 24. Available online: https://pubmed.ncbi.nlm.nih.gov/22471742/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Obel, N.; Thomsen, H.F.; Kronborg, G.; Larsen, C.S.; Hildebrandt, P.R.; Sørensen, H.T.; Gerstoft, J. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clin. Infect. Dis. 2007, 44, 1625–1631. Available online: https://pubmed.ncbi.nlm.nih.gov/17516408/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Fuster, V.; Rydén, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Le Heuzey, J.-Y.; Kay, G.N.; Lowe, J.E.; et al. 2011 ACCF/AHA/HRS Focused Updates Incorporated Into the ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2011, 57, e101–e198. Available online: https://pdf/10.1016/j.jacc.2010.09.013?download=true (accessed on 14 July 2025). [PubMed]
- Liu, D.; Han, X.; Zhang, Z.; Tse, G.; Shao, Q.; Liu, T. Role of Heat Shock Proteins in Atrial Fibrillation: From Molecular Mechanisms to Diagnostic and Therapeutic Opportunities. Cells 2023, 12, 151. Available online: https://www.mdpi.com/2073-4409/12/1/151/htm (accessed on 14 July 2025). [CrossRef]
- Chen, P.S.; Tan, A.Y. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. Off. J. Heart Rhythm. Soc. 2006, 4, S61. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC1852524/ (accessed on 14 July 2025). [CrossRef]
- Chen, Y.H.; Xu, S.J.; Bendahhou, S.; Wang, X.L.; Wang, Y.; Xu, W.Y.; Jin, H.-W.; Sun, H.; Su, X.-Y.; Zhuang, Q.-N.; et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 2003, 299, 251–254. Available online: https://pubmed.ncbi.nlm.nih.gov/12522251/ (accessed on 14 July 2025). [CrossRef]
- Roselli, C.; Chaffin, M.D.; Weng, L.C.; Aeschbacher, S.; Ahlberg, G.; Albert, C.M.; Almgren, P.; Alonso, A.; Anderson, C.D.; Aragam, K.G.; et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 2018, 50, 1225–1233. Available online: https://pubmed.ncbi.nlm.nih.gov/29892015/ (accessed on 14 July 2025). [CrossRef]
- Nielsen, J.B.; Thorolfsdottir, R.B.; Fritsche, L.G.; Zhou, W.; Skov, M.W.; Graham, S.E.; Herron, T.J.; McCarthy, S.; Schmidt, E.M.; Sveinbjornsson, G.; et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018, 50, 1234–1239. Available online: https://pubmed.ncbi.nlm.nih.gov/30061737/ (accessed on 14 July 2025). [CrossRef]
- Shoemaker, M.B.; Husser, D.; Roselli, C.; Al Jazairi, M.; Chrispin, J.; Kühne, M.; Neumann, B.; Knight, S.; Sun, H.; Mohanty, S.; et al. Genetic Susceptibility for Atrial Fibrillation in Patients Undergoing Atrial Fibrillation Ablation. Circ. Arrhythm. Electrophysiol. 2020, 13, e007676. Available online: https://pubmed.ncbi.nlm.nih.gov/32078373/ (accessed on 14 July 2025). [CrossRef]
- Gudbjartsson, D.F.; Holm, H.; Gretarsdottir, S.; Thorleifsson, G.; Walters, G.B.; Thorgeirsson, G.; Gulcher, J.; Mathiesen, E.B.; Njølstad, I.; Nyrnes, A.; et al. A sequence variant in ZFHX3 on 16q22 associates with a trial fibrillation and ischemic stroke. Nat. Genet. 2009, 41, 876–878. Available online: https://pubmed.ncbi.nlm.nih.gov/19597491/ (accessed on 14 July 2025). [CrossRef]
- Ellinor, P.T.; Lunetta, K.L.; Glazer, N.L.; Pfeufer, A.; Alonso, A.; Chung, M.K.; Sinner, M.; De Bakker, P.; Mueller, M.; Lubitz, S.; et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat. Genet. 2010, 42, 240–244. Available online: https://pubmed.ncbi.nlm.nih.gov/20173747/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Tamargo, J.; Caballero, R.; Delpón, E. Cancer Chemotherapy and Cardiac Arrhythmias: A Review. Drug Saf. 2015, 38, 129–152. Available online: https://pubmed.ncbi.nlm.nih.gov/25577497/ (accessed on 14 July 2025). [CrossRef]
