Association between Statin Use and Chemotherapy-Induced Cardiotoxicity: A Meta-Analysis
Abstract
1. Introduction
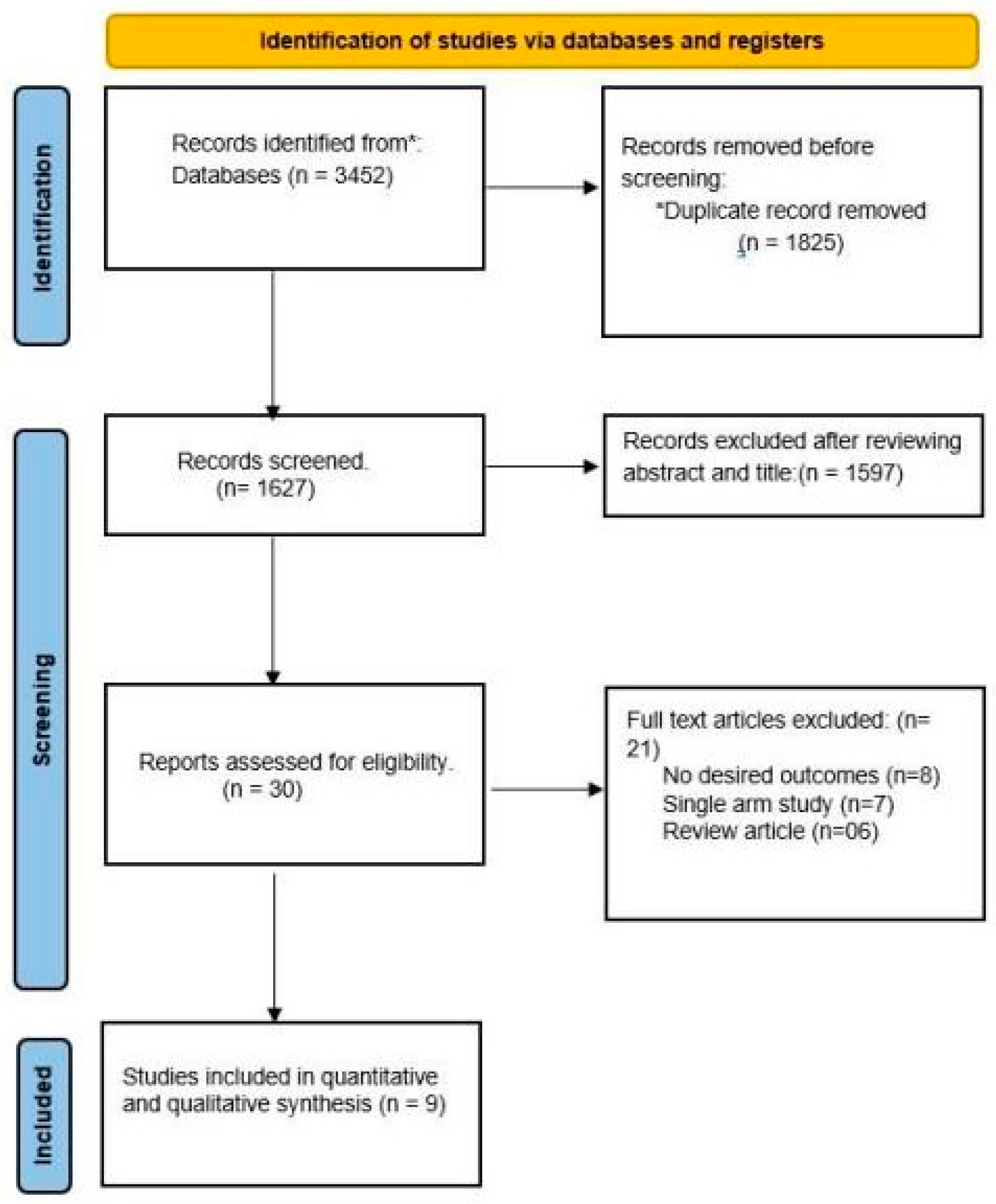
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
3. Clinical Outcomes
4. Data Extraction and Quality Assessment
5. Statistical Analysis
6. Results
6.1. Baseline Characteristics of Patients in Included Studies
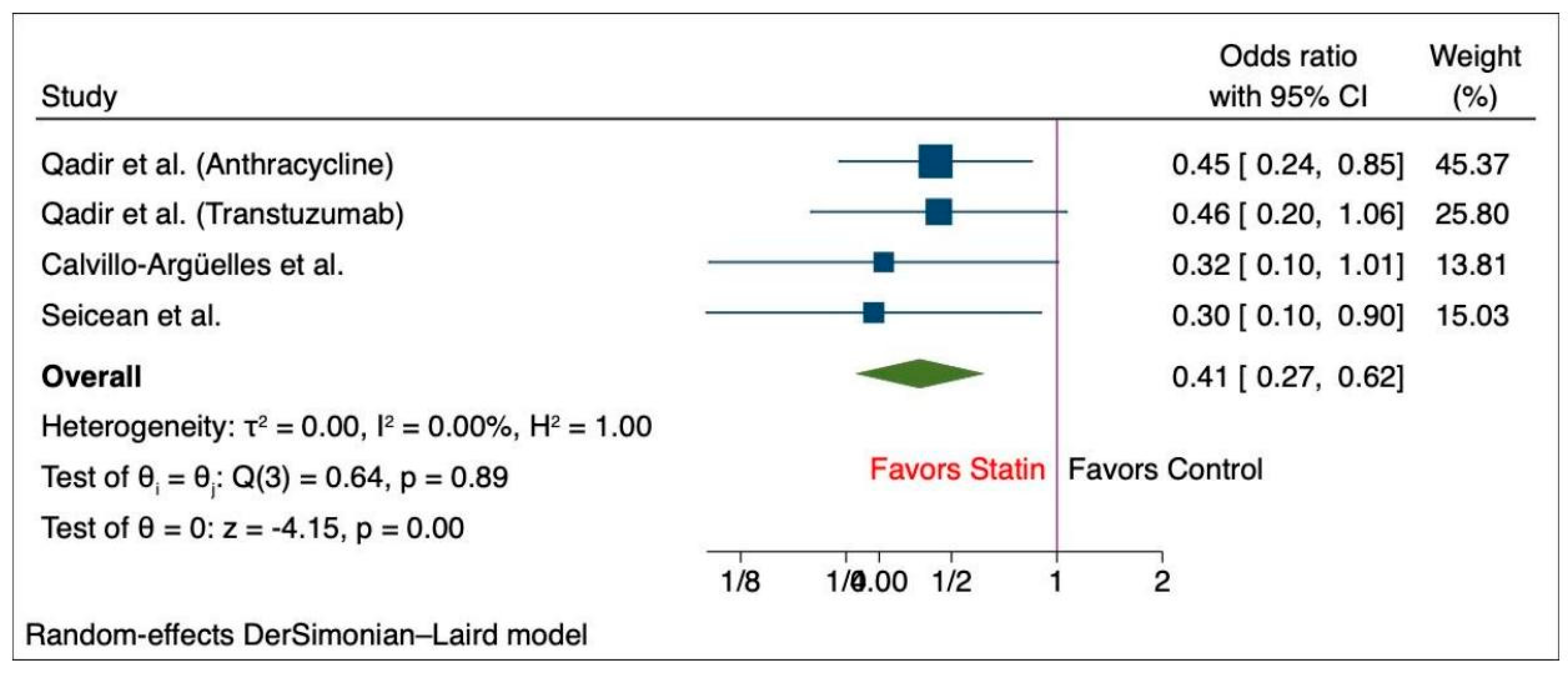
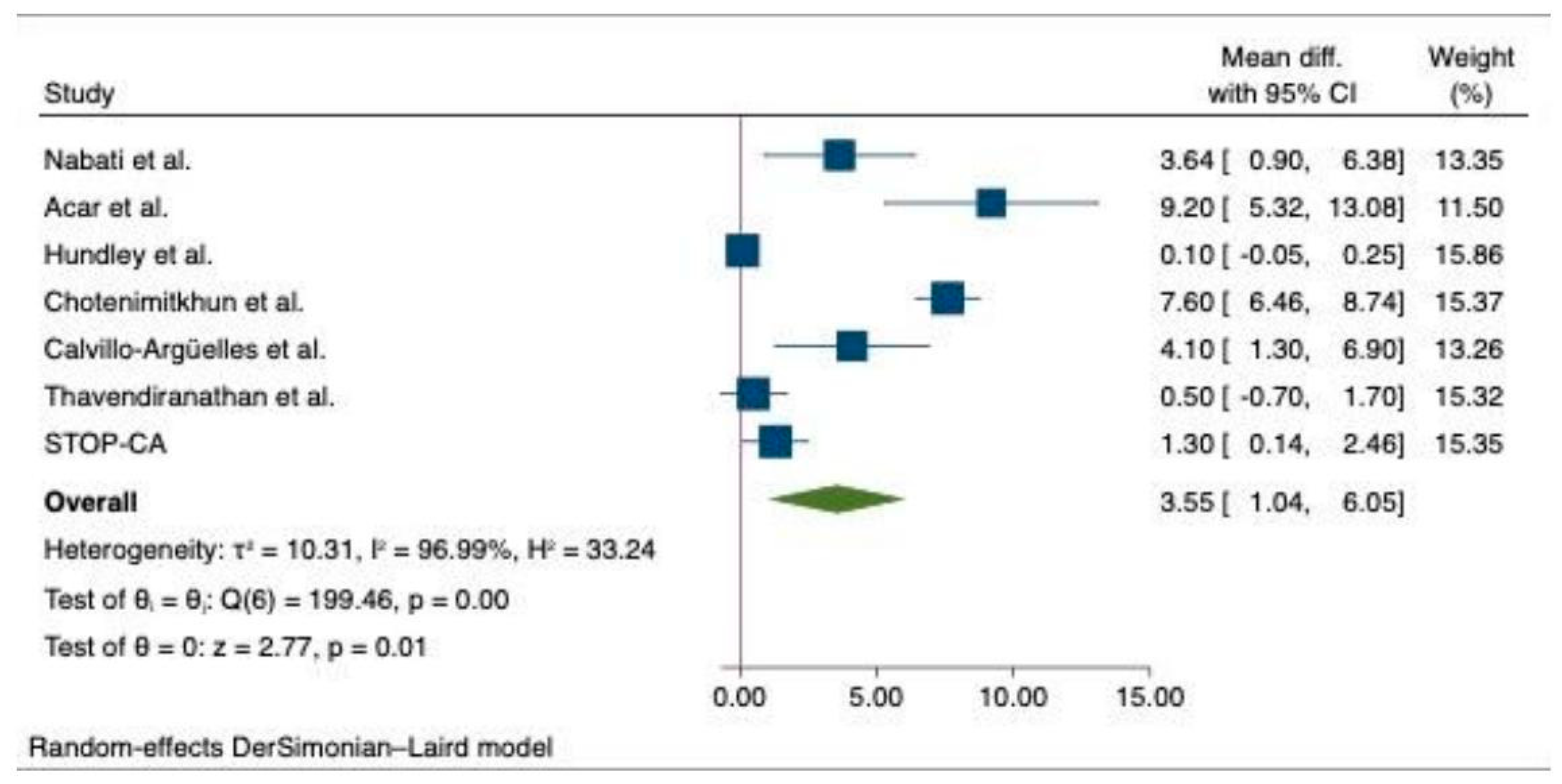
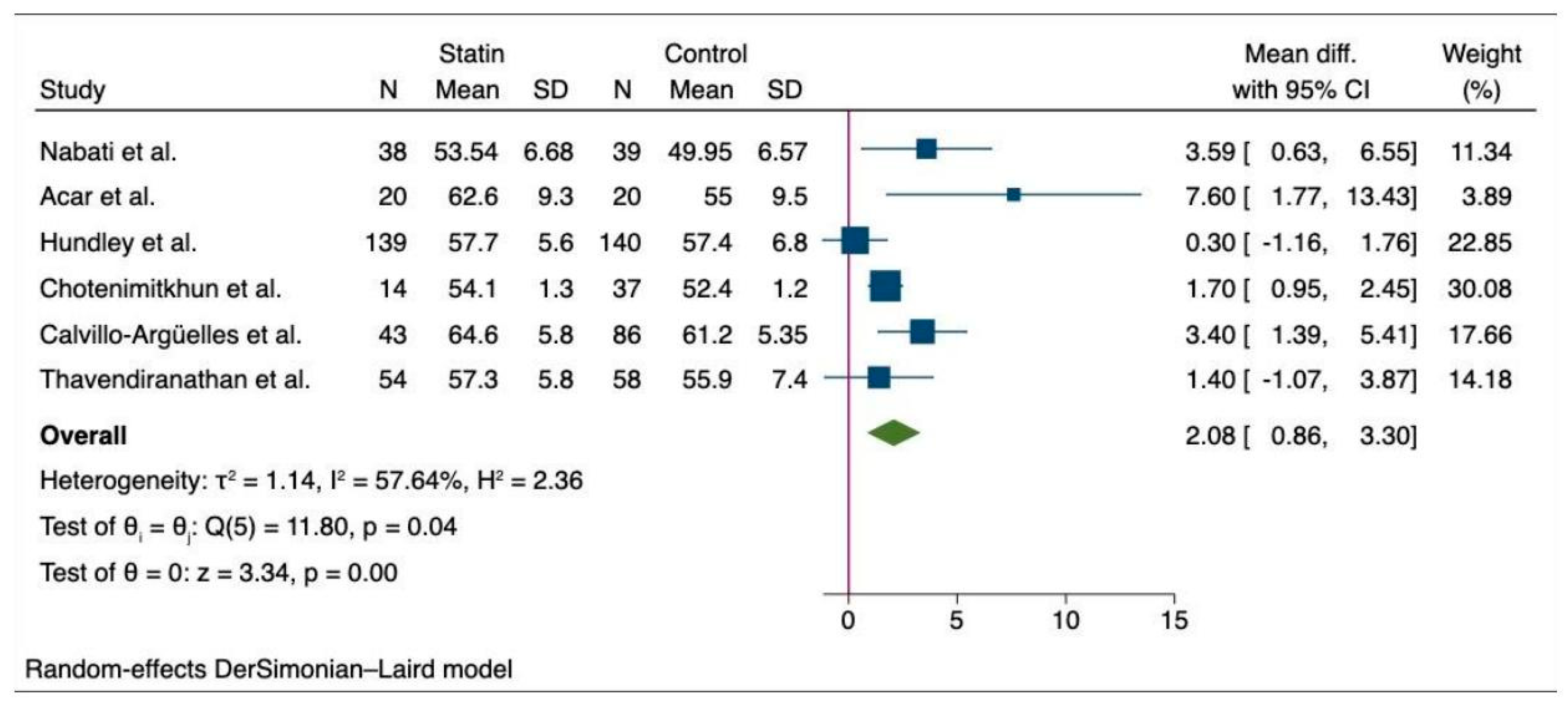
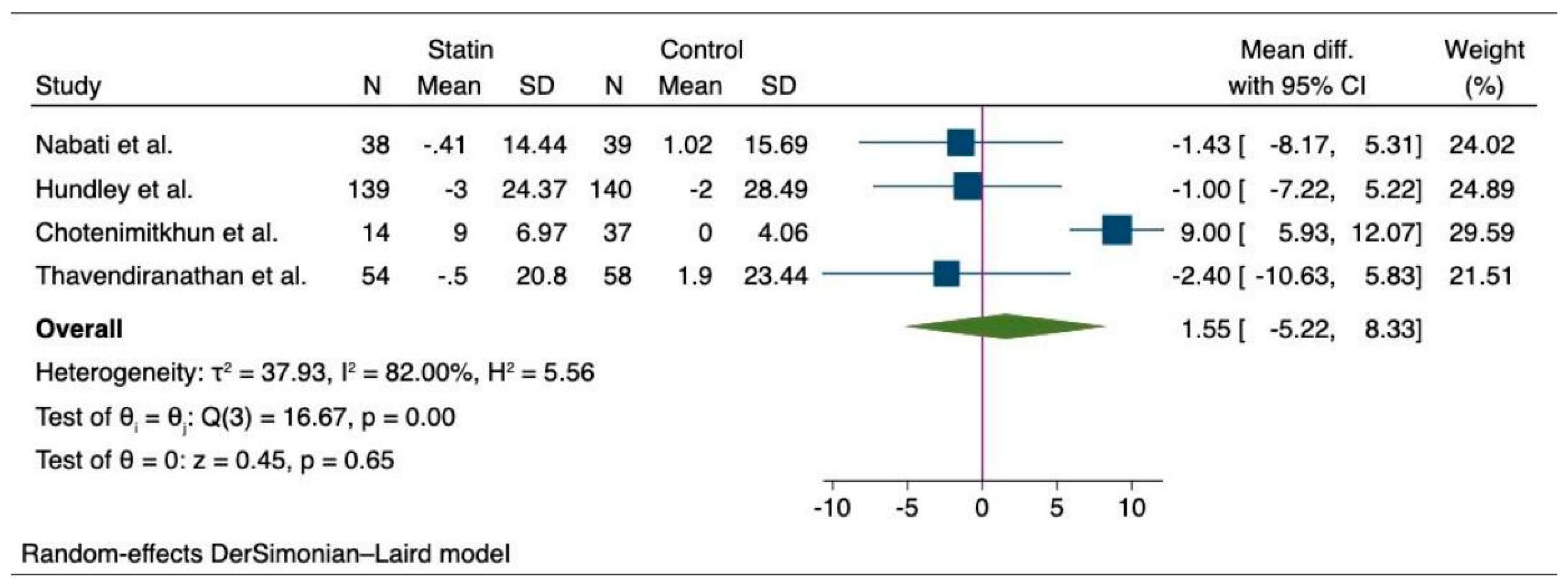
6.2. Meta-Analysis of the Outcomes among Cancer Patients with Statin and Non-Statin Users
7. Quality Assessment, Sensitivity Analysis, and Publication Bias
8. Discussion
9. Strengths and Limitations
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Facts & Figures 2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html (accessed on 27 January 2024).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Auseon, A.J. Chemotherapy-Induced Takotsubo Cardiomyopathy. Heart Fail. Clin. 2013, 9, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac Dysfunction in the Trastuzumab Clinical Trials Experience. