Aspects of Histopathological and Ultrastructural Retinal Changes in Chronic Exposure to Hydroxychloroquine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Retinal Tissue for Optical Microscopy (OM)
2.2. Preparation of Retinal Tissue for Electronic Microscopy (EM)
2.3. Statistics and Morphometric Analysis
3. Results
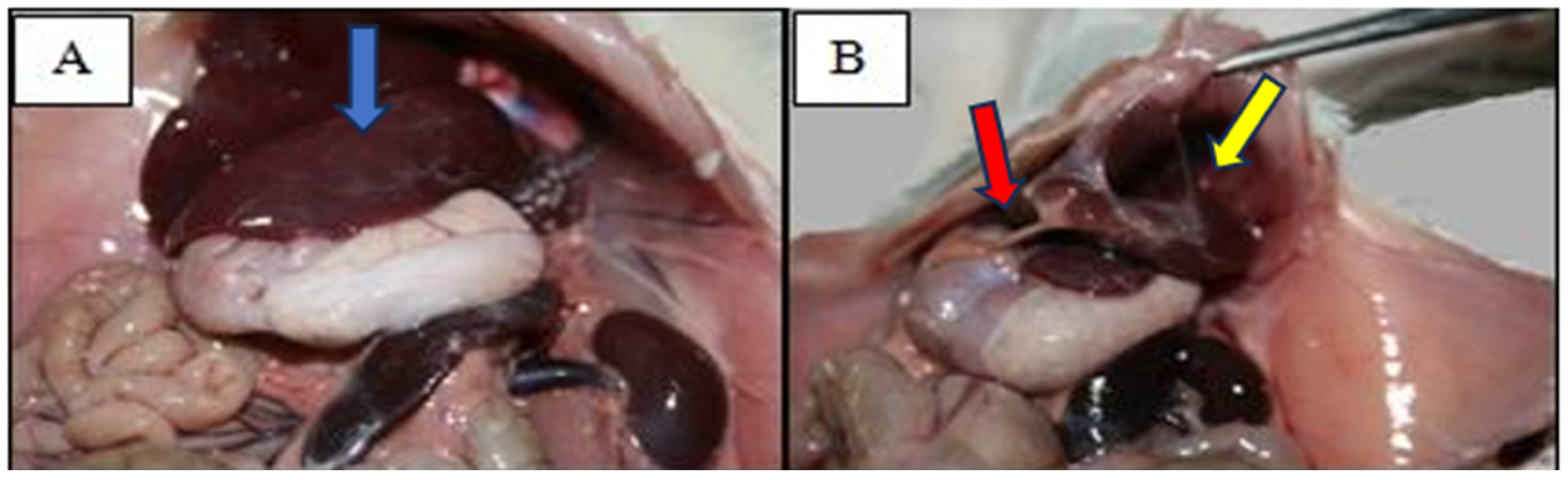
3.1. Pathological Examination
3.2. Retinal Microtopography
3.3. Structural Analysis of the Retina Using Optical Microscopy
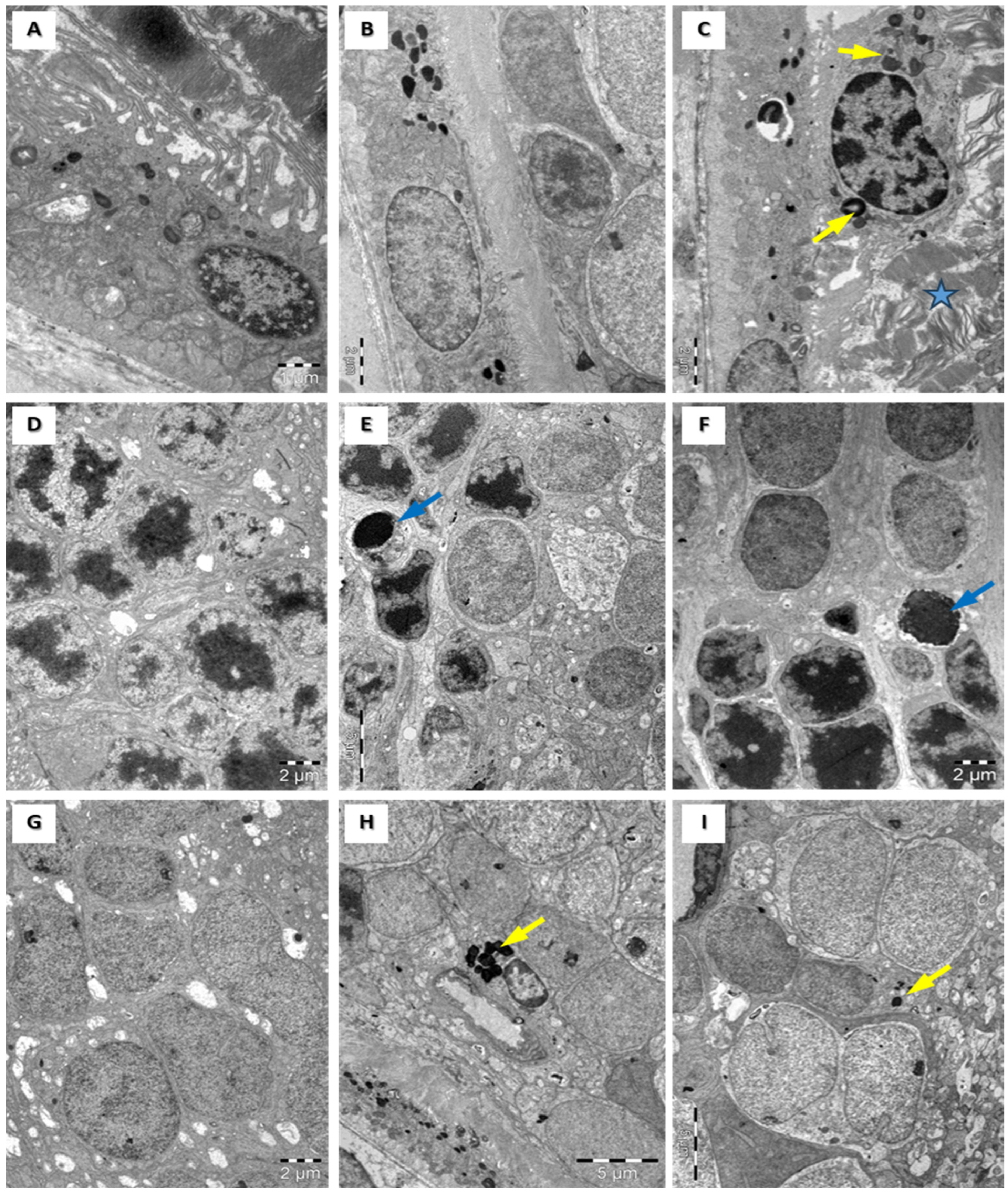
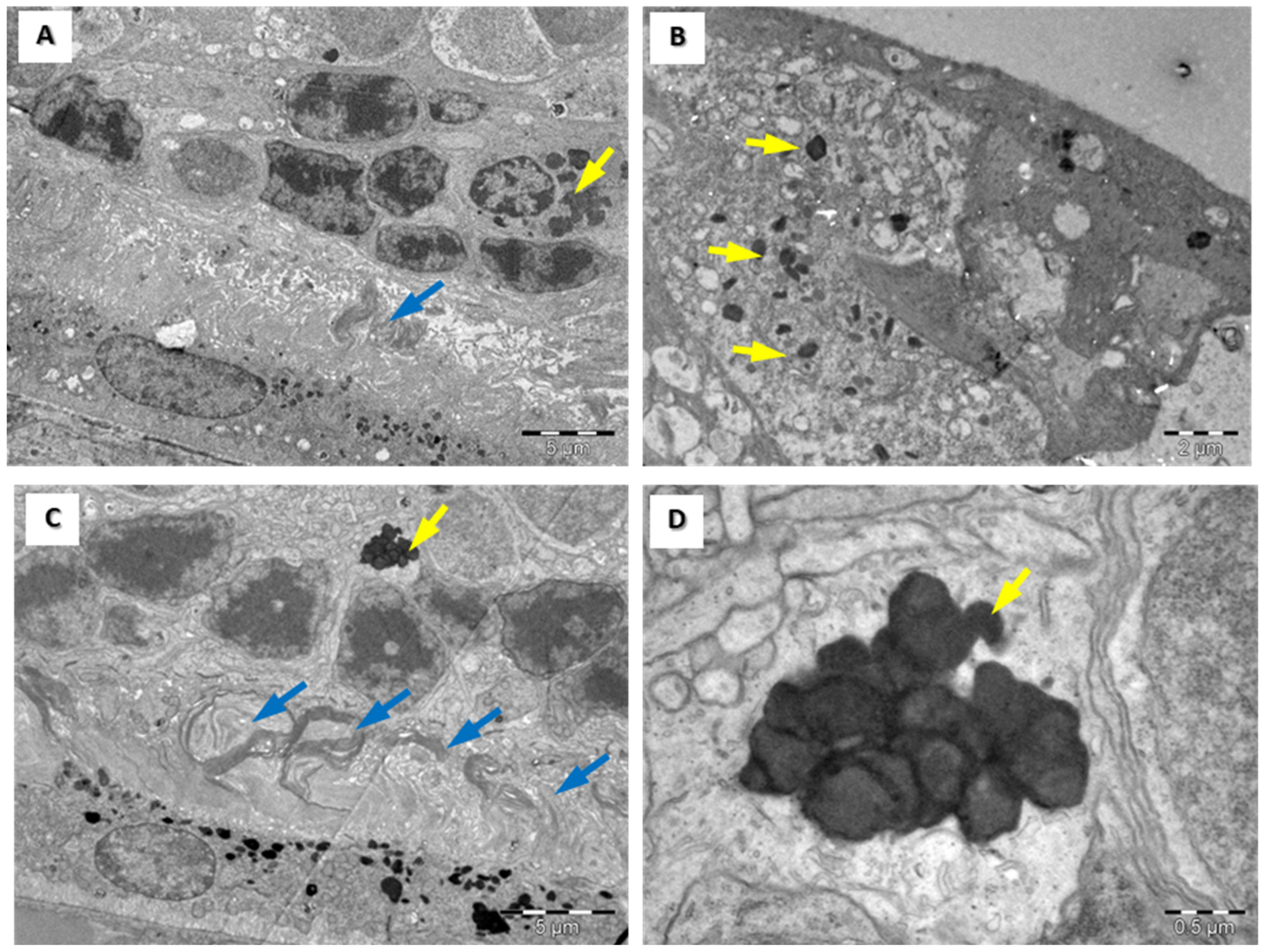
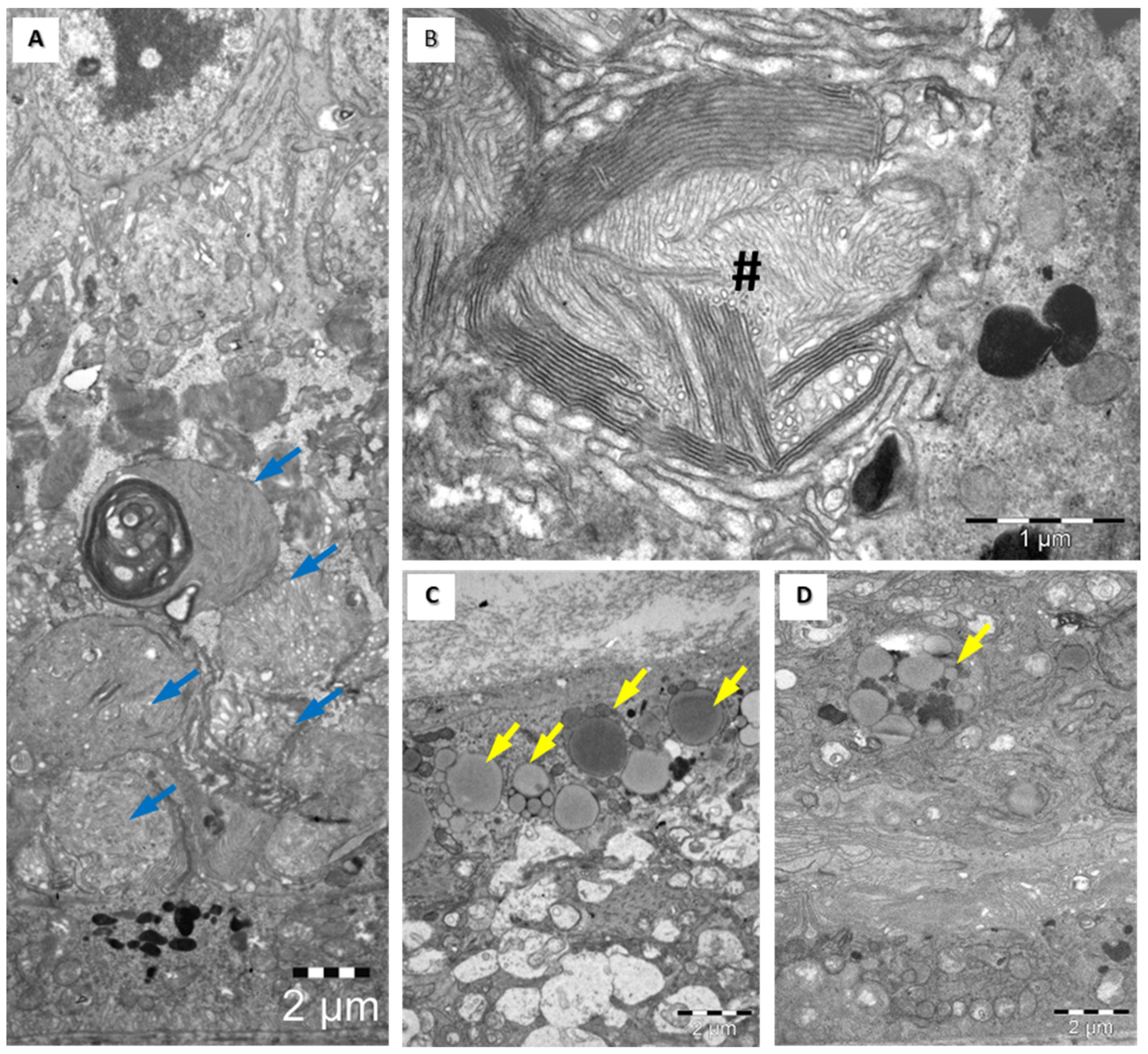
3.4. Structural Assessment of the Retina with Electronic Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geamănu Pancă, A.; Popa-Cherecheanu, A.; Marinescu, B.; Geamănu, C.D.; Voinea, L.M. Retinal Toxicity Associated with Chronic Exposure to Hydroxychloroquine and Its Ocular Screening. Review. J. Med. Life 2014, 7, 322–326. [Google Scholar] [PubMed]
- Yam, J.C.S.; Kwok, A.K.H. Ocular Toxicity of Hydroxychloroquine. Hong Kong Med. J. 2006, 12, 294–304. [Google Scholar] [PubMed]
- Geamanu, A. Modificari Structurale Retiniene Datorate Efectului Expunerii La Hidroclorochina; Editura Universitara “Carol Davila”, Ed.; Editura Universitara “Carol Davila” Bucharest: Bucuresti, Romania, 2020. [Google Scholar]