- Dean, Y.E.; Dahshan, H.; Motawea, K.R.; Khalifa, Z.; Tanas, Y.; Rakha, I.; Hasan, W.; Kishk, M.; Mahmoud, A.; Elsayed, A.; et al. Anthracyclines and the risk of arrhythmias: A systematic review and meta-analysis. Medicine 2023, 102, E35770. Available online: https://pubmed.ncbi.nlm.nih.gov/37986405/ (accessed on 25 June 2025). [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Bretagne, M.; Lebrun-Vignes, B.; Groarke, J.D.; Johnson, D.B.; Yang, T.; Reddy, N.M.; Funck-Brentano, C.; Brown, J.R.; et al. Cardiovascular Toxicities Associated with Ibrutinib. J. Am. Coll. Cardiol. 2019, 74, 1667–1678. Available online: https://pubmed.ncbi.nlm.nih.gov/31558250/ (accessed on 14 July 2025). [CrossRef]
- Lenneman, C.G.; Sawyer, D.B. Cardio-oncology: An update on cardiotoxicity of cancer-related treatment. Circ. Res. 2016, 118, 1008–1020. Available online: https://www.ahajournals.org/doi/pdf/10.1161/CIRCRESAHA.115.303633?download=true (accessed on 11 July 2025). [CrossRef] [PubMed]
- Turagam, M.K.; Mirza, M.; Werner, P.H.; Sra, J.; Kress, D.C.; Tajik, A.J.; Jahangir, A. Circulating biomarkers predictive of postoperative atrial fibrillation. Cardiol. Rev. 2016, 24, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Frantz, S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ. Res. 2015, 116, 354–367. Available online: https://pubmed.ncbi.nlm.nih.gov/25593279/ (accessed on 14 July 2025). [CrossRef]
- Pirozzi, F.; Poto, R.; Aran, L.; Cuomo, A.; Galdiero, M.R.; Spadaro, G.; Abete, P.; Bonaduce, D.; Marone, G.; Tocchetti, C.G.; et al. Cardiovascular Toxicity of Immune Checkpoint Inhibitors: Clinical Risk Factors. Curr. Oncol. Rep. 2021, 23, 13. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7790474/ (accessed on 14 July 2025). [CrossRef]
- Oancea, A.F.; Morariu, P.C.; Godun, M.; Dobreanu, S.D.; Mihnea, M.; Iosep, D.G.; Buburuz, A.M.; Mitu, O.; Burlacu, A.; Floria, D.-E.; et al. Atrial Fibrillation Risk Scores as Potential Predictors of Significant Coronary Artery Disease in Chronic Coronary Syndrome: A Novel Diagnostic Approach. Life 2025, 15, 1134. Available online: https://www.mdpi.com/2075-1729/15/7/1134/htm (accessed on 1 September 2025). [CrossRef]
- Alonso, A.; Krijthe, B.P.; Aspelund, T.; Stepas, K.A.; Pencina, M.J.; Moser, C.B.; Sinner, M.F.; Sotoodehnia, N.; Fontes, J.D.; Janssens, A.C.J.W.; et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The CHARGE-AF consortium. J. Am. Heart Assoc. 2013, 2, e000102. Available online: https://pubmed.ncbi.nlm.nih.gov/23537808/ (accessed on 14 July 2025). [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Vasan, R.S.; Leip, E.P.; Wolf, P.A.; D’Agostino, R.B.; Murabito, J.M.; Kannel, W.B.; Benjamin, E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation 2003, 107, 2920–2925. Available online: https://www.ahajournals.org/doi/pdf/10.1161/01.CIR.0000072767.89944.6E?download=true (accessed on 25 September 2025). [CrossRef] [PubMed]
- Sánchez, F.J.; Pueyo, E.; Diez, E.R. Strain Echocardiography to Predict Postoperative Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1355. Available online: https://pubmed.ncbi.nlm.nih.gov/35163278/ (accessed on 25 September 2025). [CrossRef]
- Gong, M.; Cheung, A.; Wang, Q.S.; Li, G.; Goudis, C.A.; Bazoukis, G.; Lip, G.Y.H.; Baranchuk, A.; Korantzopoulos, P.; Letsas, K.P.; et al. Galectin-3 and risk of atrial fibrillation: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23104. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.M.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-Adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [PubMed]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, Y.; Dong, J.; Wang, N.; Zhan, Z.; Zhao, Y.; Jiang, S. Assessment of Risk Factors for Drug Resistance of Dual Anti Platelet Therapy After PCI. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221083674. [Google Scholar] [CrossRef]
- Ozkan, M.; Tatar, S.; Serhat Tokgöz, O. RESEARCH Open Access Diastolic global longitudinal strain and acute ischemic stroke: A hidden relationship? BMC Cardiovasc. Disord. 2025, 25, 1–12. Available online: https://doi.org/10.1186/s12872-025-04841-2 (accessed on 14 July 2025).
