A Systematic Literature Review on Inflammatory Markers in the Saliva of Patients with Multiple Sclerosis: A Cause or a Consequence of Periodontal Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Selection Criteria
- -
- Research examining the existence of inflammatory indicators in saliva among individuals diagnosed with MS.
- -
- Research investigating the correlation between PDs and MS.
- -
- English-language scholarly articles, including original research papers, observational studies (such as cross-sectional, case–control, or cohort studies), systematic reviews, and meta-analyses.
- -
- Articles with accessible full-text versions.
- -
- Studies that do not specifically examine the relationship between inflammatory indicators or PDs and MS.
- -
- Articles written in languages other than English—this includes abstracts, conference papers, editorials, and commentaries.
- -
- Studies using non-human subjects.
- -
- Case reports, reviews, and papers that do not present original data or lack appropriate information regarding inflammatory indicators.
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis and Analysis
3. Results
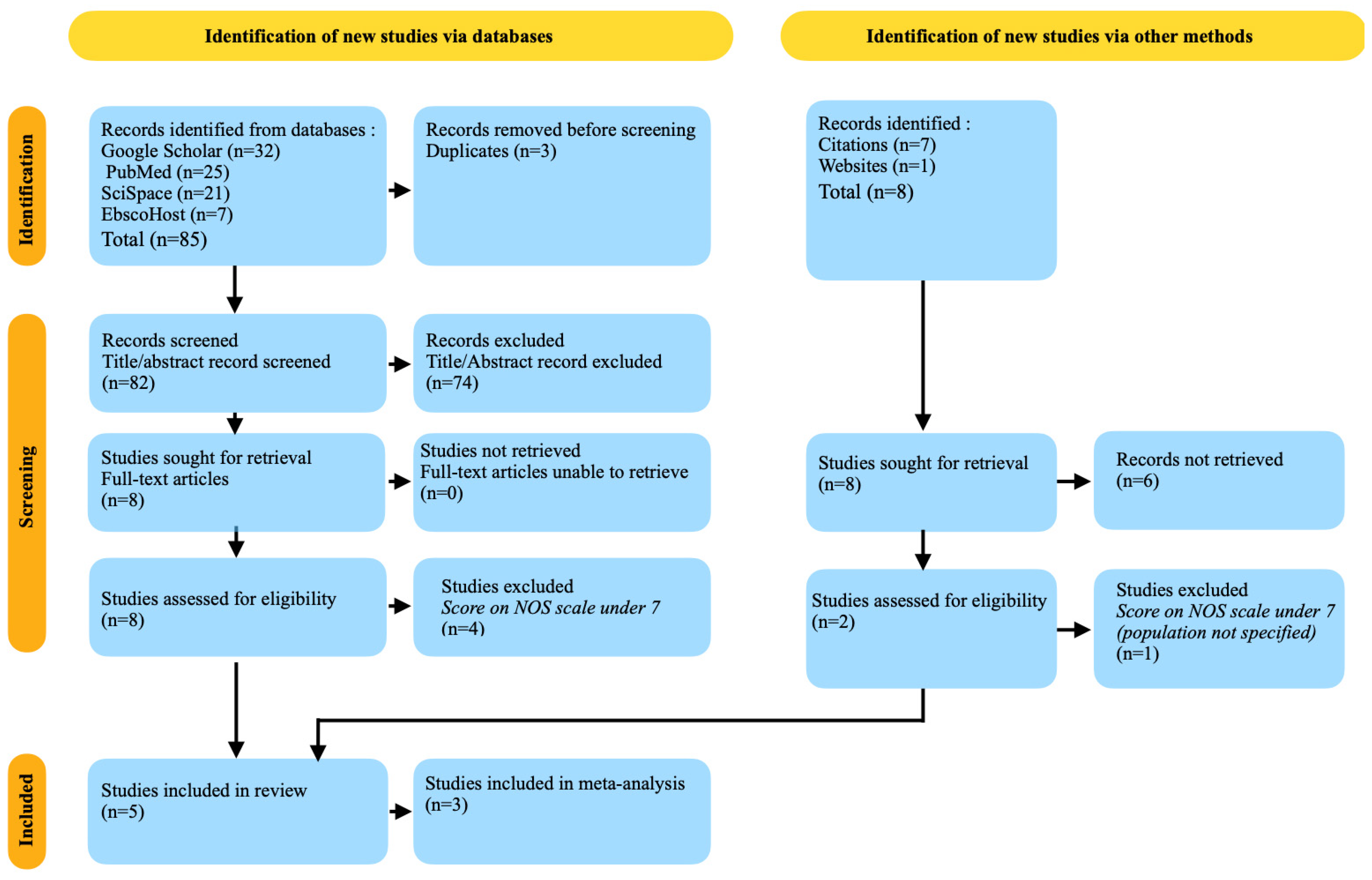
3.1. Databases Research Results
3.2. Other Sources’ Research Results
3.3. Risk of Bias
3.4. Strength of Evidence
3.5. Statistical Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balasa, R.; Barcutean, L.; Balasa, A.; Motataianu, A.; Roman-Filip, C.; Manu, D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum. Immunol. 2020, 81, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, A.F. Summery, perspective, and future studies on neurodegeneration and regeneration in neurological disorders. In Molecular Aspects of Neurodegeneration, Neuroprotection, and Regeneration in Neurological Disorders; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and treatment of multiple sclerosis: A review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Deeb, O.; Salameh, S.; Atallah, A. Exploring the Effect of Genetic, Environmental and Lifestyle Factors on Multiple Sclerosis Susceptibility; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Xiaodong, M. Elucidating the Mechanisms of Disease-Triggering Myelin-Specific Autoantibodies. Ph.D. Thesis, Georg-August Universität, Göttingen, Germany, 2022. [Google Scholar] [CrossRef]
- Bassi, M.S.; Iezzi, E.; Centonze, D. Multiple sclerosis: Inflammation, autoimmunity and plasticity. Handb. Clin. Neurol. 2022, 184, 457–470. [Google Scholar] [CrossRef]
