Intraoperative Application of Hyaluronic Acid in Achilles Tendon Repair: A Retrospective Cohort Study on Short-Term Functional Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Surgical Technique
2.4. Postoperative Follow-Up and Rehabilitation
2.5. Functional Outcome Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATRS | Achilles Tendon Total Rupture Score |
| BMI | Body mass index |
| HA | Hyaluronic acid |
| MCS | Mental Component Summary |
| PCS | Physical Component Summary |
| SF-36 | Short Form 36 |
References
- Huttunen, T.T.; Kannus, P.; Rolf, C.; Felländer-Tsai, L.; Mattila, V.M. Acute achilles tendon ruptures: Incidence of injury and surgery in Sweden between 2001 and 2012. Am. J. Sports Med. 2014, 42, 2419–2423. [Google Scholar] [CrossRef]
- Westin, O.; Nilsson Helander, K.; Grävare Silbernagel, K.; Samuelsson, K.; Brorsson, A.; Karlsson, J. Patients with an Achilles tendon re-rupture have long-term functional deficits and worse patient-reported outcome than primary ruptures. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3063–3072. [Google Scholar] [CrossRef]
- Oliva, F.; Maffulli, N.; Via, A.G.; Maffulli, G.D.; Tarantino, U. Achilles tendon rupture and dysmetabolic diseases: A multicentric, epidemiologic study. J. Clin. Med. 2022, 11, 3698. [Google Scholar] [CrossRef]
- Gür, V.; Yapici, F.; Küçük, U.; Subaşi, İ.Ö.; Gökgöz, M.B.; Karaköse, R.; Koçkara, N. Patients with Achilles Tendon Rupture Are Prone to Develop Ventricular Arrhythmia. J. Clin. Med. 2023, 12, 3583. [Google Scholar] [CrossRef]
- Węgłowski, R.; Borowski, B.; Bronikowska, A.; Piech, P.; Staśkiewicz, G.; Jarecki, J. The Role of Metabolic Disorders and Laboratory Abnormalities in Wound Healing and Recovery in Geriatric and Non-Geriatric Orthopedic Patients in Poland—Prospective Research. J. Clin. Med. 2024, 14, 5317. [Google Scholar] [CrossRef] [PubMed]
- Paczesny, Ł.; Zabrzyński, J.; Domżalski, M.; Gagat, M.; Termanowski, M.; Szwedowski, D.; Łapaj, Ł.; Kruczyński, J. Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture—A Pilot Study. J. Clin. Med. 2021, 10, 2370. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.K.; Mahmoud, K.; d’Hooghe, P.; Bariteau, J.; Labib, S.A.; Myerson, M.S. Outcomes and Complications of Open Versus Minimally Invasive Repair of Acute Achilles Tendon Ruptures: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2023, 51, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Bernardi, G.; Farsetti, P.; Gasparini, M.; Marsilio, E.; Piccirilli, E.; Tarantino, U.; Rugiero, C.; Gaj, E.; Lupariello, D.; et al. I.S.Mu.L.T. Achilles tendon ruptures guidelines. Muscles Ligaments Tendons J. 2018, 8, 310–363. [Google Scholar] [CrossRef]