- Yahalomi, T.; Pikkel, Y.; Arnon, R.; Porat, D.; Pikkel, J. The HIT Study-The Hydroxychloroquine Effect in the Treatment of Patients with Age-Related Macular Degeneration: A Randomized Controlled Trial. Medicina 2023, 59, 551. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L. V A Role for Local Inflammation in the Formation of Drusen in the Aging Eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef] [PubMed]
- Pasadhika, S.; Fishman, G. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye 2010, 24, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, A.; Naeser, P.; Andersson, A. Effects of long-term chloroquine exposure on the phospholipid metabolism in retina and pigment epithelium of the mouse. Acta Ophthalmol. 1990, 68, 125–130. [Google Scholar] [CrossRef] [PubMed]
- in t’ Veld, B.A.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; van Duijn, C.M.; Stijnen, T.; Breteler, M.M.; Stricker, B.H. Nonsteroidal Antiinflammatory Drugs and the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2001, 345, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Schulzer, M.; McGeer, E.G. Arthritis and Anti-Inflammatory Agents as Possible Protective Factors for Alzheimer’s Disease: A Review of 17 Epidemiologic Studies. Neurology 1996, 47, 425–432. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Sibley, J. Sparing of Age-Related Macular Degeneration in Rheumatoid Arthritis. Neurobiol. Aging 2005, 26, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Melles, R.B.; Marmor, M.F. Rapid Macular Thinning Is an Early Indicator