- Pietsch, A.M.; Viehöver, A.; Diem, R.; Weiler, M.; Korporal-Kuhnke, M.; Wildemann, B.; Sam, G.; Hayes, J.M.; Fösleitner, O.; Jende, J.M.; et al. Quantification and Proximal-to-Distal Distribution Pattern of Tibial Nerve Lesions in Relapsing-Remitting Multiple Sclerosis. Clin. Neuroradiol. 2022, 33, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Schepici, G.; Silvestro, S.; Trubiani, O.; Bramanti, P.; Mazzon, E. Salivary Biomarkers: Future Approaches for Early Diagnosis of Neurodegenerative Diseases. Brain Sci. 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Tsimpiris, A.; Tsolianos, I.; Grigoriadis, A.; Tsimtsiou, Z.; Goulis, D.G.; Grigoriadis, N. Association of chronic periodontitis with multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2023, 77, 104874. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kiprowska, M.; Kansara, T.; Kansara, P.; Li, P. Neuroinflammation: A Distal Consequence of Periodontitis. J. Dent. Res. 2022, 101, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting Probiotics in Rheumatoid Arthritis. Nutrients 2021, 13, 3376. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, R.D.; Baharuddin, N.A.; Rahman, M.T.; Teughels, W.; Lamont, R.J. Periodontitis and Systemic Diseases. In Immunology for Dentistry; Wiley: Hoboken, NJ, USA, 2023; pp. 114–133. [Google Scholar] [CrossRef]
- Kedlaya, M.N.; Puzhankara, L.; Prasad, R.; Raj, A. Periodontal Disease Pathogens, Pathogenesis, and Therapeutics: The CRISPR-Cas Effect. CRISPR J. 2023, 6, 90–98. [Google Scholar] [CrossRef]
- Brown, J.L.; Townsend, E.; Short, R.D.; Williams, C.; Woodall, C.; Nile, C.J.; Ramage, G. Assessing the inflammatory response to in vitro polymicrobial wound biofilms in a skin epidermis model. npj Biofilms Microbiomes 2022, 8, 19. [Google Scholar] [CrossRef]
- Debnath, K.; Nisha, S.; Das, D.; Goswami, N.; Barai, S. Periodontal Health-The Gordian Knot in Public Health: The Indian Standpoint. J. Clin. Diagn. Res. 2023, 17, 14. [Google Scholar] [CrossRef]
- Dede, F.Ö.; Doğan, Ş.B. Chemokines in Periodontal Diseases. In Chemokines Updates; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Ha, J.Y.; Seok, J.; Kim, S.J.; Jung, H.J.; Ryu, K.Y.; Nakamura, M.; Jang, I.S.; Hong, S.H.; Lee, Y.; Lee, H.J. Periodontitis promotes bacterial extracellular vesicle-induced neuroinflammation in the brain and trigeminal ganglion. PLoS Pathog. 2023, 19, e1011743. [Google Scholar] [CrossRef]