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Pennesi, G.; Donatelli, F.; Cammarota, R.; De Flora, S.; Noonan, D.M. Cardiotoxicity of Anticancer Drugs: The Need for Cardio-Oncology and Cardio-Oncological Prevention. JNCI J. Natl. Cancer Inst. 2010, 102, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Dolci, A.; Dominici, R.; Cardinale, D.; Sandri, M.T.; Panteghini, M. Biochemical Markers for Prediction of Chemotherapy-Induced Cardiotoxicity: Systematic Review of the Literature and Recommendations for Use. Am. J. Clin. Pathol. 2008, 130, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Abdel-Qadir, H.; Fischer, H.D.; Camacho, X.; Amir, E.; Austin, P.C.; Lee, D.S. Breast Cancer Therapy-Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Lamantia, G.; Colombo, N.; Civelli, M.; De Giacomi, G.; Rubino, M.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Anthracycline-Induced Cardiomyopathy: Clinical Relevance and Response to Pharmacologic Therapy. J. Am. Coll. Cardiol. 2010, 55, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Vooletich, M.T.; Durand, J.-B.; Woods, M.L.; Davis, J.R.; Valero, V.; Lenihan, D.J. Reversibility of Trastuzumab-Related Cardiotoxicity: New Insights Based on Clinical Course and Response to Medical Treatment. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 7820–7826. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Hundley, W.G. Understanding Cardiovascular Injury after Treatment for Cancer: An Overview of Current Uses and Future Directions of Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2013, 15, 66. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Bobrowski, D.; Zhou, L.; Austin, P.C.; Calvillo-Argüelles, O.; Amir, E.; Lee, D.S.; Thavendiranathan, P. Statin Exposure and Risk of Heart Failure After Anthracycline- or Trastuzumab-Based Chemotherapy for Early Breast Cancer: A Propensity Score—Matched Cohort Study. J. Am. Heart Assoc. 2021, 10, e018393. [Google Scholar] [CrossRef]
- Acar, Z.; Kale, A.; Turgut, M.; Demircan, S.; Durna, K.; Demir, S.; Meriç, M.; Ağaç, M.T. Efficiency of Atorvastatin in the Protection of Anthracycline-Induced Cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Statins and Cardiovascular Diseases: From Cholesterol Lowering to Pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, J.A.M.; Linschoten, M.; Cramer, M.J.; Doevendans, P.A.; Asselbergs, F.W.; Teske, A.J. Early- and Late Anthracycline-Induced Cardiac Dysfunction: Echocardiographic Characterization and Response to Heart Failure Therapy. Cardio-Oncol. Lond. Engl. 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-Argüelles, O.; Abdel-Qadir, H.; Michalowska, M.; Billia, F.; Suntheralingam, S.; Amir, E.; Thavendiranathan, P. Cardioprotective Effect of Statins in Patients With HER2-Positive Breast Cancer Receiving Trastuzumab Therapy. Can. J. Cardiol. 