Effectiveness of Electroencephalography Neurofeedback for Improving Working Memory and Episodic Memory in the Elderly: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. EEG Band Definition
2.4. Data Extraction and Outcome Definition
2.5. Assessment of Bias
2.6. Data Analysis
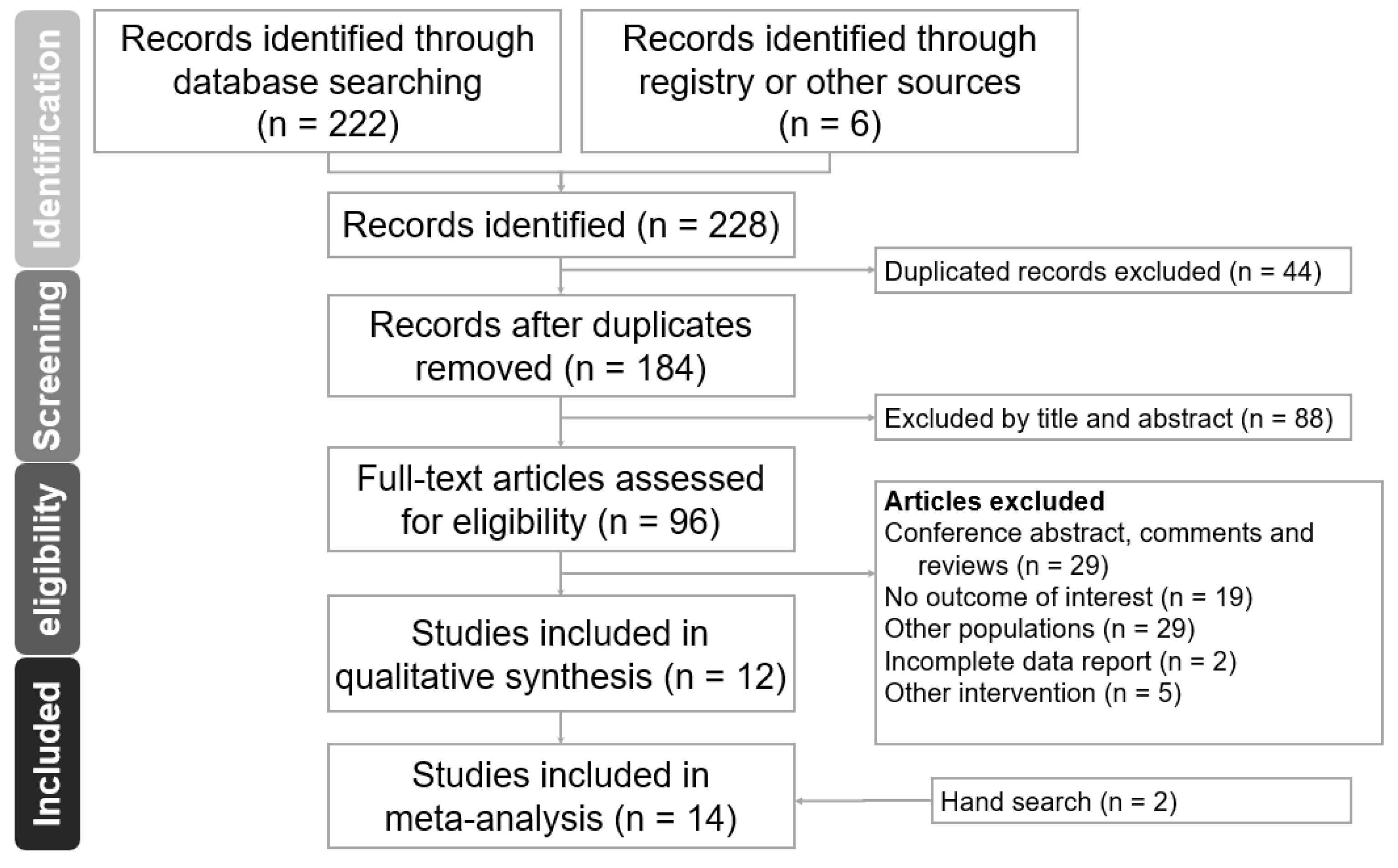
3. Results
3.1. Study Characteristics
3.2. Methodology Quality of the Included Studies
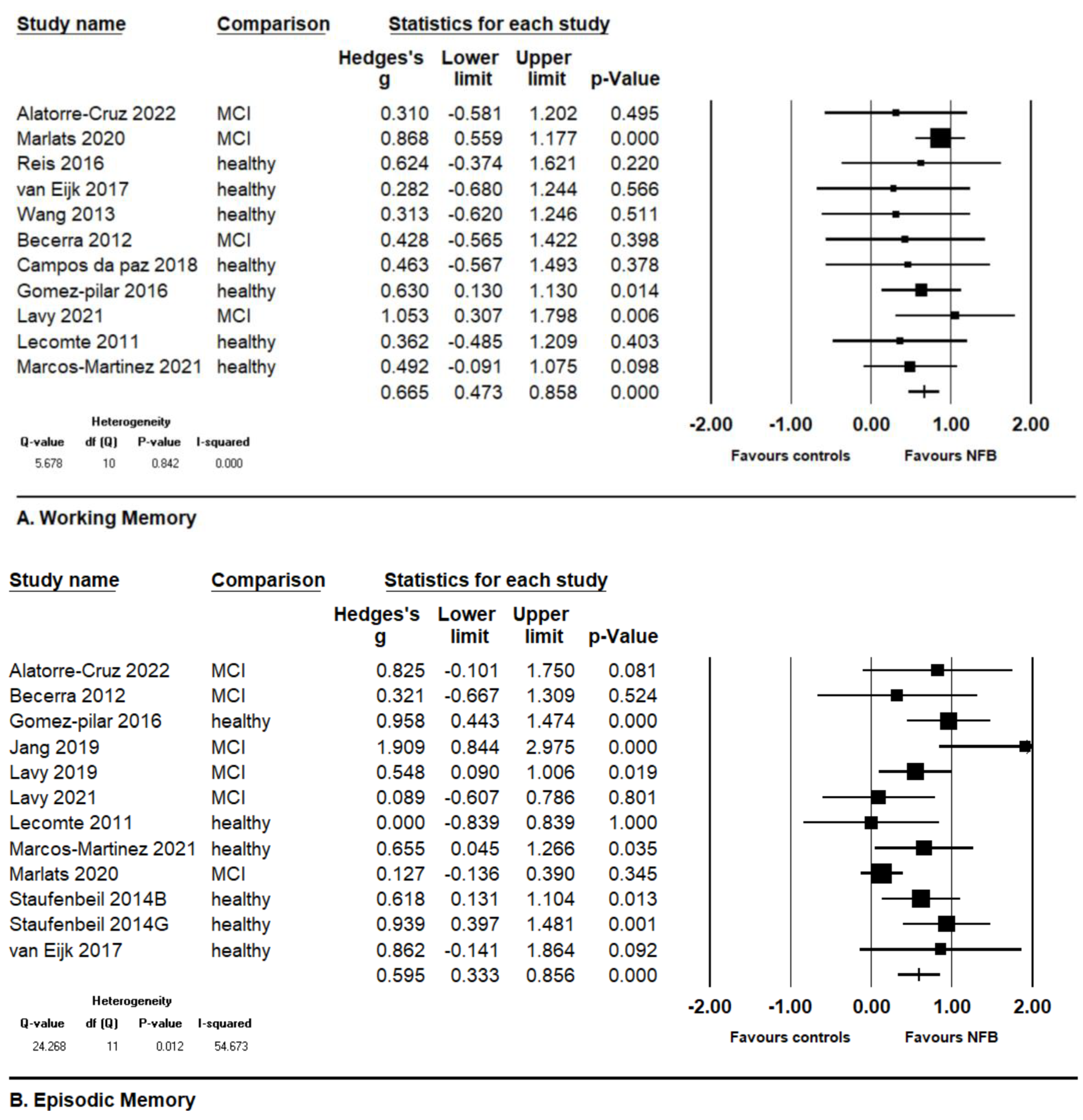
3.3. Co-Primary Outcomes: Working Memory and Episodic Memory
3.4. Subgroup Analyses
3.4.1. Controlled Studies vs. Single-Arm Pre–Post Studies
3.4.2. Healthy Elderly vs. Elderly with MCI
3.4.3. More Than 10 Sessions vs. Less Than 10 Sessions
3.4.4. More Than 300 Min Total Training Time vs. Less Than 300 Min
3.4.5. SMR Up-Training vs. Alpha Up-Training
3.5. Meta-Regression
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef]
- Heinrich, H.; Gevensleben, H.; Strehl, U. Annotation: Neurofeedback—Train your brain to train behaviour. J. Child Psychol. Psychiatry 2007, 48, 3–16. [Google Scholar] [CrossRef]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-loop brain training: The science of neurofeedback. Nat. Rev. Neurosci. 2017, 18, 86–100. [Google Scholar] [CrossRef]
- Kopčanová, M.; Tait, L.; Donoghue, T.; Stothart, G.; Smith, L.; Flores-Sandoval, A.A.; Davila-Perez, P.; Buss, S.; Shafi, M.M.; Pascual-Leone, A.; et al. Resting-state EEG signatures of Alzheimer’s disease are driven by periodic but not aperiodic changes. Neurobiol. Dis. 2024, 190, 106380. [Google Scholar] [CrossRef] [PubMed]
- Meghdadi, A.H.; Karić, M.S.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef]
- Eskikurt, G.; Duru, A.D.; Ermutlu, N.; İşoğlu-Alkaç, Ü. Evaluation of Brain Electrical Activity of Visual Working Memory with Time-Frequency Analysis. Clin. EEG Neurosci. 2024; e-published. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Stern, C.E. Theta rhythm and the encoding and retrieval of space and time. Neuroimage 2014, 85 Pt 2, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.-R.; Snaedal, J.; Wahlund, L.-O.; Waldemar, G.; et al. EEG Theta Power Is an Early Marker of Cognitive Decline in Dementia due to Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.H.; Hsueh, J.J.; Shaw, F.Z. Neurofeedback of Alpha Activity on Memory in Healthy Participants: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2020, 14, 562360. [Google Scholar] [CrossRef]