- Franciotti, R.; Pignatelli, P.; Carrarini, C.; Romei, F.M.; Mastrippolito, M.; Gentile, A.; Mancinelli, R.; Fulle, S.; Piattelli, A.; Onofrj, M.; et al. Exploring the Connection between Porphyromonas gingivalis and Neurodegenerative Diseases: A Pilot Quantitative Study on the Bacterium Abundance in Oral Cavity and the Amount of Antibodies in Serum. Biomolecules 2021, 11, 845. [Google Scholar] [CrossRef]
- Kalhan, A.C.; Wong, M.L.; Allen, F.; Gao, X. Periodontal disease and systemic health: An update for medical practitioners. Ann. Acad. Med. Singap. 2022, 51, 567–574. [Google Scholar] [CrossRef]
- Bai, X.B.; Zhou, L.J.; Lin, W.Z.; Zhu, Y.Q. Research progress on association of neurological disorders and periodontal diseases. Zhonghua Kou Qiang Yi Xue Za Zhi = Zhonghua Kouqiang Yixue Zazhi Chin. J. Stomatol. 2022, 57, 529–534. [Google Scholar] [CrossRef]
- Al Johani, K.; Fudah, M.; Al-Zahrani, M.; Abed, H.; Srivastava, K.C.; Shrivastava, D.; Cicciù, M.; Minervini, G. Multiple Sclerosis—A Demyelinating Disorder and Its Dental Considerations—A Literature Review with Own Case Report. Brain Sci. 2023, 13, 1009. [Google Scholar] [CrossRef]
- Jung, S.; Gavriiloglou, M.; Séverac, F.; Haumesser, L.; Sayeh, A.; Chatelus, E.; Martin, T.; Huck, O. Influence of systemic sclerosis on periodontal health: A case–control study. J. Clin. Periodontol. 2023, 50, 1348–1359. [Google Scholar] [CrossRef]
- Tkachuk, V.A.; Aguirre, R.; Cao, J.; Nadur, D.; Isa, S.; Aguirre, M.E.B.; Perachino, P.; Contentti, E.C. Oral health-related quality of life in multiple sclerosis and neuromyelitis optica spectrum disorder patients from Argentina (P5-3.007). Neurology 2023, 100, 4162. [Google Scholar] [CrossRef]
- Nangle, M.R.; Manchery, N.; Swayne, A.; Boocock, H.; Blum, S.; Henry, J.D. Oral health-related quality of life is more strongly correlated with mental health than with oral health in relapsing–remitting multiple sclerosis. J. Oral Rehabil. 2022, 50, 62–68. [Google Scholar] [CrossRef]
- Martin, A.; Emorine, T.; Megdiche, I.; Créange, A.; Kober, T.; Massire, A.; Bapst, B. Accurate Diagnosis of Cortical and Infratentorial Lesions in Multiple Sclerosis Using Accelerated Fluid and White Matter Suppression Imaging. Investig. Radiol. 2022, 58, 337–345. [Google Scholar] [CrossRef]
- Hegen, H.; Arrambide, G.; Gnanapavan, S.; Kaplan, B.; Khalil, M.; Saadeh, R.; Teunissen, C.; Tumani, H.; Villar, L.M.; Willrich, M.A.V.; et al. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A consensus statement. Mult. Scler. J. 2022, 29, 182–195. [Google Scholar] [CrossRef]
- Rohin, G.; Karlie, R.S.; Prabhu, D.E. Physiology, Blood Brain Barrier; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557721/ (accessed on 24 January 2024).
