1. Introduction
Colorectal cancer is the second most common cancer in Europe with over 500,000 new diagnoses made annually [
1]. Statistically, about one-third of colorectal cancer cases are located in the rectum with a similar number of cases being diagnosed at advanced stages [
2].
The surgical approach is fundamental for cancer treatment [
3]. Surgery is useful in both palliative and curative care; therefore, there is a great need to improve the understanding of possible outcomes and prognostic factors [
4]. The continuous progress in this field introduces greater complexity in treatment selection [
5]. The preferable choices for early rectal cancer are either removal by local excision or chemoradiotherapy followed by local excision. If a complete response is confirmed using only chemoradiotherapy, then a “watch and wait” strategy is optional [
6]. However, advanced rectal cancer typically requires a radical surgical approach, determined by the tumor’s size and exact location, along with combined neoadjuvant chemoradiotherapy. This approach can increase the likelihood of preserving the anal sphincter by reducing the size and stage of the tumor, thereby securing a more adequate circumferential margin [
7].
One of the biggest concerns of patients dealing with low rectal cancer is permanent stoma, which influences the surgical decision to extend into intersphincteric space and perform anastomosis [
8]. Laparoscopic surgery can also be useful for operable low rectal tumors yielding minimal postoperative complications, low local recurrence rates and reduced incidence of low anterior resection syndrome [
9]. Moreover, Laparoscopic surgery was associated with shorter hospital stays and reduced opioid use. Compared to laparoscopic procedures, open surgery significantly increased the likelihood of blood transfusion, ICU admission, and mechanical ventilation [
10].
There is a spectrum of factors that collectively determine the success of rectal cancer surgery (intervention and following patient quality of life) and can alter postoperative outcomes [
11]. Common short-term postoperative complications following rectal cancer surgery include anastomotic leaks, infections, bleeding, and wound dehiscence [
12]. These complications can lead to heightened morbidity, extended hospital admissions, and the requirement for further interventions [
13]. Postoperative complications could worsen the prognosis as well. Cytokines released by inflammation caused by either anastomotic leakage, abdominal abscess or pneumonia can cause tumor progression or metastasis. In addition, recovery from postoperative complications takes time, often delaying the initiation of adjuvant chemotherapy [
14].
Surgical outcomes in rectal cancer are intrinsically tied to a range of patient-specific and tumor-related factors. The most recognized risk factors include the age and male gender of the patient, poor blood supply to the anastomosis, excessive tension, preoperative chemoradiotherapy, obesity, emergency surgery and coexisting medical conditions [
15]. Several modifiable prognostic factors include timely neoadjuvant treatment, high surgeon volume, high tie of the inferior mesenteric artery, extension of mesorectal excision, and improved techniques for intersphincteric resection and anastomosis [
16]. The use of protective ileostomy remains debated, as its leak-reducing benefits must be weighed against significant stoma-related morbidity, including dehydration, wound complications, and reoperation after reversal [
17].
This study aimed to compare laparoscopic and open surgical techniques and identify prognostic factors associated with anastomotic leakage following rectal cancer resection.
4. Discussion
This retrospective study analyzed postoperative outcomes in patients undergoing laparoscopic and open rectal cancer surgery, with a focus on anastomotic leakage, reoperation rates, lymph node harvest and comorbidities.
The optimal surgical approach to minimize anastomotic leakage remains debated. A meta-analysis done by Zheng et al. [
18] found that a laparoscopic approach reduces the incidence of anastomotic leakage because of better pelvis exposure. Another meta-analysis done by Arezzo et al. [
19] found no significant differences in open versus laparoscopic technique used regarding anastomotic leakage (
p = 0.128). The study by J. Lujan et al. [
20] showed no significant differences in complications or reoperations between the open and laparoscopic surgery groups. In our study the overall anastomotic leakage rate did not differ significantly between surgical techniques (
p = 0.78), while the reoperation rate was significantly higher in the laparoscopic group (
p = 0.027). The greater reoperation rate in our cohort may reflect a more frequent use of primary anastomosis in laparoscopic cases.
We found that laparoscopic surgeries were significantly longer than open surgeries (213.0 ± 65.9 vs. 201.3 ± 60.35 min,
p = 0.05), which is consistent with previous studies. A meta-analysis by Trastulli et al. [
21] also reported longer operative times for laparoscopic surgery, although with high heterogeneity. The authors attributed this variability to differences in surgeons’ experience with laparoscopic techniques.
The number of harvested lymph nodes was significantly higher in open surgery (13.1 vs. 11.2,
p = 0.01). This difference may be explained by the more extensive manual exposure and tactile feedback during open dissection. However, other studies have reached opposite conclusions. Boutros et al. [
22] have proposed that laparoscopic surgery for rectal cancer may provide better visualization and more accurate pelvic dissection with less tissue manipulation. Improved retraction and easier access to the most proximal portion of the inferior mesenteric vessels contribute to greater harvested lymph nodes in the laparoscopic group, according to this study. On the other hand, Yamamoto et al. [
23] conducted a similar study, but with patients who had a BMI over 25. They found that the median number of lymph nodes harvested in the laparoscopic group was significantly lower compared to the open surgery group (17.5 vs. 21.0
p = 0.0047). These results correlate with our study, where the median BMI of our cohort was over 25 in both the laparoscopic and open groups. These findings suggest that for patients with a BMI higher than 25, more lymph nodes can be harvested using an open surgery approach.