- Narayan, S.M.; Krummen, D.E.; Shivkumar, K.; Clopton, P.; Rappel, W.J.; Miller, J.M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation with or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012, 60, 628–636. [Google Scholar] [CrossRef]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular disease among survivors of adult-onset cancer: A community-based retrospective cohort study. J. Clin. Oncol. 2016, 34, 1122–1130. Available online: https://pubmed.ncbi.nlm.nih.gov/26834065/ (accessed on 25 June 2025). [CrossRef]
- Koniari, I.; Apostolakis, E.; Rogkakou, C.; Baikoussis, N.G.; Dougenis, D. Pharmacologic prophylaxis for atrial fibrillation following cardiac surgery: A systematic review. J. Cardiothorac. Surg. 2010, 5, 121. [Google Scholar] [CrossRef]
- Totzeck, M.; Schuler, M.; Stuschke, M.; Heusch, G.; Rassaf, T. Cardio-oncology—Strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019, 280, 163–175. Available online: https://pubmed.ncbi.nlm.nih.gov/30661849/ (accessed on 13 July 2025). [CrossRef] [PubMed]
- Chung, M.K. Atrial fibrillation after cardiac surgery. Atr. Fibrillation 2004, 226, 279–337. Available online: https://pubmed.ncbi.nlm.nih.gov/9351718/ (accessed on 25 June 2025).
- Masuda, Y.; Luo, H.D.; Kang, G.S.; Teoh, K.L.K.; Kofidis, T. Meta-analysis of the benefit of beta-blockers for the reduction of isolated atrial fibrillation incidence after cardiac surgery. JTCVS Open. 2020, 3, 66–85. Available online: https://pubmed.ncbi.nlm.nih.gov/36003876/ (accessed on 20 July 2025). [CrossRef]
- Ali, I.M.; Sanalla, A.A.; Clark, V. Beta-blocker effects on postoperative atrial fibrillation 1. Eur. J. Cardio Thorac. Surg. 1997, 11, 1154–1157. Available online: https://academic.oup.com/ejcts/article/11/6/1154/465617 (accessed on 20 July 2025). [CrossRef] [PubMed]
- Puscas, A.; Harpa, M.M.; Brinzaniuc, K.; Al-Hussei, H.; Al-Hussei, H.; Banceu, C.; Opris, C.; Ghiragosian, C.; Flamind, S.; Balan, R.; et al. Evaluation of Perioperative Beta-Blockers and Factors Associated with Postoperative Atrial Fibrillation in Cardiac Surgery: A Single Center Experience. Rev. Cardiovasc. Med. 2023, 24, 370. [Google Scholar] [CrossRef]
- Gibbison, B.; Murphy, G.; O’brien, B.; Pufulete, M. An Update on Guidelines to Prevent and Manage Atrial Fibrillation After Cardiac Surgery and a Survey of Practice in the UK. J. Cardiothorac. Vasc. Anesth. 2024, 38, 2307–2313. Available online: http://creativecommons.org/licenses/by/4.0/ (accessed on 20 July 2025). [CrossRef]
- Waterford, S.D.; Prescher, L.; Ad, M.; Santore, L.A.; Spellman, C.; Ad, N. Amiodarone and postoperative atrial fibrillation. Vessel Plus 2022, 6, 28. Available online: https://www.oaepublish.com/articles/2574-1209.2021.115 (accessed on 20 July 2025). [CrossRef]