of Hydroxychloroquine Retinal Toxicity. Ophthalmology 2022, 129, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Manschot, W.A.; Lee, W.R. Retinal neovascularisation arising from hyalinised blood vessels. Graefe’s Arch. Clin. Exp. Ophthalmol. 1984, 222, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Duncker, G.; Schmiederer, M.; Bredehorn, T. Chloroquine-Induced Lipidosis in the Rat Retina: A Functional and Morphological Study. Ophthalmologica 1995, 209, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fukatsu, R.; Tsuzuki, K.; Aizawa, Y.; Hayashi, Y.; Sasaki, N.; Takamaru, Y.; Fujii, N.; Takahata, N. Amyloid Precursor Protein, A Beta and Amyloid-Associated Proteins Involved in Chloroquine Retinopathy in Rats--Immunopathological Studies. Brain Res. 1997, 764, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Duncker, G.; Bredehorn, T. Chloroquine-Induced Lipidosis in the Rat Retina: Functional and Morphological Changes after Withdrawal of the Drug. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Pasadhika, S.; Fishman, G.A.; Choi, D.; Shahidi, M. Selective Thinning of the Perifoveal Inner Retina as an Early Sign of Hydroxychloroquine Retinal Toxicity. Eye 2010, 24, 756–762; quiz 763. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Hendy, R.J. Irreversible Lysosomal Damage Induced by Chloroquine in the Retinae of Pigmented and Albino Rats. Exp. Mol. Pathol. 1970, 12, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, J.V. Cellular Pathology, Metabolic and Storage Diseases, 1st ed.; Cellular Pathobiology, Metabolic and Storage Diseases; McGraw-Hill: New York, NY, USA.