- Isola, G. Saliva biotechnology as a diagnostic tool for periodontal diseases: New challenges for clinical practice. Front. Biosci. 2022, 14, 2022. [Google Scholar] [CrossRef]
- Fehlhofer, J.; Ries, J.; Nickel, F.T.; Rothhammer, V.; Schwab, S.; Kesting, M.; Buchbender, M. Expression of Inflammatory Mediators in Biofilm Samples and Clinical Association in Multiple Sclerosis Patients in Remission—A Pilot Study. Life 2024, 14, 367. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Casati, S.; Goldoni, R.; Thomaz, D.V.; Kehr, N.S.; Galimberti, D.; Del Fabbro, M.; Tartaglia, G.M. Salivary biomarkers: Novel noninvasive tools to diagnose chronic inflammation. Int. J. Oral Sci. 2023, 15, 27. [Google Scholar] [CrossRef]
- Manchery, N.; Henry, J.D.; Nangle, M.R. A systematic review of oral health in people with multiple sclerosis. Community Dent. Oral Epidemiol. 2020, 48, 89–100. [Google Scholar] [CrossRef]
- Shi, F.; Liu, W.; Yao, Y.; Zhang, Q.; Chen, Z.; Xian, Y.; Sujanamulk, B. Predictive salivary biomarkers for early diagnosis of periodontal diseases—Current and future developments. Turk. J. Biochem. 2023, 48, 335–344. [Google Scholar] [CrossRef]
- Buchbender, M.; Fehlhofer, J.; Proff, P.; Möst, T.; Ries, J.; Hannig, M.; Neurath, M.F.; Gund, M.; Atreya, R.; Kesting, M. Expression of inflammatory mediators in biofilm samples and clinical association in inflammatory bowel disease patients—A preliminary study. Clin. Oral Investig. 2022, 26, 1217–1228. [Google Scholar] [CrossRef]
- Suzuki, A.; Iwata, J. Molecular Regulatory Mechanism of Exocytosis in the Salivary Glands. Int. J. Mol. Sci. 2018, 19, 3208. [Google Scholar] [CrossRef]
- Athikarisamy, S.; Patole, S. Reporting of Meta-Analysis (PRISMA). In Principles and Practice of Systematic Reviews and Meta-Analysis; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Stang, A.; Jonas, S.; Poole, C. Case study in major quotation errors: A critical commentary on the Newcastle-Ottawa scale. Eur. J. Epidemiol. 2018, 33, 1025–1031. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- De Santis, K.K.; Pieper, D.; Lorenz, R.C.; Wegewitz, U.; Siemens, W.; Matthias, K. User experience of applying AMSTAR 2 to appraise systematic reviews of healthcare interventions: A commentary. BMC Med. Res. Methodol. 2023, 23, 63. [Google Scholar] [CrossRef]
- StataCorp Stata MP 16.0 Free Download. Available online: https://filecr.com/windows/stata-mp/?id=178670608000 (accessed on 11 March 2024).