- Chatterjee, S.; Cangut, B.; Rea, A.; Salenger, R.; Arora, R.C.; Grant, M.C.; Morton-Bailey, V.; Hirji, S.; Engelman, D.T. Enhanced Recovery After Surgery Cardiac Society turnkey order set for prevention and management of postoperative atrial fibrillation after cardiac surgery: Proceedings from the American Association for Thoracic Surgery ERAS Conclave 2023. JTCVS Open. 2024, 18, 118–122. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. New Engl. J. Med. 1998, 339, 659–666. Available online: https://pubmed.ncbi.nlm.nih.gov/9725923/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; Neven, K.; Köktürk, B.; Konstantinidou, M.; Metzner, A.; et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: Lessons from a 5-year follow-up. Circulation 2010, 122, 2368–2377. Available online: https://www.researchgate.net/publication/49629605_Long-Term_Results_of_Catheter_Ablation_in_Paroxysmal_Atrial_Fibrillation_Lessons_From_a_5-Year_Follow-Up (accessed on 20 July 2025). [CrossRef]
- Alexandre, J.; Cautela, J.; Ederhy, S.; Damaj, G.L.; Salem, J.E.; Barlesi, F.; Farnault, L.; Charbonnier, A.; Mirabel, M.; Champiat, S.; et al. Cardiovascular toxicity related to cancer treatment: A pragmatic approach to the american and european cardio-oncology guidelines. J. Am. Heart Assoc. 2020, 9, e018403. Available online: https://pubmed.ncbi.nlm.nih.gov/32893704/ (accessed on 25 June 2025). [CrossRef]
- Patel, M.R.; Hellkamp, A.S.; Lokhnygina, Y.; Piccini, J.P.; Zhang, Z.; Mohanty, S.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: Analysis from the ROCKET AF trial (Rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J. Am. Coll. Cardiol. 2013, 61, 651–658. Available online: https://pubmed.ncbi.nlm.nih.gov/23391196/ (accessed on 13 July 2025).
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2017, 35, 893–911. Available online: https://pubmed.ncbi.nlm.nih.gov/27918725/ (accessed on 13 July 2025). [CrossRef] [PubMed]
- Zamorano, J.L.; Gottfridsson, C.; Asteggiano, R.; Atar, D.; Badimon, L.; Bax, J.J.; Cardinale, D.; Cardone, A.; Feijen, E.A.M.; Ferdinandy, P.; et al. The cancer patient and cardiology. Eur. J. Heart Fail. 2020, 22, 2290–2309. [Google Scholar] [CrossRef]
- Feng, Z.; Bhat, R.R.; Yuan, X.; Freeman, D.; Baslanti, T.; Bihorac, A.; Li, X. Intelligent Perioperative System: Towards Real-time Big Data Analytics in Surgery Risk Assessment. In Proceedings of the 2017 IEEE 15th International Conference on Dependable, Autonomic and Secure Computing, 15th International Conference on Pervasive Intelligence and Computing, 3rd International Conference on Big Data Intelligence and Computing and Cyber Science and Technology Congress (DASC/PiCom/DataCom/CyberSciTech), Orlando, FL, USA, 6–10 November 2017; Available online: http://arxiv.org/abs/1709.10192 (accessed on 20 July 2025).