2019, 35, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Neilan, T.G.; Quinaglia, T.; Onoue, T.; Mahmood, S.S.; Drobni, Z.D.; Gilman, H.K.; Smith, A.; Heemelaar, J.C.; Brahmbhatt, P.; Ho, J.S.; et al. Atorvastatin for Anthracycline-Associated Cardiac Dysfunction: The STOP-CA Randomized Clinical Trial. JAMA 2023, 330, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Houbois, C.; Marwick, T.H.; Kei, T.; Saha, S.; Runeckles, K.; Huang, F.; Shalmon, T.; Thorpe, K.E.; Pezo, R.C.; et al. Statins to Prevent Early Cardiac Dysfunction in Cancer Patients at Increased Cardiotoxicity Risk Receiving Anthracyclines. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Nabati, M.; Janbabai, G.; Esmailian, J.; Yazdani, J. Effect of Rosuvastatin in Preventing Chemotherapy-Induced Cardiotoxicity in Women With Breast Cancer: A Randomized, Single-Blind, Placebo-Controlled Trial. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Taha, A.M.; Joshi, A.; Deb, N.; Kanagala, S.G.; Nebuwa, C.; Ang, S.P.; Halder, A.; Rajak, K.; Jha, M.; et al. Implantable cardioverter defibrillators for primary prevention in patients with ischemic and non-ischemic cardiomyopathy: A meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102198. