Effect of Third Molar Surgery on Sleep Health Parameters of Young Adults: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Registration
2.2. Study Population
2.3. Clinical Procedures
2.4. Baseline and Follow-Up Measurements
2.4.1. Daytime Sleepiness
2.4.2. Sleep Quality
2.4.3. Insomnia Severity
2.4.4. Subjective Sleep Assessment
2.4.5. Pain Perception
2.5. Sample Size Calculation
2.6. Statistical Analysis
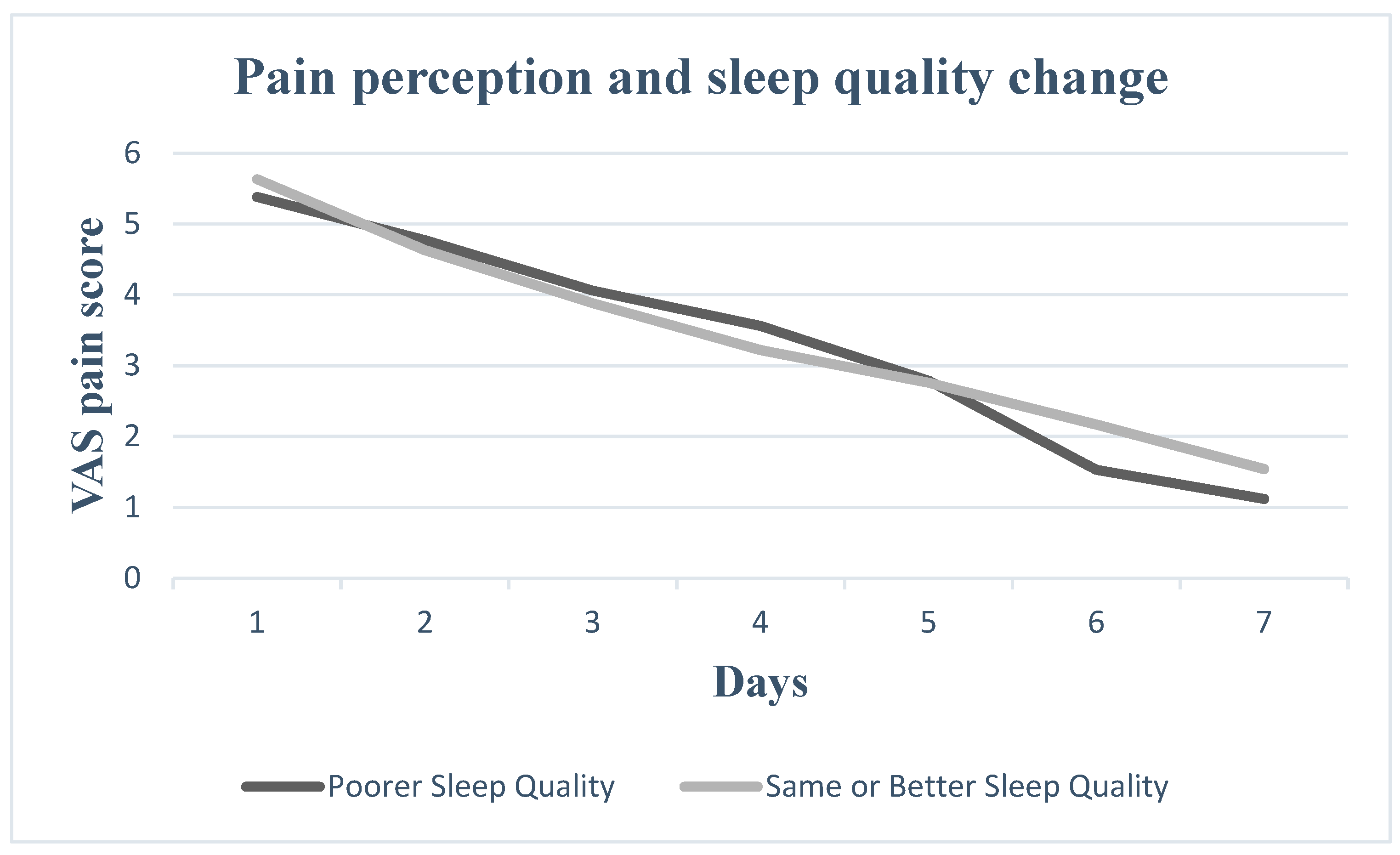
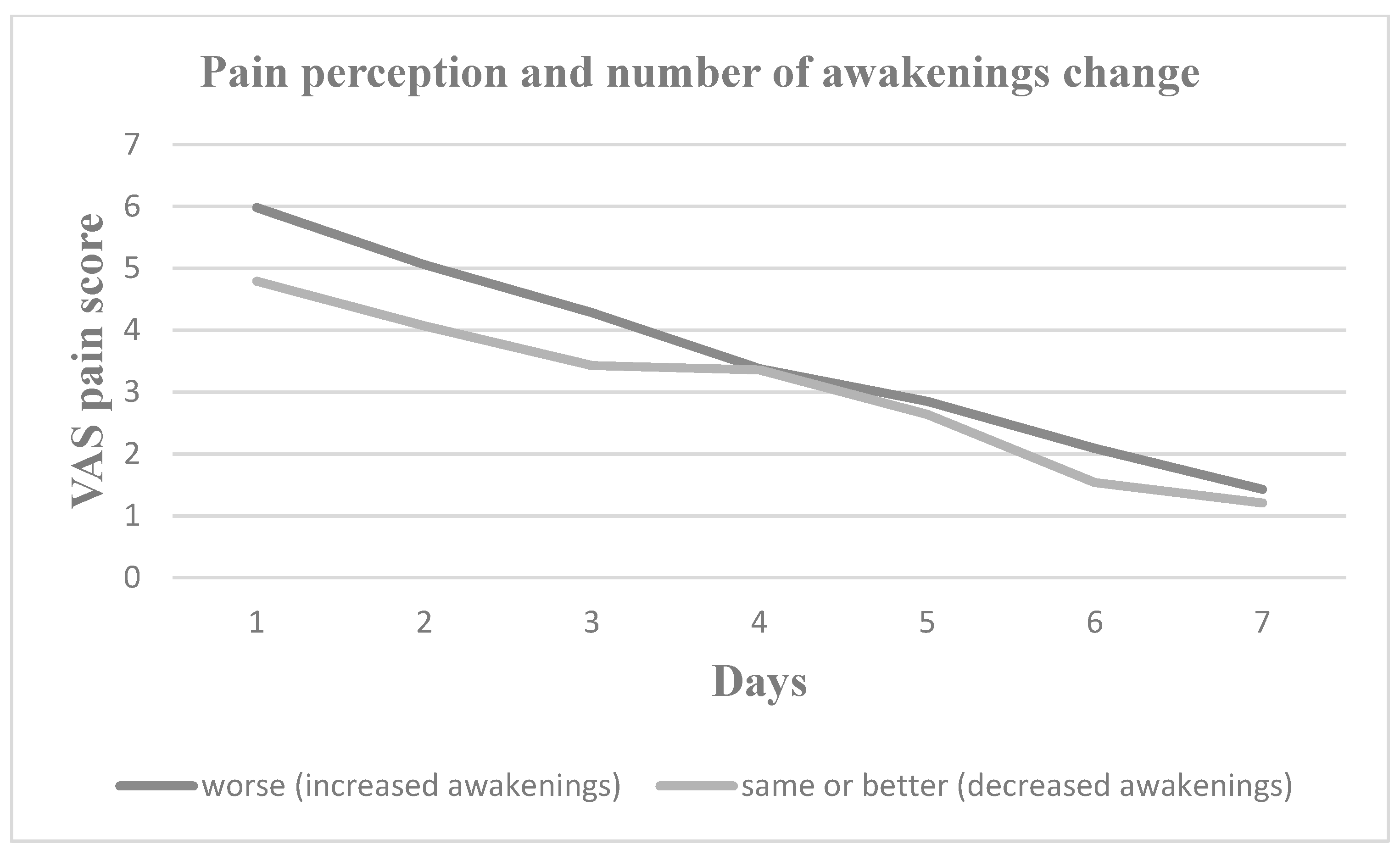
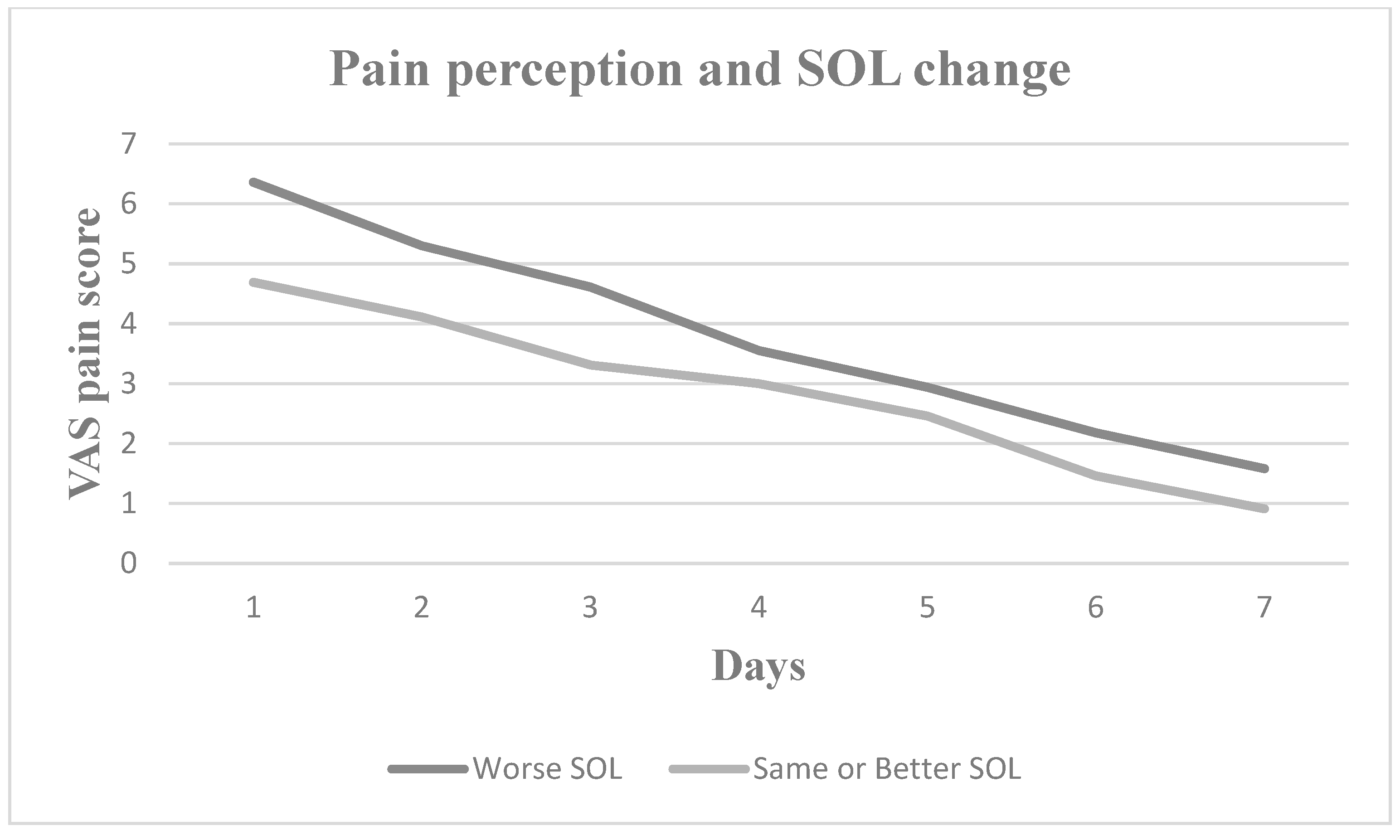
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kautto, A.; Vehkalahti, M.M.; Ventä, I. Age of patient at the extraction of the third molar. Int. J. Oral Maxillofac. Surg. 2018, 47, 947–951. [Google Scholar] [CrossRef] [PubMed]
- American Association of Oral and Maxillofacial Surgeons. White Paper on the Management of Third Molar Teeth; American Association of Oral and Maxillofacial Surgeons: Rosemont, IL, USA, 2016. [Google Scholar]
- Gojayeva, G.; Tekin, G.; Kose, N.S.; Dereci, O.; Kosar, Y.C.; Caliskan, G. Evaluation of complications and quality of life of patient after surgical extraction of mandibular impacted third molar teeth. BMC Oral Health 2024, 24, 131. [Google Scholar] [CrossRef] [PubMed]
- Sologova, D.; Diachkova, E.; Gor, I.; Sologova, S.; Grigorevskikh, E.; Arazashvili, L.; Petruk, P.; Tarasenko, S. Antibiotics Efficiency in the Infection Complications Prevention after Third Molar Extraction: A Systematic Review. Dent. J. 2022, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Dudde, F.; Barbarewicz, F.; Henkel, K.-O. Risk factor analysis for perioperative complications in impacted third molar surgery—A single center experience. Oral Maxillofac. Surg. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Rodrigues, L.; Miranda, E.F.P.; Souza, T.O.; de Paiva, H.N.; Falci, S.G.M.; Galvão, E.L. Third molar removal and its impact on quality of life: Systematic review and meta-analysis. Qual. Life Res. 2018, 27, 2477–2489. [Google Scholar] [CrossRef] [PubMed]