- Carmont, M.R.; Rossi, R.; Scheffler, S.; Mei-Dan, O.; Beaufils, P. Percutaneous & Mini Invasive Achilles tendon repair. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2011, 3, 28. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Spiezia, F.; Denaro, V. Minimally invasive surgery for Achilles tendon pathologies. Open Access J. Sports Med. 2010, 1, 95–103. [Google Scholar] [CrossRef]
- Carmont, M.R.; Maffulli, N. Modified percutaneous repair of ruptured Achilles tendon. Knee Surg. Sports Traumatol. Arthrosc. 2008, 16, 199–203. [Google Scholar]
- Boksh, K.; Elbashir, M.; Thomas, O.; Divall, P.; Mangwani, J. Platelet-Rich Plasma in acute Achilles tendon ruptures: A systematic review and meta-analysis. Foot 2022, 53, 101923. [Google Scholar] [CrossRef]
- Arnaud-Franco, Á.; Lara-Arias, J.; Marino-Martínez, I.A.; Cienfuegos-Jiménez, O.; Barbosa-Quintana, Á.; Peña-Martínez, V.M. Effect of Adipose-Derived Mesenchymal Stem Cells (ADMSCs) Application in Achilles-Tendon Injury in an Animal Model. Curr. Issues Mol. Biol. 2022, 44, 5827–5838. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Sánchez, M.; Vaquerizo, V.; Malanga, G.A.; Fiz, N.; Azofra, J.; Rogers, C.J.; Samitier, G.; Sampson, S.; Seijas, R.; et al. Platelet-Rich Plasma Applications for Achilles Tendon Repair: A Bridge between Biology and Surgery. Int. J. Mol. Sci. 2021, 22, 824. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, J.; Wang, X.; Peng, S.; Ning, J.; Qian, Y.; Fan, C. MicroRNA-21-3p engineered umbilical cord stem cell-derived exosomes inhibit tendon adhesion. J. Inflamm. Res. 2020, 13, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.; Lui, Y.H.; Kapacee, Z.; Kadler, K.E.; Ferguson, M.W.; McGrouther, D.A. The cellular biology of flexor tendon adhesion formation: An old problem in a new paradigm. Am. J. Pathol. 2009, 175, 1938–1951. [Google Scholar]
- Altman, R.D.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Chen, K. The bioengineering application of hyaluronic acid in tissue regeneration and repair. Int. J. Biol. Macromol. 2024, 270, 132454. [Google Scholar] [CrossRef]
- López-Ruiz, E.; Jiménez, G.; Álvarez de Cienfuegos, L.; Antic, C.; Sabata, R.; A Marchal, J.; Gálvez-Martín, P. Advances of hyaluronic acid in stem cell therapy and tissue engineering, including current clinical trials. Eur. Cell Mater. 2019, 37, 186–213. [Google Scholar] [CrossRef]