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Ang, S.P.; Ishak, A.; Nasir, Y.M.; Chia, J.E.; Naz, S.; Jaiswal, A. Comparison of Outcome among Type 2 vs Type 1 Myocardial Infarction: A Systematic Review and Meta-Analysis. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2023, 71, 223–234. [Google Scholar] [CrossRef]
- Jaiswal, V.; Ang, S.P.; Huang, H.; Momi, N.K.; Hameed, M.; Naz, S.; Batra, N.; Ishak, A.; Doshi, N.; Gera, A.; et al. Association between Nonalcoholic Fatty Liver Disease and Atrial Fibrillation and Other Clinical Outcomes: A Meta-Analysis. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2023, 71, 591–602. [Google Scholar] [CrossRef]
- Jaiswal, V.; Ang, S.P.; Agrawal, V.; Hameed, M.; Saleeb, M.R.A.; Jaiswal, A.; Shah, M.; Lao, N.M.; Chia, J.E.; Paudel, K.; et al. Association between Heart Failure and the Incidence of Cancer: A Systematic Review and Meta-Analysis. Eur. Heart J. Open 2023, 3, oead073. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 January 2024).
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Stata|FAQ: Citing Stata Software, Documentation, and FAQs. Available online: https://www.stata.com/support/faqs/resources/citing-software-documentation-faqs/ (accessed on 11 September 2023).