- Colorado-Bonnin, M.; Valmaseda-Castellón, E.; Berini-Aytés, L.; Gay-Escoda, C. Quality of life following lower third molar removal. Int. J. Oral Maxillofac. Surg. 2006, 35, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, S.; Gacic, B.; Konstantinovic, V.S.; Valls Ontañón, A.; Sapundzhiev, A.; Pavlov, N.; Pechalova, P.; Szalma, J.; Mottl, R.; Tamme, T.; et al. Patient’s perception of recovery following surgical removal of mandibular third molars. A prospective european multi-center study. J. Cranio-Maxillofac. Surg. 2023, 51, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Braimah, R.O.; Ndukwe, K.C.; Owotade, F.J.; Aregbesola, S.B. Oral health related quality of life (OHRQoL) following third molar surgery in Sub-Saharan Africans: An observational study. Pan Afr. Med. J. 2016, 25, 97. [Google Scholar] [CrossRef]
- Ramar, K.; Malhotra, R.K.; Carden, K.A.; Martin, J.L.; Abbasi-Feinberg, F.; Aurora, R.N.; Kapur, V.K.; Olson, E.J.; Rosen, C.L.; Rowley, J.A.; et al. Sleep is essential to health: An American Academy of Sleep Medicine position statement. J. Clin. Sleep Med. 2021, 17, 2115–2119. [Google Scholar] [CrossRef]
- Chattu, V.K.; Manzar, M.D.; Kumary, S.; Burman, D.; Spence, D.W.; Pandi-Perumal, S.R. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare 2018, 7, 1. [Google Scholar] [CrossRef]
- Becker, S.P.; Jarrett, M.A.; Luebbe, A.M.; Garner, A.A.; Burns, G.L.; Kofler, M.J. Sleep in a large, multi-university sample of college students: Sleep problem prevalence, sex differences, and mental health correlates. Sleep Health 2018, 4, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rampes, S.; Ma, K.; Divecha, Y.A.; Alam, A.; Ma, D. Postoperative sleep disorders and their potential impacts on surgical outcomes. J. Biomed. Res. 2020, 34, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Dette, F.; Cassel, W.; Urban, F.; Zoremba, M.; Koehler, U.; Wulf, H.; Graf, J.; Steinfeldt, T. Occurrence of rapid eye movement sleep deprivation after surgery under regional anesthesia. Obstet. Anesthesia Dig. 2013, 116, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Chouchou, F.; Khoury, S.; Chauny, J.