- Mondal, K.; Porter, H.; Cole, J., 2nd; Pandya, H.K.; Basu, S.K.; Khanam, S.; Chiu, C.Y.; Shah, V.; Stephenson, D.J.; Chalfant, C.E.; et al. Hydroxychloroquine Causes Early Inner Retinal Toxicity and Affects Autophagosome-Lysosomal Pathway and Sphingolipid Metabolism in the Retina. Mol. Neurobiol. 2022, 59, 3873–3887, Erratum in Mol. Neurobiol. 2022, 59, 6611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahon, G.J.; Anderson, H.R.; Gardiner, T.A.; McFarlane, S.; Archer, D.B.; Stitt, A.W. Chloroquine causes lysosomal dysfunction in neural retina and RPE: Implications for retinopathy. Curr. Eye Res. 2004, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kader, D.H.A.; Sayed, S.S.E.; El-Ghamrawy, T.A. Chloroquine-Induced Retinopathy in the Rat. Immunohistochemical and Ultrastructural Study. J. Med. Sci. 2007, 7, 1225–1238. [Google Scholar] [CrossRef]

| Treatment Duration (Months) | HCQ 50 mg/Kg (Group A) Total = 24 Rats | HCQ 100 mg/Kg (Group B) Total = 24 Rats | HCQ 200 mg/Kg (Group C) Total = 24 Rats | Saline 0.4 mL (Control Group) Total = 24 Rats |
|---|---|---|---|---|
| 1 | 1 A (n = 6) | 1 B (n = 6) | 1 C (n = 6) | 1 M (n = 6) |
| 2 | 2 A (n = 6) | 2 B (n = 6) | 2 C (n = 6) | 2 M (n = 6) |
| 3 | 3 A (n = 6) | 3 B (n = 6) | 3 C (n = 6) | 3 M (n = 6) |