- Liu, Y.; Skardal, A.; Shu, X.Z.; Prestwich, G.D. Prevention of peritendinous adhesions using a hyaluronan-derived hydrogel film following partial-thickness flexor tendon injury. J. Orthop. Res. 2008, 26, 562–569. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V. The use of hyaluronic acid after tendon surgery and in tendinopathies. Biomed. Res. Int. 2014, 2014, 783632. [Google Scholar] [CrossRef]
- Ozgenel, G.Y.; Etöz, A. Effects of repetitive injections of hyaluronic acid on peritendinous adhesions after flexor tendon repair: A preliminary randomized, placebo-controlled clinical trial. Ulus. Travma Acil Cerrahi Derg. 2012, 18, 11–17. [Google Scholar] [CrossRef]
- Kolodzinskyi, M.N.; Zhao, C.; Sun, Y.L.; An, K.-N.; Thoreson, A.R.; Amadio, P.C.; Moran, S.L. The effects of hylan g-f 20 surface modification on gliding of extrasynovial canine tendon grafts in vitro. J. Hand Surg. Am. 2013, 38, 231–236. [Google Scholar] [PubMed]
- Işik, S.; Oztürk, S.; Gürses, S.; Yetmez, M.; Güler, M.; Selmanpakoglu, N.; Günhan, Ö. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: Experimental research in chickens. Br. J. Plast. Surg. 1999, 52, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Park, E.S.; Choi, C.Y.; Cha, H.G.; Hwang, Y.; Nam, S.M. Hyaluronic Acid Treatment Improves Healing of the Tenorrhaphy Site by Suppressing Adhesions through Extracellular Matrix Remodeling in a Rat Model. Polymers 2021, 13, 928. [Google Scholar] [CrossRef]
- Kaux, J.F.; Samson, A.; Crielaard, J.M. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2016, 5, 264–269. [Google Scholar] [CrossRef]
- Müller, S.A.; Evans, C.H.; Heisterbach, P.E.; Majewski, M. The Role of the Paratenon in Achilles Tendon Healing: A Study in Rats. Am. J. Sports Med. 2018, 46, 1214–1219. [Google Scholar] [CrossRef]
- Kaya, M.E.; Celik, D.; Kiliçoglu, Ö.; Ozdincler, A.R.; Nilsson-Helander, K. The Turkish version of the Achilles tendon Total Rupture Score: Cross-cultural adaptation, reliability and validity. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2427–2432. [Google Scholar] [CrossRef]
- Analay, A.Y.; Celik, D.; Ogut, R.T. Translation, Cross-Cultural Adaptation, Reliability, and Validity of Turkish Version of the American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scale. J. Foot Ankle Surg. 2016, 55, 1139–1142. [Google Scholar] [CrossRef]
- Kaya, B.B.; İçağasıoğlu, A. Reliability and validity of the Turkish version of the Short Form-36 (SF-36) in patients with rheumatoid arthritis. J. Surg. Med. 2018, 2, 11–16. [Google Scholar]