- Sophie, K. Statistical Methods in Research; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Goldsmith, C.H. Sensitivity Analysis: Introduction; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Kahale, L.A.; Elkhoury, R.; El Mikati, I.; Pardo-Hernandez, H.; Khamis, A.M.; Schünemann, H.J.; Haddaway, N.R.; Akl, E.A. Tailored PRISMA 2020 flow diagrams for living systematic reviews: A methodological survey and a proposal. F1000Research 2022, 10, 192. [Google Scholar] [CrossRef]
- Lo, C.K.L.; Dominik, M.; Mark, L. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Mirzaii-Dizgah, M.-H.; Mirzaii-Dizgah, M.-R.; Mirzaii-Dizgah, I. Serum and Saliva Myelin Basic Protein as Multiple Sclerosis Biomarker. Basic Clin. Neurosci. J. 2021, 12, 309–314. [Google Scholar] [CrossRef]
- Koshkzari, R.; Mirzaii-Dizgah, I.; Moghaddasi, M.; Mirzaii-Dizgah, M.-R. Saliva and Serum Acetylcholinesterase Activity in Multiple Sclerosis. Mol. Neurobiol. 2023, 60, 2884–2888. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Izcovich, A.; Chu, D.K.; Mustafa, R.A.; Guyatt, G.; Brignardello-Petersen, R. A guide and pragmatic considerations for applying GRADE to network meta-analysis. BMJ 2023, 381, e074495. [Google Scholar] [CrossRef]
- Bezerra, C.T.; Grande, A.J.; Galvao, V.K.; dos Santos, D.H.M.; Atallah, A.N.; Silva, V. Assessment of the strength of recommendation and quality of evidence: GRADE checklist. A descriptive study. Sao Paulo Med. J. 2022, 140, 829–836. [Google Scholar] [CrossRef]
- Vural, N.; Duyan, M.; Saridas, A.; Ertas, E.; Kalkan, A. The predictive value of inflammatory biomarkers in the detection of multiple sclerosis attacks. Emerg. Care J. 2023, 19, 11314. [Google Scholar] [CrossRef]
- Brickman, R. Biological Markers in the Diagnosis of Multiple Sclerosis. Microreviews Cell Mol. Biol. 2012, 1, 1–3. [Google Scholar]
- Jafari, A.; Babajani, A.; Rezaei-Tavirani, M. Multiple Sclerosis Biomarker Discoveries by Proteomics and Metabolomics Approaches. Biomark. Insights 2021, 16, 11772719211013352. [Google Scholar] [CrossRef]
- Alizadeh-Ghodsi, M.; Zavvari, A.; Ebrahimi-Kalan, A.; Shiri-Shahsavar, M.R.; Yousefi, B. The hypothetical roles of arsenic in multiple sclerosis by induction of inflammation and aggregation of tau protein: A commentary. Nutr. Neurosci. 2016, 21, 92–96. [Google Scholar] [CrossRef]
- Filipoiu, D.C.; Bungau, S.G.; Endres, L.; Negru, P.A.; Bungau, A.F.; Pasca, B.; Radu, A.F.; Tarce, A.G.; Bogdan, M.A.; Behl, T.; et al. Characterization of the Toxicological Impact of Heavy Metals on Human Health in Conjunction with Modern Analytical Methods. Toxics 2022, 10, 716. [Google Scholar] [CrossRef]
- Di Bari, M.; Reale, M.; Di Nicola, M.; Orlando, V.; Galizia, S.; Porfilio, I.; Costantini, E.; D’angelo, C.; Ruggieri, S.; Biagioni, S.; et al. Dysregulated Homeostasis of Acetylcholine Levels in Immune Cells of RR-Multiple Sclerosis Patients. Int. J. Mol. Sci. 2016, 17, 2009. [Google Scholar] [CrossRef]
- Balakesavan, P.; Gokhale, S.R.; Deshmukh, V.; Williams, R.C. Impact of Periodontal Disease on Overall Health. Res. Dev. Med. Med. Sci. 2023, 10, 1–14. [Google Scholar] [CrossRef]
- Grut, V.; Biström, M.; Salzer, J.; Stridh, P.; Lindam, A.; Alonso-Magdalena, L.; Andersen, O.; Jons, D.; Gunnarsson, M.; Vrethem, M.; et al. Systemic inflammation and risk of multiple sclerosis—A presymptomatic case-control study. Mult. Scler. J.-Exp. Transl. Clin. 2022, 8, 20552173221139768. [Google Scholar] [CrossRef]
 ; moderate risk
; moderate risk  ; low risk
; low risk  . (b) Summary plot of review authors’ judgments about each risk of bias item presented as percentages across all included studies: high risk
. (b) Summary plot of review authors’ judgments about each risk of bias item presented as percentages across all included studies: high risk  ; moderate risk
; moderate risk  ; low risk
; low risk  [9,10,30,45,46].