| 4 | 4 A (n = 6) | 4 B (n = 6) | 4 C (n = 6) | 4 M (n = 6) |
| Total: 96 RATS | Group A (50 mg/kg) | Group B (100 mg/kg) | Group C (200 mg/kg) | Control Group M (Saline 0.4 mL) |
|---|---|---|---|---|
| Rats sacrificed every month (1, 2, 3, 4) | 6 | 6 | - | 6 |
| Deceased rats (spontaneously) within 48 h of the experiment | 0 | 0 | 24 | 0 |
| Groups | AVERAGES | |||
|---|---|---|---|---|
| Total Retinal Thickness (µm) ± SD | Inner Retinal Thickness (µm) ± SD | Outer Retinal Thickness (µm) ± SD | Retinal Pigment Epithelium Thickness (µm) ± SD | |
| Control group 1 M | 150.00 ± 1.10 | 88.00 ± 1.26 | 62.00 ± 1.10 | 13.50 ± 1.05 |
| Subgroup 1 A | 151.00 ± 0.89 *** | 88.67 ± 1.03 | 62.33 ± 1.21 | 13.67 ± 0.82 |
| Subgroup 1 B | 149.83 ± 1.33 | 87.83 ± 1.33 | 62.00 ± 1.10 | 13.50 ± 1.05 |
| Control group 2 M | 150.00 ± 1.10 | 87.00 ± 0.89 | 64.00 ± 0.89 | 13.00 ± 1.41 |
| Subgroup 2 A | 154.00 ± 0.63 ** | 91.00 ± 1.10 * | 63.00 ± 1.10 ** | 13.50 ± 0.82 |
| Subgroup 2 B | 137.50 ± 1.76 * | 75.83 ± 0.75 * | 61.67 ± 1.97 ** | 11.17 ± 1.17 ** |
| Control group 3 M | 150.00 ± 1.10 | 88.00 ± 1.26 | 62.00 ± 2.00 | 13.00 ± 0.89 |
| Subgroup 3 A | 157.50 ± 1.05 * | 93.17 ± 0.75 * | 64.33 ± 1.03 ** | 15.17 ± 1.17 * |
| Subgroup 3 B | 133.80 ± 0.75 * | 74.67 ± 0.82 * | 59.17 ± 1.17 * | 9.50 ± 0.55 * |
| Control group 4 M | 151.00 ± 1.79 | 87.00 ± 0.84 | 64.00 ± 1.79 | 13.00 ± 1.55 * |