- Noback, P.C.; Jang, E.S.; Cuellar, D.O.; Seetharaman, M.; Malagoli, E.; Greisberg, J.K.; Vosseller, J.T. Risk factors for achilles tendon rupture: A matched case control study. Injury 2017, 48, 2342–2347. [Google Scholar] [CrossRef]
- Chen, D.; Liu, J.; Zhu, Z.; Zhang, Z.; Liu, D.; Zheng, L. Hyperuricemia as an independent risk factor for achilles tendon rupture in male: A case-control study. J. Orthop. Surg. Res. 2024, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Rui, Y.F.; Li, G.; Wang, C. Alterations of tendons in diabetes mellitus: What are the current findings? Int. Orthop. 2015, 39, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Humbyrd, C.J.; Bae, S.; Kucirka, L.M.; Segev, D.L. Incidence, Risk Factors, and Treatment of Achilles Tendon Rupture in Patients With End-Stage Renal Disease. Foot Ankle Int. 2018, 39, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Marsilio, E.; Asparago, G.; Frizziero, A.; Berardi, A.C.; Maffulli, N. The Impact of Hyaluronic Acid on Tendon Physiology and Its Clinical Application in Tendinopathies. Cells 2021, 10, 3081. [Google Scholar] [CrossRef]
- Osti, L.; Berardocco, M.; di Giacomo, V.; Di Bernardo, G.; Oliva, F.; Berardi, A.C. Hyaluronic acid increases tendon derived cell viability and collagen type I expression in vitro: Comparative study of four different Hyaluronic acid preparations by molecular weight. BMC Musculoskelet. Disord. 2015, 16, 334. [Google Scholar] [CrossRef]
- Frizziero, A.; Oliva, F.; Vittadini, F.; Vetrano, M.; Bernetti, A.; Giordan, N.; Vulpiani, M.C.; Santilli, V.; Masiero, S.; Maffulli, N. Efficacy of ultrasound-guided hyaluronic acid injections in achilles and patellar tendinopathies: A prospective multicentric clinical trial. Muscles Ligaments Tendons J. 2019, 9, 305–313. [Google Scholar] [CrossRef]
- Heikkinen, J.; Lantto, I.; Flinkkilä, T.; Ohtonen, P.; Pajala, A.; Siira, P.; Leppilahti, J. Augmented Compared with Nonaugmented Surgical Repair After Total Achilles Rupture: Results of a Prospective Randomized Trial with Thirteen or More Years of Follow-up. J. Bone Joint Surg. Am. 2016, 98, 85–92. [Google Scholar] [CrossRef]
- Jaakkola, J.I.; Hutton, W.C.; Beskin, J.L.; Lee, G.P. Achilles tendon rupture repair: Biomechanical comparison of the triple bundle technique versus the Krakow locking loop technique. Foot Ankle Int. 2000, 21, 14–17. [Google Scholar] [CrossRef]
- Oesman, I.; Canintika, A.F. Absorbable vs. Nonabsorbable Sutures for Achilles Tendon Repair: A Systematic Review and Meta-analysis. Foot Ankle Orthop. 2023, 8, 24730114231201842. [Google Scholar] [CrossRef]