- Yeh, W.H.; Ju, Y.J.; Liu, Y.T.; Wang, T.Y. Systematic Review and Meta-Analysis on the Effects of Neurofeedback Training of Theta Activity on Working Memory and Episodic Memory in Healthy Population. Int. J. Environ. Res. Public Health 2022, 19, 11037. [Google Scholar] [CrossRef]
- Becerra, J.; Fernandez, T.; Roca-Stappung, M.; Diaz-Comas, L.; Galan, L.; Bosch, J.; Espino, M.; Moreno, A.J.; Harmony, T. Neurofeedback in healthy elderly human subjects with electroencephalographic risk for cognitive disorder. J. Alzheimers Dis. 2012, 28, 357–367. [Google Scholar] [CrossRef]
- Lavy, Y.; Dwolatzky, T.; Kaplan, Z.; Guez, J.; Todder, D. Mild Cognitive Impairment and Neurofeedback: A Randomized Controlled Trial. Front. Aging Neurosci. 2021, 13, 657646. [Google Scholar] [CrossRef] [PubMed]
- Marlats, F.; Bao, G.; Chevallier, S.; Boubaya, M.; Djabelkhir-Jemmi, L.; Wu, Y.H.; Lenoir, H.; Rigaud, A.S.; Azabou, E. SMR/Theta Neurofeedback Training Improves Cognitive Performance and EEG Activity in Elderly With Mild Cognitive Impairment: A Pilot Study. Front. Aging Neurosci. 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Su, I.C.; Shih, C.Y.; Liu, Y.C.; Su, Y.K.; Wei, L.; Luh, H.T.; Huang, H.C.; Tsai, P.S.; Fan, Y.C.; et al. Effects of Neurofeedback on Cognitive Function, Productive Activity, and Quality of Life in Patients With Traumatic Brain Injury: A Randomized Controlled Trial. Neurorehabil. Neural Repair 2023, 37, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jessee, W.; Hoyng, S.; Borhani, S.; Liu, Z.; Zhao, X.; Price, L.K.; High, W.; Suhl, J.; Cerel-Suhl, S. Sharpening Working Memory With Real-Time Electrophysiological Brain Signals: Which Neurofeedback Paradigms Work? Front. Aging Neurosci. 2022, 14, 780817. [Google Scholar] [CrossRef]