[9,10,30,45,46].
 ; moderate risk
; moderate risk  ; low risk
; low risk  . (b) Summary plot of review authors’ judgments about each risk of bias item presented as percentages across all included studies: high risk
. (b) Summary plot of review authors’ judgments about each risk of bias item presented as percentages across all included studies: high risk  ; moderate risk
; moderate risk  ; low risk
; low risk  [9,10,30,45,46].
[9,10,30,45,46].
| Search Combinations |
|---|
| multiple sclerosis, saliva, inflammatory markers, periodontal diseases |
| multiple sclerosis, saliva, inflammatory markers |
| saliva, inflammatory markers, periodontal diseases |
| multiple sclerosis, periodontal diseases |
| Authors | Year of Publication | Type of Study | Material and Method | Details/Findings | Population Characteristics | Number of Patients | Presence of Multiple Sclerosis | Presence of Periodontal Disease | Type of Inflamatory Markers |
|---|---|---|---|---|---|---|---|---|---|
| Jakob Fehlhofer et al. [30] | 2024 | Case series cohort study | Dental exams, mRNA analysis of inflammatory mediators in plaque samples | Found higher expression of MMP-9 and higher PD in MS patients; no significant difference in IL-2 and IL-10 expression | MS patients in remission compared to healthy controls | Not specified | Yes | Examined (in periodontal pockets) | IL-2, IL-6, IL-10, MMP-7, MMP-9, CD-90 |
| Mohammad Hossein Mirzaii-Dizgah et al. [45] | 2021 | Case-control study | Assayed MBP in serum and saliva | MBP was lower in serum and stimulated saliva of MS patients; significant diagnostic ability for MBP to discriminate MS | 29 healthy women and 32 definitive relapsing-remitting MS patients | 61 (32 MS patients + 29 controls) | Yes | Not directly studied | Myelin Basic Protein (MBP) |
| Athanasios Tsimpiris et al. [10] | 2023 | Systematic review and meta-analysis | Literature search and meta-analysis | High prevalence of CP found among MS patients compared to healthy controls | Included studies with adults having MS or healthy controls | 3376 (868 MS patients + 2508 controls) | Yes | Chronic periodontitis (CP) | Not directly studied (focus on CP association with MS) |
| Roghayeh Koshkzari et al. [46] | 2023 | Case-control study | Assayed acetylcholinesterase activity in saliva and serum | Cholinesterase activity significantly reduced in MS group; identified cutoff values for differentiating MS patients | 30 women with multiple sclerosis and 30 healthy females | 60 (30 MS patients + 30 controls) | Yes | Not directly studied | Acetylcholinesterase Activity |
| Giovanni Schepici et al. [9] | 2020 | Review | Literature review | Overview of studies identifying salivary biomarkers in neurodegenerative diseases, including MS | Studies with adults having neurodegenerative diseases including MS | Not directly studied | Discussed | Not directly studied | Beta-amyloid1–42, TAU, alpha-synuclein, DJ-1 |
| Main Author, Title of Research, Year of Publication | Selection | Comparability | Outcome/Exposure | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | ||
| Jakob Fehlhofer et al., 2024, [30] | 1p | 1p | 1p | 0p | 2p | 1p | 1p | 0p | 7p |
| Mohammad Hossein Mirzaii-Dizgah et al., 2021, [45] | 1p | 1p | 1p | 1p | 1p | 1p | 1p | 0p | 7p |
| Roghayeh Koshkzari et al., 2023, [46] | 1p | 1p | 1p | 1p | 1p | 1p | 1p | 1p | 8p |
| Amstar 2 Critical Criteria | Athanasios Tsimpiris et al. [10] | Giovanni Schepici et al. [9] |