- Jildeh, T.R.; Okoroha, K.R.; Marshall, N.E.; Abdul-Hak, A.; Zeni, F.; Moutzouros, V. Infection and Rerupture After Surgical Repair of Achilles Tendons. Orthop. J. Sports Med. 2018, 6, 2325967118774302. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, J.; Liu, X.; Li, Y.; Pan, S.; Liu, S. Antiadhesion Biomaterials in Tendon Repair: Application Status and Future Prospect. Tissue Eng. Part B Rev. 2025, 31, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cui, J.; Shen, Y.; Guo, W.; Cai, P.; Chen, Y.; Yuan, Z.; Liu, M.; El-Newehy, M.; El-Hamshary, H.; et al. Current Advancements and Strategies of Biomaterials for Tendon Repair. Front. Bioeng. Biotechnol. 2023, 11, 1145123. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Pan, H.; Xu, Y.; Yu, H.; Wang, P. Recent Advances in Tendon Tissue Engineering Strategy. Front. Bioeng. Biotechnol. 2023, 11, 1115312. [Google Scholar] [CrossRef] [PubMed]


| Parameters | HA Group (n = 32) | Control Group (n = 32) | p-Value |
|---|---|---|---|
| Mean age (years) | 39.2 ± 6.1 | 40.1 ± 5.8 | 0.645 |
| Gender (Male, %) | 22 (68.8%) | 21 (65.6%) | 0.790 |
| BMI (kg/m2) | 27.4 ± 3.2 | 27.8 ± 3.1 | 0.712 |
| Side of injury, right (n, %) | 17 (53.1%) | 18 (56.3%) | 0.802 |
| Limb dominance, right (n, %) | 26 (81.3%) | 25 (78.1%) | 0.756 |
| Smoking status (n, %) | 7 (21.9%) | 8 (25.0%) | 0.767 |
| Time interval from injury to surgery (days) | 4.2 ± 1.6 | 4.4 ± 1.7 | 0.632 |
| Comorbidities (n, %) | 6 (18.8%) | 6 (18.8%) | 1.000 |
| Diabetes mellitus (n, %) | 3 (9.4%) | 3 (9.4%) | 1.000 |
| Hypertension (n, %) | 2 (6.3%) | 2 (6.3%) | 1.000 |
| Cardiovascular disease (n, %) | 1 (3.1%) | 0 (0%) | 0.313 |
| Chronic respiratory disease (n, %) | 0 (0%) | 1 (3.1%) | 0.313 |
| Measure | Time | HA (Mean ± SD) | Control (Mean ± SD) | Mean Diff (95% CI) | p-Value |
|---|---|---|---|---|---|
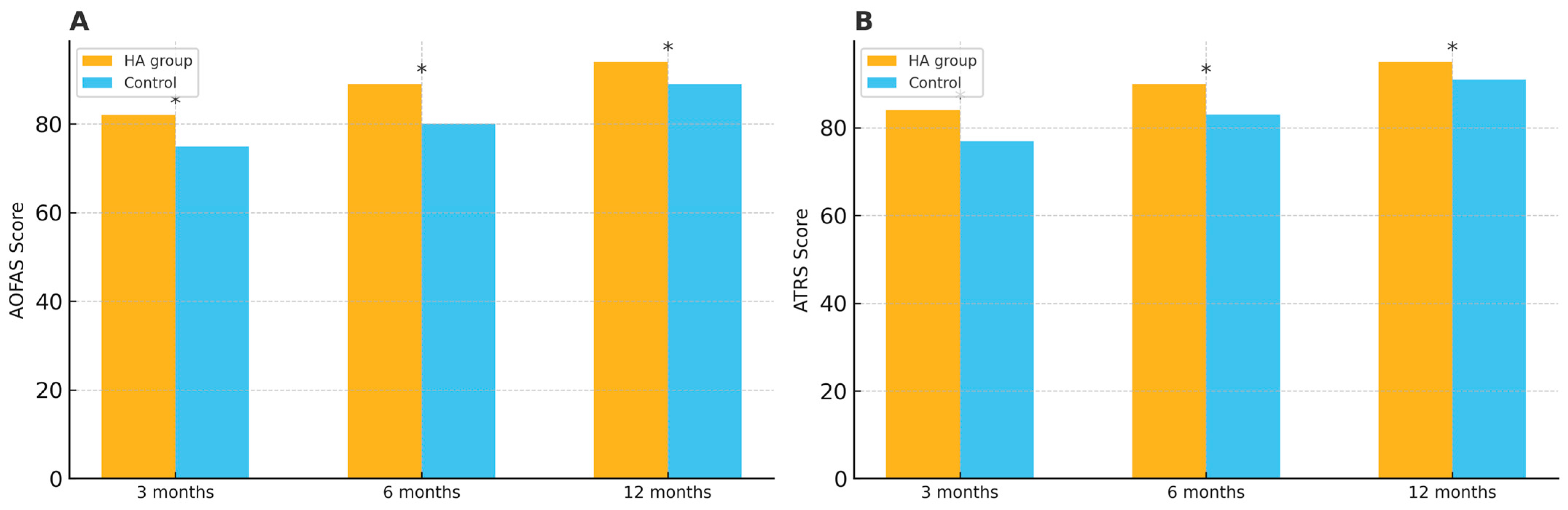
| AOFAS score | 3 months | 82 ± 6.1 | 75 ± 5.8 | 7.0 (4.0–10.0) | 0.021 |
| 6 months | 89 ± 5.3 | 80 ± 5.5 | 9.0 (6.3–11.7) | 0.008 | |
| 12 months | 94 ± 4.7 | 89 ± 5.1 | 5.0 (2.5–7.5) | 0.034 | |
| ATRS | 3 months | 84 ± 7.2 | 77 ± 6.9 | 7.0 (3.5–10.5) | 0.021 |
| 6 months | 90 ± 6.5 | 83 ± 6.1 | 7.0 (3.8–10.2) | 0.008 | |
| 12 months | 95 ± 5.9 | 91 ± 5.6 | 4.0 (1.1–6.9) | 0.034 |
| Measure | Time | HA (Mean ± SD) | Control (Mean ± SD) | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|---|
| SF-36 PCS | 6 months | 78.2 ± 4.8 | 71.1 ± 5.4 | 7.1 (4.3–9.9) | 0.013 |