- Hundley, W.G.; D’Agostino, R.; Crotts, T.; Craver, K.; Hackney, M.H.; Jordan, J.H.; Ky, B.; Wagner, L.I.; Herrington, D.M.; Yeboah, J.; et al. Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid. 2022, 1, EVIDoa2200097. [Google Scholar] [CrossRef] [PubMed]
- Chotenimitkhun, R.; D’Agostino, R.; Lawrence, J.A.; Hamilton, C.A.; Jordan, J.H.; Vasu, S.; Lash, T.L.; Yeboah, J.; Herrington, D.M.; Hundley, W.G. Chronic Statin Administration May Attenuate Early Anthracycline-Associated Declines in Left Ventricular Ejection Function. Can. J. Cardiol. 2015, 31, 302–307. [Google Scholar] [CrossRef]
- Seicean, S.; Seicean, A.; Plana, J.C.; Budd, G.T.; Marwick, T.H. Effect of Statin Therapy on the Risk for Incident Heart Failure in Patients with Breast Cancer Receiving Anthracycline Chemotherapy: An Observational Clinical Cohort Study. J. Am. Coll. Cardiol. 2012, 60, 2384–2390. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive Heart Failure in Patients Treated with Doxorubicin: A Retrospective Analysis of Three Trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement with Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Madeddu, C.; Deidda, M.; Piras, A.; Cadeddu, C.; Demurtas, L.; Puzzoni, M.; Piscopo, G.; Scartozzi, M.; Mercuro, G. Pathophysiology of Cardiotoxicity Induced by Nonanthracycline Chemotherapy. J. Cardiovasc. Med. Hagerstown Md 2016, 17 (Suppl. 1), S12–S18. [Google Scholar] [CrossRef]
- Rowinsky, E.K.; McGuire, W.P.; Guarnieri, T.; Fisherman, J.S.; Christian, M.C.; Donehower, R.C. Cardiac Disturbances during the Administration of Taxol. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Haugnes, H.S.; Wethal, T.; Aass, N.; Dahl, O.; Klepp, O.; Langberg, C.W.; Wilsgaard, T.; Bremnes, R.M.; Fosså, S.D. Cardiovascular Risk Factors and Morbidity in Long-Term Survivors of Testicular Cancer: A 20-Year Follow-up Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4649–4657. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Nohria, A.; Prsic, A.; Liu, P.-Y.; Okamoto, R.; Creager, M.A.; Selwyn, A.; Liao, J.K.; Ganz, P. Statins Inhibit Rho Kinase Activity in Patients with Atherosclerosis. Atherosclerosis 2009, 205, 517. [Google Scholar] [CrossRef]
- Gnad, R.; Kaina, B.; Fritz, G. Rho GTPases Are Involved in the Regulation of NF-kappaB by Genotoxic Stress. Exp. Cell Res. 2001, 264, 244–249. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease: From Marvel to Menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Massaro, M.; Zampolli, A.; Scoditti, E.; Carluccio, M.A.; Storelli, C.; Distante, A.; De Caterina, R. Statins Inhibit Cyclooxygenase-2 and Matrix Metalloproteinase-9 in Human Endothelial Cells: Anti-Angiogenic Actions Possibly Contributing to Plaque Stability. Cardiovasc. Res. 2010, 86, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Leenders, G.J.; Smeets, M.B.; van den Boomen, M.; Berben, M.; Nabben, M.; van Strijp, D.; Strijkers, G.J.; Prompers, J.J.; Arslan, F.; Nicolay, K.; et al. Statins Promote Cardiac Infarct Healing by Modulating Endothelial Barrier Function Revealed by Contrast-Enhanced Magnetic Resonance Imaging. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 186–194. [Google Scholar] [CrossRef]
- Graaf, M.R.; Beiderbeck, A.B.; Egberts, A.C.G.; Richel, D.J.; Guchelaar, H.-J. The Risk of Cancer in Users of Statins. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 2388–2394. [Google Scholar] [CrossRef]
- Ren, Q.-W.; Yu, S.-Y.; Teng, T.-H.K.; Li, X.; Cheung, K.-S.; Wu, M.-Z.; Li, H.-L.; Wong, P.-F.; Tse, H.-F.; Lam, C.S.P.; et al. Statin Associated Lower Cancer Risk and Related Mortality in Patients with Heart Failure. Eur. Heart J. 2021, 42, 3049–3059. [Google Scholar] [CrossRef]
