Implementation of ORBEYE®-Exoscope in the Operative Treatment of Spinal Dural Arteriovenous Fistula
Abstract
1. Introduction
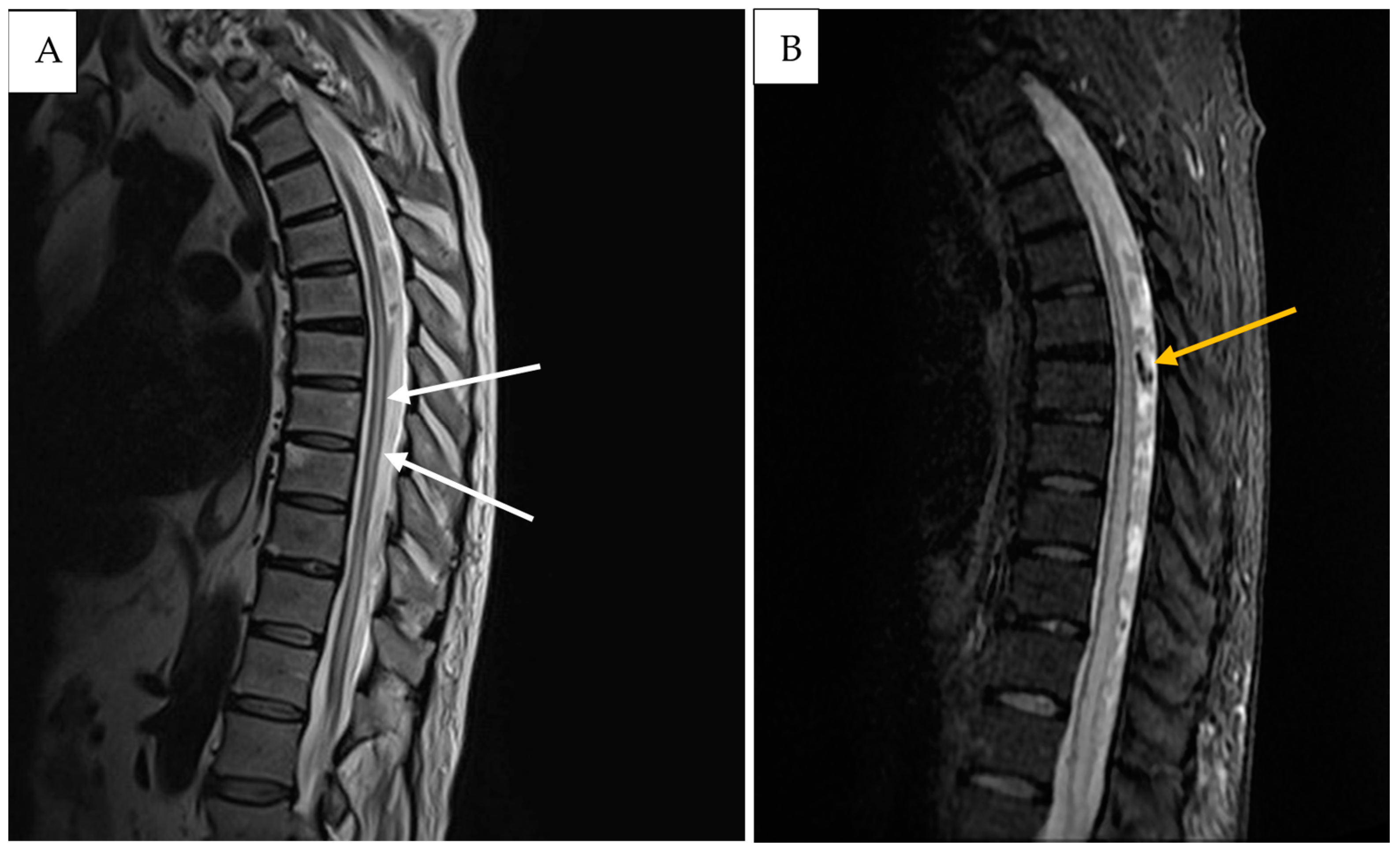
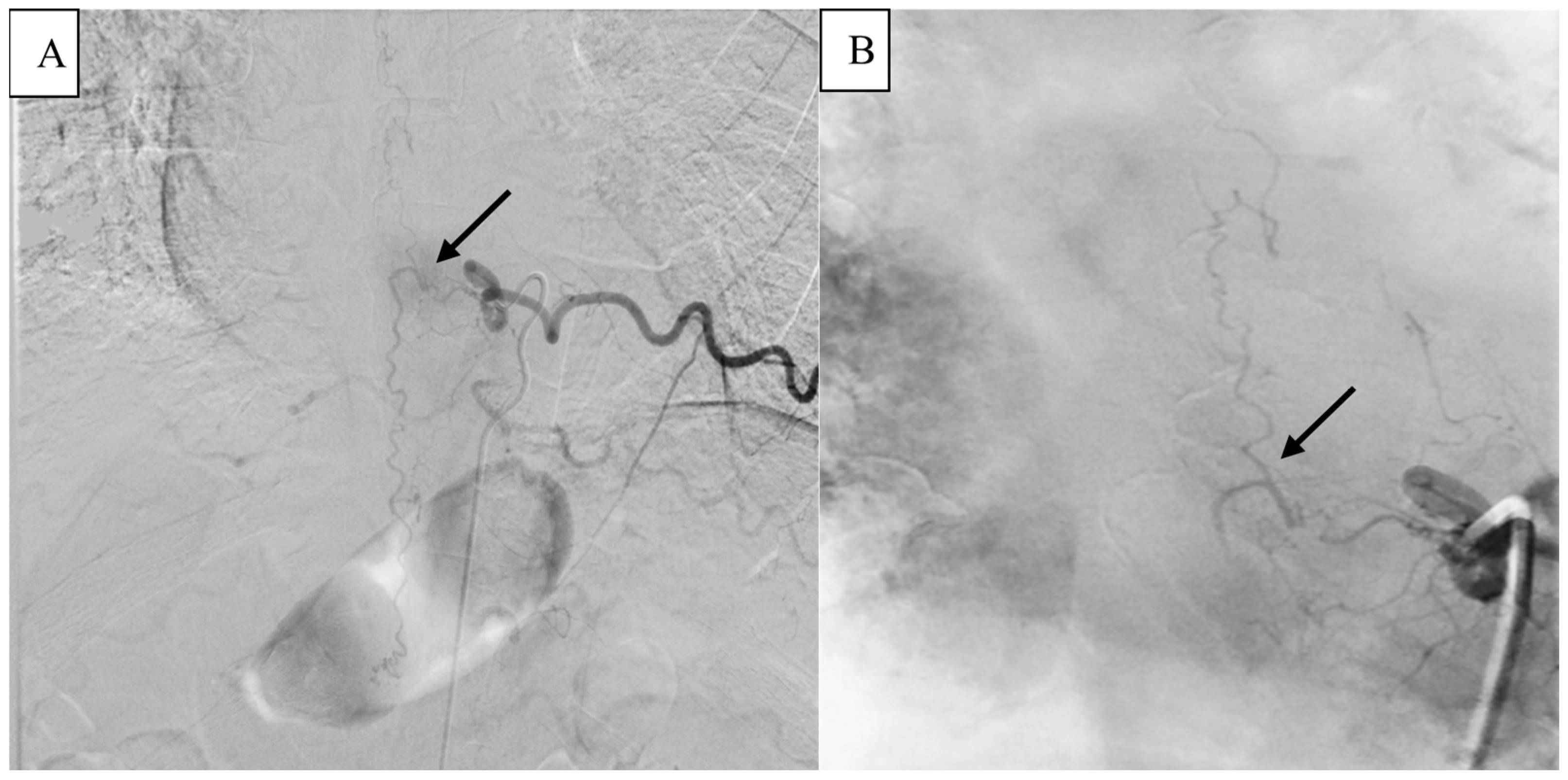
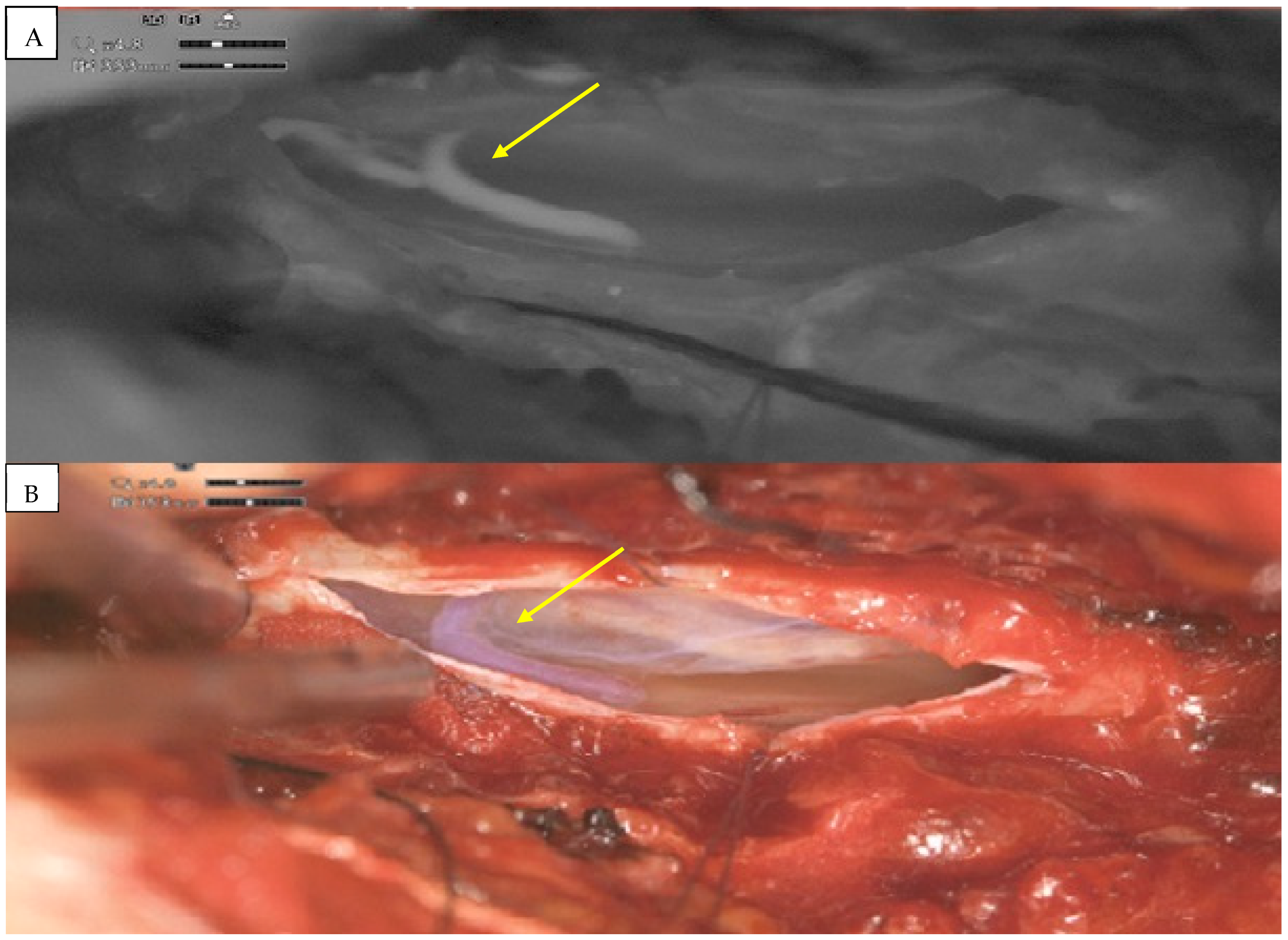
2. Detailed Case Description
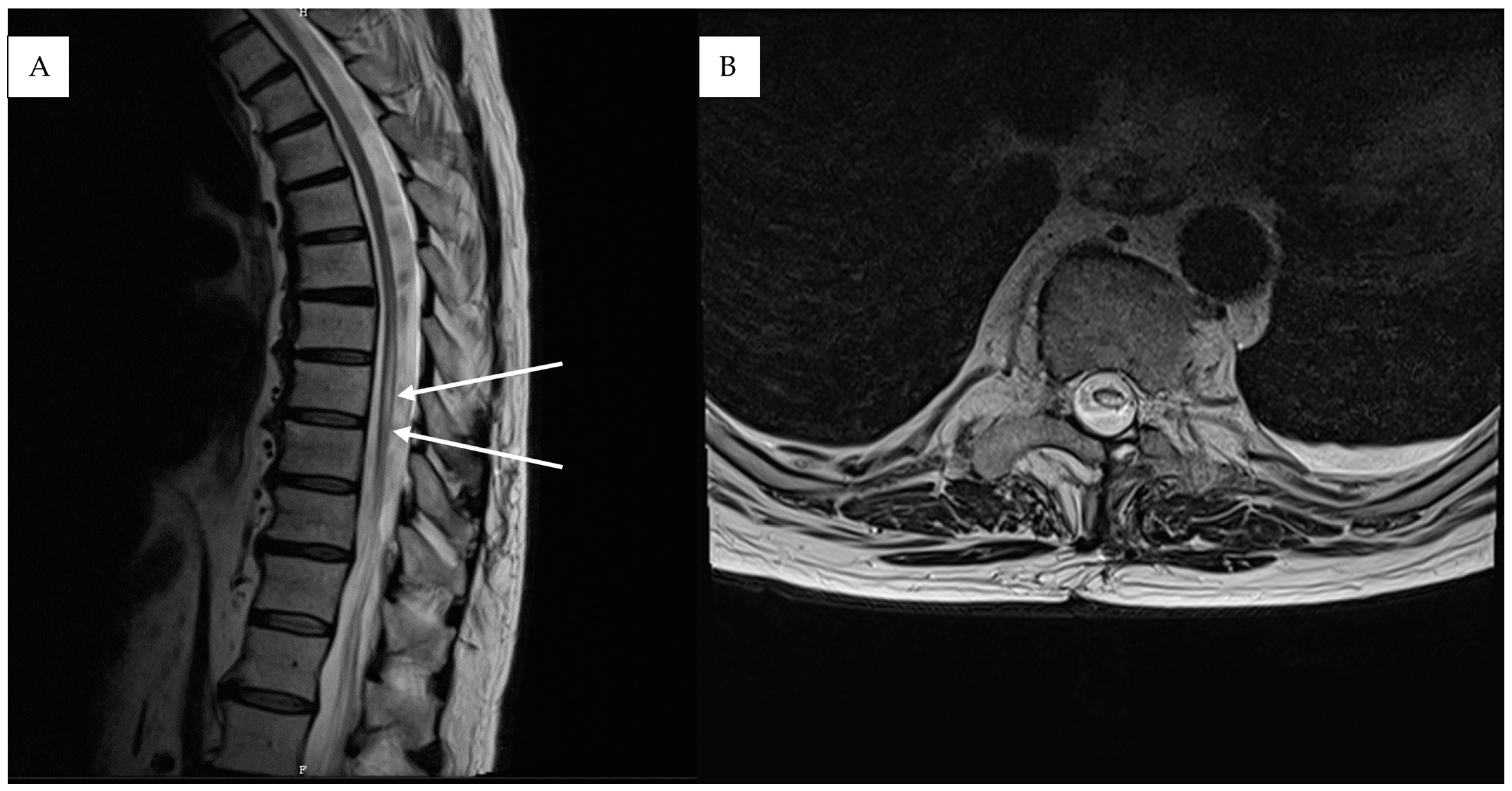
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krings, T.; Lasjaunias, P.L.; Hans, F.J.; Mull, M.; Nijenhuis, R.J.; Alvarez, H.; Backes, W.H.; Reinges, M.H.; Rodesch, G.; Gilsbach, J.M.; et al. Imaging in spinal vascular disease. Neuroimaging Clin. N. Am. 2007, 17, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Kendall, B.E.; Logue, V. Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology 1977, 13, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Hnenny, L.; Ahmed, U.; Meguro, K.; Kelly, M.E. Spinal dural arteriovenous fistula: A case series and review of imaging findings. Spinal Cord Ser. Cases 2017, 3, 17024. [Google Scholar] [CrossRef]
- Rashad, S.; Abdel-Bary, M.; Aziz, W.; Hassan, T. Management of spinal dural arterio-venous fistulas. Report of 12 cases and review of literature. Clin. Neurol. Neurosurg. 