Fewer pelvic drains were placed in the laparoscopic group (91.9% vs. 94.4%
p = 0.046). Pelvic drains can also increase postoperative pain and bowel obstruction due to their foreign body reaction, or act as a gateway for wound infection. Therefore, the routine use of pelvic drains remains controversial [
24]. A study by Denost et al. [
25] concluded that pelvic drainage did not decrease reoperation rates or detect early anastomotic leakage. However, surgeons should consider using pelvic drains in patients undergoing longer, higher-volume surgeries [
26]. This is consistent with our findings, as significantly more patients with T1 tumors—typically requiring less extensive and shorter surgeries—were operated on laparoscopically. This could be the reason why pelvic drains were used significantly less frequently in the laparoscopic group.
Our study found several factors associated with anastomotic leakage. The ASA score is a useful method for patient performance status evaluation using multiple characteristics. Therefore, association between ASA scores and postoperative complication rates was observed [
27]. According to a study by Bakker et al. [
28] an ASA score of III–IV increased the odds of anastomotic leakage significantly. These findings correlate with our study, where we found that a significant number of patients with an ASA IV score experienced anastomotic leakage (57.1%). A study by Moon et al. [
29] found that NOAC use was associated with significantly higher levels of anastomotic leakage, small bowel obstruction, infection, and renal complications. These findings correlate with our results.
Conversely, patients with arterial hypertension had a significantly lower leakage rate. Previous studies have often reported hypertension as a risk factor for surgical complications. Post et al. [
30] found that high preoperative diastolic blood pressure and profound intraoperative hypotension, in addition to complex surgery marked by a blood loss of more than 250 mL, are associated with an increased risk of developing anastomotic leakage. However, it is possible that hypertensive patients in our cohort were more closely monitored and medically optimized pre- and postoperatively, resulting in more stable hemodynamic conditions and improved tissue perfusion contributing to improved short-term outcomes.
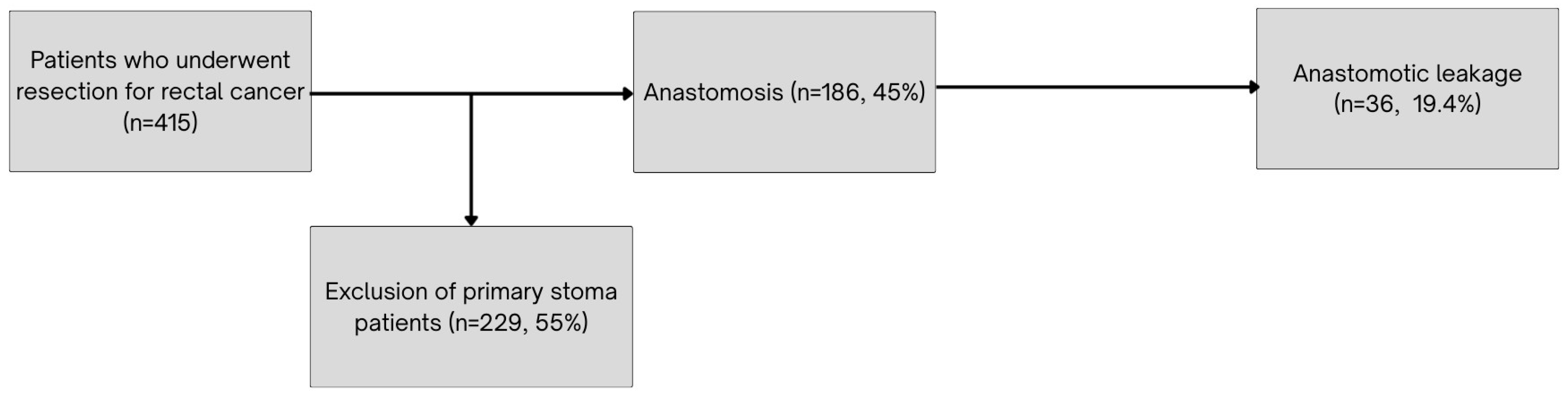
The anastomotic leakage rate observed in our cohort (19.4%) is relatively high compared to rates reported in large multicenter studies. In the COLOR II trial, leakage occurred in 13% of patients after laparoscopic and 10% after open surgery [
31]. Similarly, the Dutch TME trial reported a leakage rate of 10.4% following low anterior resection [
32] while the ALARM international study found an overall leakage incidence of 8.6% across multiple centers and countries [
33]. The elevated rate in our study may be explained by a higher proportion of ASA IV patients, more frequent use of NOAC, and fewer diverting stomas. In our cohort, only 20% of patients received a diverting stoma, compared to approximately 35% in the COLOR II trial [
31]. Additionally, our findings align with recent evidence from a large meta-analysis [
34] and multi-institutional study [
35], both of which demonstrate that diverting ileostomy significantly lowers the risk and severity of anastomotic leakage. These data, combined with our own, support a more proactive approach to fecal diversion in high-risk rectal cancer patients.
This study has several limitations. First, due to its retrospective design, it relied on the accuracy and completeness of existing medical records, which may have led to unrecorded confounding factors such as nutritional status, smoking history, or exact intraoperative decision-making processes. Secondly, while statistical associations were observed, the subgroup sample sizes, such as patients using NOACs or having arterial hypertension, were relatively small, potentially affecting statistical power. Third, the analysis was limited to short-term postoperative outcomes. Important long-term outcomes such as local recurrence, disease-free survival, and quality of life were not evaluated. Fourth, TME quality assessment was only implemented as a routine practice starting in 2022; therefore, data for preceding years are incomplete or missing.