-M.; Denis, R.; Lavigne, G.J. Postoperative sleep disruptions: A potential catalyst of acute pain? Sleep Med. Rev. 2014, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.; Bali, D.; Sharma, A.; Verma, G. Is Pederson Index a True Predictive Difficulty Index for Impacted Mandibular Third Molar Surgery? A Meta-analysis. J. Maxillofac. Oral Surg. 2012, 12, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Tsara, V.; Serasli, E.; Amfilochiou, A.; Constantinidis, T.; Christaki, P. Greek Version of the Epworth Sleepiness Scale. Sleep Breath. 2004, 8, 91–95. [Google Scholar] [CrossRef]
- McDaid, C.; Durée, K.H.; Griffin, S.C.; Weatherly, H.L.; Stradling, J.R.; Davies, R.J.; Sculpher, M.J.; Westwood, M.E. A systematic review of continuous positive airway pressure for obstructive sleep apnoea–hypopnoea syndrome. Sleep Med. Rev. 2009, 13, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. Sleep propensity varies with behaviour and the situation in which it is measured: The concept of somnificity. J. Sleep Res. 2002, 11, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, G.C.; Papadopoulou, C.N.; Papapetrou, A.; Patiraki, E. Psychometric evaluation and feasibility of the Greek Pittsburgh Sleep Quality Index (GR-PSQI) in patients with cancer receiving chemotherapy. Support. Care Cancer 2011, 19, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. The diagnostic validity of the Athens Insomnia Scale. J. Psychosom. Res. 2003, 55, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. JCSM Pub. Lication Am. Acad. Sleep Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mendoza, J.; Vgontzas, A.N. Insomnia and its impact on physical and mental health. Curr. Psychiatry Rep. 2013, 15, 418. [Google Scholar] [CrossRef]
- Schroeder, A.R.; Newman, T.B.; Girod, S.; Hashemi, S.; Häberle, A.D. Estimated Cumulative Incidence of Wisdom Tooth Extractions in Privately Insured US Patients. Front. Dent. Med. 2022, 3, 937165. [Google Scholar] [CrossRef]