| Subgroup 4 A | 162.00 ± 0.63 * | 95.00 ± 0.89 * | 67.00 ± 1.26 * | 17.17 ± 0.75 * |
| Subgroup 4 B | 115.00 ± 0.63 * | 55.17 ± 0.98 * | 59.83 ± 0.98 * | 9.00 ± 0.89 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geamănu, A.; Baciu, A.E.; Pirvulescu, R.; Iancu, R.; Anton, N.; Popa-Cherecheanu, A.; Ghita, A.M.; Romanitan, M.O. Aspects of Histopathological and Ultrastructural Retinal Changes in Chronic Exposure to Hydroxychloroquine. Medicina 2024, 60, 846. https://doi.org/10.3390/medicina60060846
Geamănu A, Baciu AE, Pirvulescu R, Iancu R, Anton N, Popa-Cherecheanu A, Ghita AM, Romanitan MO. Aspects of Histopathological and Ultrastructural Retinal Changes in Chronic Exposure to Hydroxychloroquine. Medicina. 2024; 60(6):846. https://doi.org/10.3390/medicina60060846
Chicago/Turabian StyleGeamănu, Aida, Ancuţa Elena Baciu, Ruxandra Pirvulescu, Raluca Iancu, Nicoleta Anton, Alina Popa-Cherecheanu, Aurelian Mihai Ghita, and Mihaela Oana Romanitan. 2024. "Aspects of Histopathological and Ultrastructural Retinal Changes in Chronic Exposure to Hydroxychloroquine" Medicina 60, no. 6: 846. https://doi.org/10.3390/medicina60060846
APA StyleGeamănu, A., Baciu, A. E., Pirvulescu, R., Iancu, R., Anton, N., Popa-Cherecheanu, A., Ghita, A. M., & Romanitan, M. O. (2024). Aspects of Histopathological and Ultrastructural Retinal Changes in Chronic Exposure to Hydroxychloroquine. Medicina, 60(6), 846. https://doi.org/10.3390/medicina60060846