- Chang, W.-T.; Lin, H.-W.; Lin, S.-H.; Li, Y.-H. Association of Statin Use With Cancer- and Noncancer-Associated Survival Among Patients With Breast Cancer in Asia. JAMA Netw. Open 2023, 6, e239515. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Kim, J.; Adam, R.M.; Solomon, K.R.; Freeman, M.R. Cholesterol Targeting Alters Lipid Raft Composition and Cell Survival in Prostate Cancer Cells and Xenografts. J. Clin. Investig. 2005, 115, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.; van Leeuwen, J.E.; Elbaz, M.; Branchard, E.; Penn, L.Z. Statins as Anticancer Agents in the Era of Precision Medicine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5791–5800. [Google Scholar] [CrossRef] [PubMed]
- Warita, K.; Warita, T.; Beckwitt, C.H.; Schurdak, M.E.; Vazquez, A.; Wells, A.; Oltvai, Z.N. Statin-Induced Mevalonate Pathway Inhibition Attenuates the Growth of Mesenchymal-like Cancer Cells That Lack Functional E-Cadherin Mediated Cell Cohesion. Sci. Rep. 2014, 4, 7593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mukthavaram, R.; Chao, Y.; Nomura, N.; Bharati, I.S.; Fogal, V.; Pastorino, S.; Teng, D.; Cong, X.; Pingle, S.C.; et al. In Vitro and in Vivo Anticancer Effects of Mevalonate Pathway Modulation on Human Cancer Cells. Br. J. Cancer 2014, 111, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Beckwitt, C.H.; Shiraha, K.; Wells, A. Lipophilic Statins Limit Cancer Cell Growth and Survival, via Involvement of Akt Signaling. PLoS ONE 2018, 13, e0197422. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, C.R.; Mc Menamin, Ú.; Hughes, C.M.; Murray, L.J. Statin Use and Survival from Lung Cancer: A Population-Based Cohort Study. Cancer Epidemiol. Biomarkers Prev. 2015, 24, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-W.; Lin, C.-H.; Hsiao, T.-H.; Lo, C.-C.; Hsieh, C.-Y.; Huang, C.-C.; Sher, Y.-P. Therapeutic Effects of Statins against Lung Adenocarcinoma via P53 Mutant-Mediated Apoptosis. Sci. Rep. 2019, 9, 20403. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Liu, Q.; Armstrong, G.T.; Ness, K.K.; Yasui, Y.; Devine, K.; Tonorezos, E.; Soares-Miranda, L.; Sklar, C.A.; Douglas, P.S.; et al. Exercise and Risk of Major Cardiovascular Events in Adult Survivors of Childhood Hodgkin Lymphoma: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2014, 32, 3643–3650. [Google Scholar] [CrossRef]
- Lyu, Y.L.; Kerrigan, J.E.; Lin, C.-P.; Azarova, A.M.; Tsai, Y.-C.; Ban, Y.; Liu, L.F. Topoisomerase IIbeta Mediated DNA Double-Strand Breaks: Implications in Doxorubicin Cardiotoxicity and Prevention by Dexrazoxane. Cancer Res. 2007, 67, 8839–8846. [Google Scholar] [CrossRef]
- Macedo, A.V.S.; Hajjar, L.A.; Lyon, A.R.; Nascimento, B.R.; Putzu, A.; Rossi, L.; Costa, R.B.; Landoni, G.; Nogueira-Rodrigues, A.; Ribeiro, A.L.P. Efficacy of Dexrazoxane in Preventing Anthracycline Cardiotoxicity in Breast Cancer. JACC Cardio Oncol. 2019, 1, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Whaley, F.S.; Gerber, M.C.; Weisberg, S.; York, M.; Spicer, D.; Jones, S.E.; Wadler, S.; Desai, A.; Vogel, C.; et al. Cardioprotection with Dexrazoxane for Doxorubicin-Containing Therapy in Advanced Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 1318–1332. [Google Scholar] [CrossRef]
- Dong, H.; Yao, L.; Wang, M.; Wang, M.; Li, X.; Sun, X.; Yu, X.; Guo, J.; Li, X.; Xu, Y. Can ACEI/ARB Prevent the Cardiotoxicity Caused by Chemotherapy in Early-Stage Breast Cancer?—A Meta-Analysis of Randomized Controlled Trials. Transl. Cancer Res. 2020, 9, 7034–7043. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liang, G.; Wu, Y.; Chen, L. Protective Effects of ACEI/ARB on Left Ventricular Function in Anthracycline-Induced Chronic Cardiotoxicity: A Meta-Analysis of Randomized Controlled Trials. Cardiology 2021, 146, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Sandri, M.T.; Lamantia, G.; Colombo, N.; Civelli, M.; Martinelli, G.; Veglia, F.; Fiorentini, C.; Cipolla, C.M. Prevention of High-Dose Chemotherapy-Induced Cardiotoxicity in High-Risk Patients by Angiotensin-Converting Enzyme Inhibition. Circulation 2006, 114, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bai, F.; Qin, F.; Li, J.; Liu, N.; Li, D.; Li, T.; Xie, H.; Liu, D.; Zhou, S.; et al. Beta-Blockers for the Primary Prevention of Anthracycline-Induced Cardiotoxicity: A Meta-Analysis of Randomized Controlled Trials. BMC Pharmacol. Toxicol. 