- Anjrini, A.A.; Kruger, E.; Tennant, M. Cost effectiveness modelling of a ‘watchful monitoring strategy’ for impacted third molars vs prophylactic removal under GA: An Australian perspective. Br. Dent. J. 2015, 219, 19–23. [Google Scholar] [CrossRef]
- Dodson, T.B. How Many Patients Have Third Molars and How Many Have One or More Asymptomatic, Disease-Free Third Molars? J. Oral Maxillofac. Surg. 2012, 70, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Finan, P.H.; Goodin, B.R.; Smith, M.T. The association of sleep and pain: An update and a path forward. J. Pain 2013, 14, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, K.; Voulgaris, A.; Steiropoulos, P. Obstructive Sleep Apnea and Pain. Curr. Sleep Med. Rep. 2019, 5, 95–103. [Google Scholar] [CrossRef]
- Herrero Babiloni, A.; De Koninck, B.P.; Beetz, G.; De Beaumont, L.; Martel, M.O.; Lavigne, G.J. Sleep and pain: Recent insights, mechanisms, and future directions in the investigation of this relationship. J. Neural Transm. 2020, 127, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Ibikunle, A.A.; Adeyemo, W.L.; Ladeinde, A.L. Oral health-related quality of life following third molar surgery with either oral administration or submucosal injection of prednisolone. Oral Maxillofac. Surg. 2016, 20, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Wickwire, E.M.; Barger, L.K.; Dement, W.C.; Gamble, K.; Hartenbaum, N.; Ohayon, M.M.; Pelayo, R.; Phillips, B.; Strohl, K.; et al. Sleep-deprived motor vehicle operators are unfit to drive: A multidisciplinary expert consensus statement on drowsy driving. Sleep Health 2016, 2, 94–99. [Google Scholar] [CrossRef]
- Hillman, D.R.; Lack, L.C. Public health implications of sleep loss: The community burden. Med. J. Aust. 2013, 199, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Redeker, N.S.; Caruso, C.C.; Hashmi, S.D.; Mullington, J.M.; Grandner, M.; Morgenthaler, T.I. Workplace Interventions to Promote Sleep Health and an Alert, Healthy Workforce. Sleep Med. 2019, 15, 649–657. [Google Scholar] [CrossRef]