- Laborda-Sanchez, F.; Cansino, S. The Effects of Neurofeedback on Aging-Associated Cognitive Decline: A Systematic Review. Appl. Psychophysiol. Biofeedback 2021, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trambaiolli, L.R.; Cassani, R.; Mehler, D.M.A.; Falk, T.H. Neurofeedback and the Aging Brain: A Systematic Review of Training Protocols for Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 682683. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Alatorre-Cruz, G.C.; Fernandez, T.; Castro-Chavira, S.A.; Gonzalez-Lopez, M.; Sanchez-Moguel, S.M.; Silva-Pereyra, J. One-Year Follow-Up of Healthy Older Adults with Electroencephalographic Risk for Neurocognitive Disorder After Neurofeedback Training. J. Alzheimers Dis. 2022, 85, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, J.; Park, G.; Kim, H.; Jung, E.S.; Cha, J.Y.; Kim, C.Y.; Kim, S.; Lee, J.H.; Yoo, H. Beta wave enhancement neurofeedback improves cognitive functions in patients with mild cognitive impairment: A preliminary pilot study. Medicine 2019, 98, e18357. [Google Scholar] [CrossRef]
- Lavy, Y.; Dwolatzky, T.; Kaplan, Z.; Guez, J.; Todder, D. Neurofeedback Improves Memory and Peak Alpha Frequency in Individuals with Mild Cognitive Impairment. Appl. Psychophysiol. Biofeedback 2019, 44, 41–49. [Google Scholar] [CrossRef]
- Campos da Paz, V.K.; Garcia, A.; Campos da Paz Neto, A.; Tomaz, C. SMR Neurofeedback Training Facilitates Working Memory Performance in Healthy Older Adults: A Behavioral and EEG Study. Front. Behav. Neurosci. 2018, 12, 321. [Google Scholar] [CrossRef]
- van Eijk, L.; Zwijsen, S.; Keeser, D.; Oosterman, J.; Pogarell, O.; Engelbregt, H. EEG-Neurofeedback Training and Quality of Life of Institutionalized Elderly Women: A Pilot Study. 2017. Available online: https://repository.ubn.ru.nl/handle/2066/173478 (accessed on 9 February 2024).
- Gomez-Pilar, J.; Corralejo, R.; Nicolas-Alonso, L.F.; Alvarez, D.; Hornero, R. Neurofeedback training with a motor imagery-based BCI: Neurocognitive improvements and EEG changes in the elderly. Med. Biol. Eng. Comput. 2016, 54, 1655–1666. [Google Scholar] [CrossRef]
- Lecomte, G.; Juhel, J. The effects of neurofeedback training on memory performance in elderly subjects. Psychology 2011, 2, 846. [Google Scholar] [CrossRef]