2019, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Pituskin, E.; Mackey, J.R.; Koshman, S.; Jassal, D.; Pitz, M.; Haykowsky, M.J.; Pagano, J.J.; Chow, K.; Thompson, R.B.; Vos, L.J.; et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R.; das Dores Cruz, F.; Gonçalves Brandão, S.M.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Kalil Filho, R.; Hoff, P.M.; et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef]
- Akpek, M.; Ozdogru, I.; Sahin, O.; Inanc, M.; Dogan, A.; Yazici, C.; Berk, V.; Karaca, H.; Kalay, N.; Oguzhan, A.; et al. Protective Effects of Spironolactone against Anthracycline-Induced Cardiomyopathy. Eur. J. Heart Fail. 2015, 17, 81–89. [Google Scholar] [CrossRef]
| Author | Sample Size | Study Design | Age | Year | Cancer Type | DM, n | Dyslipidemia, n | HTN, n | |
| Nabati et al. [18] | Rosuvastatin | 38 | RCT | 47.74 (9.70) | 2018 | Breast cancer | 5 | 10 | - |
| Control | 39 | 50.74 (12.440) | 7 | 8 | - | ||||
| Acar et al. [12] | Atorvastatin | 20 | RCT | 53.7 (14.2) | 2011 | Blood cancer | |||
| Control | 20 | 52.6 (17.6) | |||||||
| PREVENT (Hundley et al.) [28] | Atorvastatin | 139 | RCT | 48.5 ± 12.5 | 2022 | Breast cancer and lymphoma | |||
| Control | 140 | 49.4 ± 11.5 | |||||||
| Chotenimitkhun et al. [29] | Atorvastatin | 14 | Observational | 62 ± 2 | 2007–2010 | Breast cancer, leukemia, and lymphoma | 7 | 14 | 12 |
| Control | 37 | 43 ± 2 | 2 | 2 | 10 | ||||
| Abdel-Qadir et al. (anthracyclin) [11] | Statin | 859 | Observational | 69 (67–73) | 2007–2017 | Breast cancer (Antra) | 317 | 692 | |
| Control | 1686 | 69 (67–72 | 178 | 915 | |||||
| Abdel-Qadir et al. (transtuzumab) [11] | Statin | 520 | Observational | 71 (68–75) | 2007–2017 | Breast cancer (Trastu) | 199 | 433 | |
| Control | 851 | 70 (68–74) | 109 | 487 | |||||
| Calvillo-Argü;elles et al. [15] | Statin | 43 | Observational | 62.0 ± 9.1 | 2018 | Breast cancer | 16 | 43 | 25 |
| Control | 86 | 62.0 ± 9.0 | 4 | 9 | 19 | ||||
| Seicean et al. [30] | Statin | 67 | Observational | 61.3 (8.9) | 2005–2010 | Breast cancer | 17 | 34 | |
| Control | 561 | 50.3 (10.4) | 15 | 74 | |||||
| SPARE-HF (Thavendiranathan et al.) [17] | Atorvastatin | 54 | RCT | 55.2 (13.7) | 2017–2022 | Breast, lymphoma, leukemia, and thymoma | 3 | 2 | 11 |
| Control | 58 | 58.6 (13.4) | 4 | 3 | 18 | ||||
| STOP-CA (Neilan et al.) [16] | Statin | 150 | RCT | 50 | 2023 | Lymphoma | 0 | 14 | |
| Placebo | 150 | 49 | 2 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, V.; Ang, S.P.; Deb, N.; Hanif, M.; Batra, N.; Kanagala, S.G.; Vojjala, N.; Rajak, K.; Roy, P.; Sharath, M.; et al. Association between Statin Use and Chemotherapy-Induced Cardiotoxicity: A Meta-Analysis. Medicina 2024, 60, 580. https://doi.org/10.3390/medicina60040580
Jaiswal V, Ang SP, Deb N, Hanif M, Batra N, Kanagala SG, Vojjala N, Rajak K, Roy P, Sharath M, et al. Association between Statin Use and Chemotherapy-Induced Cardiotoxicity: A Meta-Analysis. Medicina. 2024; 60(4):580. https://doi.org/10.3390/medicina60040580
Chicago/Turabian StyleJaiswal, Vikash, Song Peng Ang, Novonil Deb, Muhammad Hanif, Nitya Batra, Sai Gautham Kanagala, Nikhil Vojjala, Kripa Rajak, Poulami Roy, Medha Sharath, and et al. 2024. "Association between Statin Use and Chemotherapy-Induced Cardiotoxicity: A Meta-Analysis" Medicina 60, no. 4: 580. https://doi.org/10.3390/medicina60040580
APA StyleJaiswal, V., Ang, S. P., Deb, N., Hanif, M., Batra, N., Kanagala, S. G., Vojjala, N., Rajak, K., Roy, P., Sharath, M., Waleed, M. S., Wajid, Z., & Mattumpuram, J. (2024). Association between Statin Use and Chemotherapy-Induced Cardiotoxicity: A Meta-Analysis. Medicina, 60(4), 580. https://doi.org/10.3390/medicina60040580