|---|---|---|
| 1. Pico elements clearly stated and research question/objective appropriately framed | Yes | Yes |
| 2.Protocol registered before commencement of the review | Yes | Partial Yes |
| 3. Explanation for excluded studies | Yes | Yes |
| 4. Comprehensive literature search | Yes | Yes |
| 5. Status of publication (i.e., grey literature) used as an inclusion criterion | Yes | No |
| 6. List of excluded studies provided and justified | No | No |
| 7. Risk of bias from individual studies included in review | Yes | No |
| 8. Appropriateness of meta-analytical methods | Partial Yes | Yes |
| 9. Consideration of risk of bias when interpreting the results | Yes | Yes |
| 10. Assessment of presence and impact of publication bias | No | No |
| Fehlhofer et al. [30] | Mirzaii-Dizgah et al. [45] | Tsimpiris et al. [10] | Koshkzari et al. [46] | Schepici et al. [9] | |
|---|---|---|---|---|---|
| Year | 2024 | 2021 | 2023 | 2023 | 2020 |
| Study type | Cohort | Case-control | Meta-analysis | Case-control | Review |
| Initial rating | Moderate | Moderate | Low | Moderate | Low |
| Comparison | MS vs. controls | MS vs. controls | MS vs. controls | MS vs. controls | MS and others |
| Outcome | Periodontal status | MBP levels | Periodontitis prevalence | Cholinesterase activity | Biomarker identification |
| Study limitations (risk of bias) | Moderate | Moderate | Low | Moderate | High |
| Inconsistency | Not significant | Not significant | Not significant | Not significant | Significant |
| Indirectness of evidence | Direct | Direct | Direct | Direct | Indirect |
| Imprecision | Moderate | Moderate | Low | Moderate | High |
| Publication bias | Undetected | Undetected | Undetected | Undetected | Possible |
| Magnitude of effect | Moderate | Moderate | High | Moderate | Low |
| Dose-response association | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| All plausible biases - confounders | Yes | Yes | No | Yes | Yes |
| Final rating | Moderate | High | Moderate | Low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcas, V.C.; Tig, I.A.; Moga, D.F.C.; Vlad, A.L.; Roman-Filip, C.; Fratila, A.M. A Systematic Literature Review on Inflammatory Markers in the Saliva of Patients with Multiple Sclerosis: A Cause or a Consequence of Periodontal Diseases. Medicina 2024, 60, 859. https://doi.org/10.3390/medicina60060859
Arcas VC, Tig IA, Moga DFC, Vlad AL, Roman-Filip C, Fratila AM. A Systematic Literature Review on Inflammatory Markers in the Saliva of Patients with Multiple Sclerosis: A Cause or a Consequence of Periodontal Diseases. Medicina. 2024; 60(6):859. https://doi.org/10.3390/medicina60060859
Chicago/Turabian StyleArcas, Vasile Calin, Ioan Andrei Tig, Doru Florian Cornel Moga, Alexandra Lavinia Vlad, Corina Roman-Filip, and Anca Maria Fratila. 2024. "A Systematic Literature Review on Inflammatory Markers in the Saliva of Patients with Multiple Sclerosis: A Cause or a Consequence of Periodontal Diseases" Medicina 60, no. 6: 859. https://doi.org/10.3390/medicina60060859
APA StyleArcas, V. C., Tig, I. A., Moga, D. F. C., Vlad, A. L., Roman-Filip, C., & Fratila, A. M. (2024). A Systematic Literature Review on Inflammatory Markers in the Saliva of Patients with Multiple Sclerosis: A Cause or a Consequence of Periodontal Diseases. Medicina, 60(6), 859. https://doi.org/10.3390/medicina60060859