| 12 months | 81.4 ± 5.0 | 76.8 ± 5.5 | 4.6 (1.8–7.4) | 0.027 | |
| SF-36 MCS | 6 months | 76.5 ± 5.1 | 72.9 ± 5.3 | 3.6 (0.6–6.6) | 0.019 |
| 12 months | 79.3 ± 5.2 | 75.9 ± 5.4 | 3.4 (0.4–6.4) | 0.031 |
| Measure | Time | HA (Mean ± SD) | Control (Mean ± SD) | Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|---|
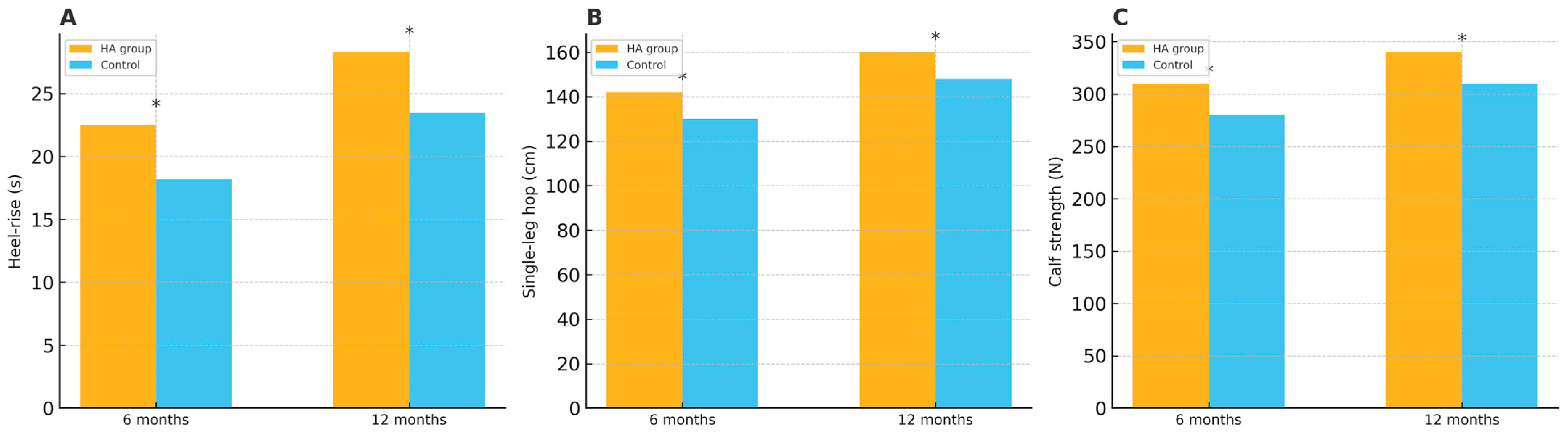
| Heel-rise Endurance (s) | 6 months | 35.4 ± 4.2 | 30.1 ± 3.9 | 5.3 (3.1–7.5) | 0.015 |
| 12 months | 41.2 ± 5.1 | 36.5 ± 4.6 | 4.7 (2.4–7.0) | 0.022 | |
| Single-leg Hopping (cm) | 6 months | 78.5 ± 6.8 | 70.3 ± 5.9 | 8.2 (4.8–11.6) | 0.018 |
| 12 months | 85.7 ± 7.3 | 79.2 ± 6.4 | 6.5 (3.2–9.8) | 0.027 | |
| Calf Muscle Strength (N) | 6 months | 120.5 ± 8.3 | 112.7 ± 7.9 | 7.8 (3.7–11.9) | 0.012 |
| 12 months | 127.8 ± 8.5 | 118.9 ± 8.1 | 8.9 (4.8–13.0) | 0.021 |
| Complication | HA (n, %) | Control (n, %) | Risk Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Re-rupture | 1 (3.1%) | 1 (3.1%) | 0.0% (−9.3% to 9.3%) | 1.000 |
| Wound infection | 1 (3.1%) | 2 (6.3%) | −3.2% (−14.6% to 8.2%) | 0.554 |
| Deep vein thrombosis | 0 (0%) | 0 (0%) | 0.0% (NA) | 1.000 |
| Delayed wound healing | 2 (6.3%) | 1 (3.1%) | +3.2% (−8.2% to 14.6%) | 0.554 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurdakul, G.; Atahan, M.O.; Askin, A.; Uzun, M.F.; Iyigun, A.; Golgelioglu, F.; Olcar, H.A. Intraoperative Application of Hyaluronic Acid in Achilles Tendon Repair: A Retrospective Cohort Study on Short-Term Functional Outcomes. Medicina 2025, 61, 1816. https://doi.org/10.3390/medicina61101816
Yurdakul G, Atahan MO, Askin A, Uzun MF, Iyigun A, Golgelioglu F, Olcar HA. Intraoperative Application of Hyaluronic Acid in Achilles Tendon Repair: A Retrospective Cohort Study on Short-Term Functional Outcomes. Medicina. 2025; 61(10):1816. https://doi.org/10.3390/medicina61101816
Chicago/Turabian StyleYurdakul, Goker, Mehmet Okan Atahan, Aydogan Askin, Mehmet Fatih Uzun, Abdullah Iyigun, Fatih Golgelioglu, and Haci Ali Olcar. 2025. "Intraoperative Application of Hyaluronic Acid in Achilles Tendon Repair: A Retrospective Cohort Study on Short-Term Functional Outcomes" Medicina 61, no. 10: 1816. https://doi.org/10.3390/medicina61101816
APA StyleYurdakul, G., Atahan, M. O., Askin, A., Uzun, M. F., Iyigun, A., Golgelioglu, F., & Olcar, H. A. (2025). Intraoperative Application of Hyaluronic Acid in Achilles Tendon Repair: A Retrospective Cohort Study on Short-Term Functional Outcomes. Medicina, 61(10), 1816. https://doi.org/10.3390/medicina61101816