2014, 125, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.J.; Spetzler, R.F. Classification and surgical management of spinal arteriovenous lesions: Arteriovenous fistulae and arteriovenous malformations. Neurosurgery 2006, 59 (Suppl. S3), 195–201. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, R.F.; Detwiler, P.W.; Riina, H.A.; Porter, R.W. Modified classification of spinal cord vascular lesions. J. Neurosurg. 2002, 96 (Suppl. S2), 145–156. [Google Scholar] [CrossRef] [PubMed]
- Krings, T.; Geibprasert, S. Spinal Dural Arteriovenous Fistulas. Am. J. Neuroradiol. 2009, 30, 639–648. [Google Scholar] [CrossRef]
- Amanieu, C.; Hermier, M.; Peyron, N.; Chabrol, A.; Deiana, G.; Manera, L. Spinal dural arteriovenous fistula. Diagn. Interv. Imaging 2014, 95, 897–902. [Google Scholar] [CrossRef]
- Jeng, Y.; Chen, D.Y.T.; Hsu, H.L.; Huang, Y.L.; Chen, C.J.; Tseng, Y.C. Spinal Dural Arteriovenous Fistula: Imaging Features and Its Mimics. Korean J. Radiol. 2015, 16, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, D.; Levitt, M.R.; Sekhar, L.N.; Kim, L.J.; Hallam, D.K.; Ghodke, B.V. Management of spinal epidural arteriovenous fistulas: Interventional techniques and results. J. NeuroInterven. Surg. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.P.W.; Ritchie, K.C.; Shankar, J.J.S. Spinal dural arteriovenous fistula: Correlation between radiological and clinical findings. J. Neurosurg. Spine. 2014, 21, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Jellema, K.; Canta, L.R.; Tijssen, C.C.; van Rooij, W.J.; Koudstaal, P.J.; van Gijn, J. Spinal dural arteriovenous fistulas: Clinical features in 80 patients. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1438–1440. [Google Scholar] [CrossRef]
- Atkinson, J.L.D.; Miller, G.M.; Krauss, W.E.; Marsh, W.R.; Piepgras, D.G.; Atkinson, P.P.; Brown, R.D.; Lane, J.I. Clinical and Radiographic Features of Dural Arteriovenous Fistula, a Treatable Cause of Myelopathy. Mayo Clin. Proc. 2001, 76, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, J.R.; Miller, G.M.; Goldman, M.S.; Marsh, W.R. Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am. J. Neuroradiol. 1995, 16, 2049–2057. [Google Scholar] [PubMed]
- Huffmann, B.C.; Spetzger, U.; Reinges, M.; Bertalanffy, H.; Thron, A.; Gilsbach, J.M. Treatment Strategies and Results in Spinal Vascular Malformations. Neurol. Med. Chir. 1998, 38, 231–237. [Google Scholar] [CrossRef]