- Vranckx, M.; Fieuws, S.; Jacobs, R.; Politis, C. Surgical experience and patient morbidity after third molar removal. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 297–302. [Google Scholar] [CrossRef]



| Variable | Measurement |
|---|---|
| Gender (male/female) | 32/43 |
| Age (mean ± SD) | 24.01 ± 3.43 years |
| BMI (mean ± SD) | 22.98 ± 3.73 kg/m2 |
| Neck circumference (mean ± SD) | 36.27 ± 4.28 cm |
| Measurement | Before the Third Molar Surgery | After the Third Molar Surgery | p |
|---|---|---|---|
| ESS score | 6.32 (±3.99) | 6.48 (±3.85) | 0.707 |
| PSQI score | 4.85 (±2.32) | 5.39 (±2.75) | 0.041 |
| AIS score | 5.56 (±3.23) | 6.91 (±4.06) | <0.001 |
| Average weekly sleep duration (h) | 7.36 (±1.15) | 7.41 (±1.16) | 0.648 |
| Average weekly sleep onset latency (min) | 25.10 (±21.66) | 26.33 (±19.27) | 0.074 |
| Weekly nocturnal awakenings | 2.01 (±3.72) | 4.19 (±5.20) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apessos, I.; Lillis, T.; Voulgaris, A.; Archontogeorgis, K.; Steiropoulos, P.; Dabarakis, N. Effect of Third Molar Surgery on Sleep Health Parameters of Young Adults: An Observational Study. Medicina 2024, 60, 858. https://doi.org/10.3390/medicina60060858
Apessos I, Lillis T, Voulgaris A, Archontogeorgis K, Steiropoulos P, Dabarakis N. Effect of Third Molar Surgery on Sleep Health Parameters of Young Adults: An Observational Study. Medicina. 2024; 60(6):858. https://doi.org/10.3390/medicina60060858
Chicago/Turabian StyleApessos, Ioulianos, Theodoros Lillis, Athanasios Voulgaris, Kostas Archontogeorgis, Paschalis Steiropoulos, and Nikolaos Dabarakis. 2024. "Effect of Third Molar Surgery on Sleep Health Parameters of Young Adults: An Observational Study" Medicina 60, no. 6: 858. https://doi.org/10.3390/medicina60060858
APA StyleApessos, I., Lillis, T., Voulgaris, A., Archontogeorgis, K., Steiropoulos, P., & Dabarakis, N. (2024). Effect of Third Molar Surgery on Sleep Health Parameters of Young Adults: An Observational Study. Medicina, 60(6), 858. https://doi.org/10.3390/medicina60060858