- Cantwell, C.D.; Mohamied, Y.; Tzortzis, K.N.; Garasto, S.; Houston, C.; Chowdhury, R.A.; Ng, F.S.; Bharath, A.A.; Peters, N.S. Rethinking multiscale cardiac electrophysiology with machine learning and predictive modelling. Comput. Biol. Med. 2019, 104, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Caldonazo, T.; Kirov, H.; Rahouma, M.; Robinson, N.B.; Demetres, M.; Gaudino, M.; Doenst, T.; Dobrev, D.; Borger, M.A.; Kiehntopf, M.; et al. Atrial fibrillation after cardiac surgery: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2023, 165, 94–103.e24. Available online: https://pubmed.ncbi.nlm.nih.gov/33952399/ (accessed on 1 September 2025). [CrossRef]
- Liao, K.M.; Yu, C.H.; Wu, Y.C.; Wang, J.J.; Liang, F.W.; Ho, C.H. Risk of Atrial Fibrillation in Patients with Different Cancer Types in Taiwan. Life 2024, 14, 621. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11122475/ (accessed on 14 July 2025). [CrossRef]
- Suero, O.R.; Ali, A.K.; Barron, L.R.; Segar, M.W.; Moon, M.R.; Chatterjee, S. Postoperative atrial fibrillation (POAF) after cardiac surgery: Clinical practice review. J. Thorac. Dis. 2024, 16, 1503–1520. Available online: https://jtd.amegroups.org/article/view/83529/html (accessed on 20 July 2025). [CrossRef] [PubMed]
- Noordzij, P.G.; Thio, M.S.Y.; Reniers, T.; Dijkstra, I.; Mondelli, G.; Langelaan, M.; Ruven, H.J.T.; Rettig, T.C.D. Improving Prediction of Postoperative Atrial Fibrillation After Cardiac Surgery Using Multiple Pathophysiological Biomarkers: A Prospective Double-Centre Study. J. Clin. Med. 2025, 14, 3737. Available online: https://www.mdpi.com/2077-0383/14/11/3737/htm (accessed on 20 July 2025). [CrossRef] [PubMed]
- Chung, S.C.; O’Brien, B.; Lip, G.Y.H.; Fields, K.G.; Muehlschlegel, J.D.; Thakur, A.; Clifton, D.; Collins, G.S.; Watkinson, P.; Providencia, R. Prognostic model for atrial fibrillation after cardiac surgery: A UK cohort study. Clin. Res. Cardiol. 2023, 112, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, P.; Zhu, F.; Wang, D.; Yang, S.; Yan, W. Efficacy and safety of sacubitril/valsartan on postoperative atrial fibrillation in adult patients undergoing cardiac surgery: A real-world observational study. Front. Cardiovasc. Med. 2024, 11, 1477858. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ad, N.; Badhwar, V.; Gillinov, A.M.; Alexander, J.H.; Moon, M.R. Anticoagulation for atrial fibrillation after cardiac surgery: Do guidelines reflect the evidence? J. Thorac. Cardiovasc. Surg. 2024, 167, 694–700. Available online: https://pubmed.ncbi.nlm.nih.gov/37037415/ (accessed on 1 September 2025). [CrossRef]
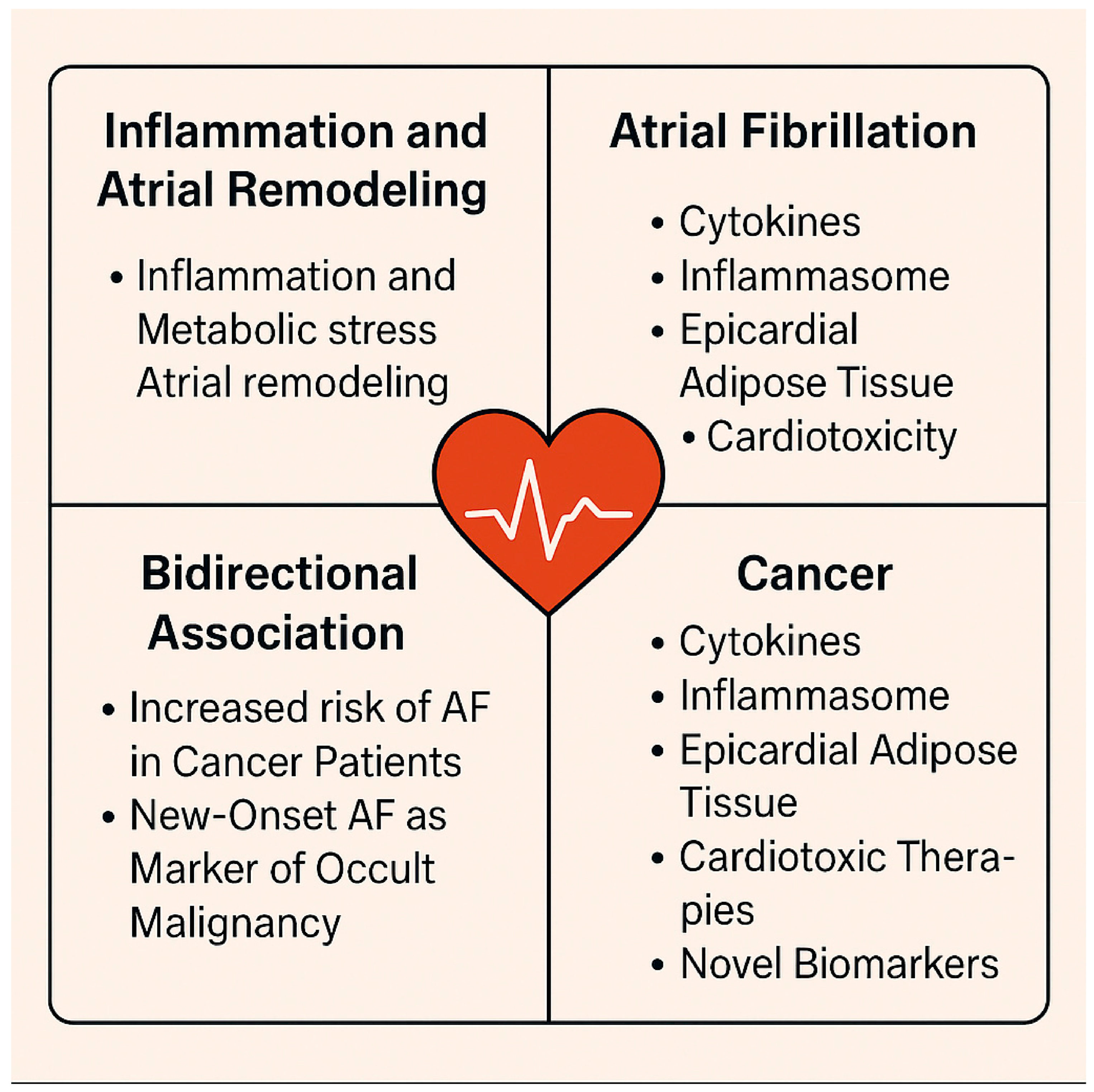
| Cancer Type | Mechanisms | Notable Treatment Affecting Risk | Prevalence/Impact |
|---|---|---|---|
| Lung Cancer | Chronic inflammation, Hypercoagulability | Chemotherapy, Radiotherapy | Highest risk among all cancers |
| Breast Cancer | Cardiotoxicity from treatment | Anthracyclines, HER2-targeted therapies | Increased risk, especially with therapy |
| Gastrointestinal Cancers | Inflammation, Metabolic disturbances | Various chemotherapy regimens | Moderate; associated with inflammation |
| Hematologic Malignancies | Systemic inflammation, Cytokine release | Chemotherapy, Immunotherapy | Variable; cytokine-mediated AF triggers |
| Prostate Cancer | Cardiovascular complications from ADT | Androgen deprivation therapy (ADT) | Low to moderate risk; ADT-related |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georghiou, G.P.; Georghiou, P.; Georgiou, A.; Triposkiadis, F. Cardiac Surgery and Postoperative Atrial Fibrillation: The Role of Cancer. Medicina 2025, 61, 1815. https://doi.org/10.3390/medicina61101815
Georghiou GP, Georghiou P, Georgiou A, Triposkiadis F. Cardiac Surgery and Postoperative Atrial Fibrillation: The Role of Cancer. Medicina. 2025; 61(10):1815. https://doi.org/10.3390/medicina61101815
Chicago/Turabian StyleGeorghiou, Georgios P., Panos Georghiou, Amalia Georgiou, and Filippos Triposkiadis. 2025. "Cardiac Surgery and Postoperative Atrial Fibrillation: The Role of Cancer" Medicina 61, no. 10: 1815. https://doi.org/10.3390/medicina61101815
APA StyleGeorghiou, G. P., Georghiou, P., Georgiou, A., & Triposkiadis, F. (2025). Cardiac Surgery and Postoperative Atrial Fibrillation: The Role of Cancer. Medicina, 61(10), 1815. https://doi.org/10.3390/medicina61101815