- Zanin, L.; di Bonaventura, R.; Agosti, E.; di Bergamo, L.T.; Daniele, D.; Saraceno, G.; Auricchio, A.M.; Sturiale, C.L.; Bergui, M.; Mardighian, D.; et al. Surgery versus endovascular treatment for spinal dural arteriovenous fistulas: A multicenter experience and systematic literature review. Neurosurg. Rev. 2024, 47, 206. [Google Scholar] [CrossRef]
- Bretonnier, M.; Hénaux, P.-L.; Gaberel, T.; Roualdes, V.; Kerdiles, G.; Le Reste, P.-J.; Morandi, X. Spinal Dural Arteriovenous Fistulas: Clinical Outcome After Surgery Versus Embolization: A Retrospective Study. World Neurosurg. 2019, 127, e943–e949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xin, W.Q. Application of hybrid operating rooms for treating spinal dural arteriovenous fistula. World J. Clin. Cases 2020, 8, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Calloni, T.; Roumy, L.G.; Cinalli, M.A.; Rocca, A.; Held, A.; Trezza, A.; Carrabba, G.G.; Giussani, C.G. Exoscope as a Teaching Tool: A Narrative Review of the Literature. Front. Surg. 2022, 9, 878293. Available online: https://www.frontiersin.org/articles/10.3389/fsurg.2022.878293 (accessed on 7 February 2024). [CrossRef]
- Belykh, E.G.; Zhao, X.; Cavallo, C.; Bohl, M.A.; Yagmurlu, K.; Aklinski, J.L.; Byvaltsev, V.A.; Sanai, N.; Spetzler, R.F.; Lawton, M.T.; et al. Laboratory Evaluation of a Robotic Operative Microscope—Visualization Platform for Neurosurgery. Cureus 2018, 10, e3072. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, M.H.M.; Ibrahim, K.; Baharudin, A.; Tamil, A.M. Early Experience, Setup, Learning Curve, Benefits, and Complications Associated with Exoscope and Three-Dimensional 4K Hybrid Digital Visualizations in Minimally Invasive Spine Surgery. Asian Spine J. 2019, 14, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.; Schneider, J.R.; Du, V.; Falting, L.; Boockvar, J.A.; Oren, J.; Levine, M.; Langer, D.J. Lessons Learned Using a High-Definition 3-Dimensional Exoscope for Spinal Surgery. Oper. Neurosurg. 2019, 16, 619. [Google Scholar] [CrossRef]
- Smith, S.; Kozin, E.D.; Kanumuri, V.V.; Barber, S.R.; Backous, D.; Nogueira, J.F.; Lee, D.J. Initial Experience with 3-Dimensional Exoscope-Assisted Transmastoid and Lateral Skull Base Surgery. Otolaryngol. Head Neck Surg. 2019, 160, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Scerrati, A.; Ricciardi, L.; Trevisi, G. The Exoscope in Neurosurgery: An Overview of the Current Literature of Intraoperative Use in Brain and Spine Surgery. J. Clin. Med. 2021, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.M.; Calvanese, F.; Vasankari, V.; Raj, R.; Gallé, C.L.C.; Niemelä, M.; Lehecka, M. Digital exoscope versus surgical microscope in spinal dural arteriovenous fistula surgery: A comparative series. Neurosurg. Focus 2024, 56, E13. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonchev, N.; Neyazi, B.; Stein, K.-P.; Sandalcioglu, I.E.; Rashidi, A. Implementation of ORBEYE®-Exoscope in the Operative Treatment of Spinal Dural Arteriovenous Fistula. Medicina 2025, 61, 101. https://doi.org/10.3390/medicina61010101
Tonchev N, Neyazi B, Stein K-P, Sandalcioglu IE, Rashidi A. Implementation of ORBEYE®-Exoscope in the Operative Treatment of Spinal Dural Arteriovenous Fistula. Medicina. 2025; 61(1):101. https://doi.org/10.3390/medicina61010101
Chicago/Turabian StyleTonchev, Nikolay, Belal Neyazi, Klaus-Peter Stein, I. Erol Sandalcioglu, and Ali Rashidi. 2025. "Implementation of ORBEYE®-Exoscope in the Operative Treatment of Spinal Dural Arteriovenous Fistula" Medicina 61, no. 1: 101. https://doi.org/10.3390/medicina61010101
APA StyleTonchev, N., Neyazi, B., Stein, K.-P., Sandalcioglu, I. E., & Rashidi, A. (2025). Implementation of ORBEYE®-Exoscope in the Operative Treatment of Spinal Dural Arteriovenous Fistula. Medicina, 61(1), 101. https://doi.org/10.3390/medicina61010101