- Marcos-Martinez, D.; Martinez-Cagigal, V.; Santamaria-Vazquez, E.; Perez-Velasco, S.; Hornero, R. Neurofeedback Training Based on Motor Imagery Strategies Increases EEG Complexity in Elderly Population. Entropy 2021, 23, 1574. [Google Scholar] [CrossRef]
- Reis, J.; Portugal, A.M.; Fernandes, L.; Afonso, N.; Pereira, M.; Sousa, N.; Dias, N.S. An Alpha and Theta Intensive and Short Neurofeedback Protocol for Healthy Aging Working-Memory Training. Front. Aging Neurosci. 2016, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Staufenbiel, S.M.; Brouwer, A.M.; Keizer, A.W.; van Wouwe, N.C. Effect of beta and gamma neurofeedback on memory and intelligence in the elderly. Biol. Psychol. 2014, 95, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Hsieh, S. Neurofeedback training improves attention and working memory performance. Clin. Neurophysiol. 2013, 124, 2406–2420. [Google Scholar] [CrossRef] [PubMed]
- Tromp, D.; Dufour, A.; Lithfous, S.; Pebayle, T.; Despres, O. Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2015, 24, 232–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wu, F.; Huang, K.; Yang, L.; Ji, L. Prediction of working memory ability based on EEG by functional data analysis. J. Neurosci. Methods 2020, 333, 108552. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Bramanti, P.; Rossini, P.M. Human brain networks in physiological aging: A graph theoretical analysis of cortical connectivity from EEG data. J. Alzheimers Dis. 2014, 41, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Marra, C.; Quaranta, D.; Vita, M.G.; Bramanti, P.; Rossini, P.M. Human brain networks in cognitive decline: A graph theoretical analysis of cortical connectivity from EEG data. J. Alzheimer’s Dis. 2014, 41, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Schneider, S.L.; Rose, M. Differential effects of ongoing EEG beta and theta power on memory formation. PLoS ONE 2017, 12, e0171913. [Google Scholar] [CrossRef] [PubMed]
- Bonner-Jackson, A.; Mahmoud, S.; Miller, J.; Banks, S.J. Verbal and non-verbal memory and hippocampal volumes in a memory clinic population. Alzheimer’s Res. Ther. 2015, 7, 61. [Google Scholar] [CrossRef] [PubMed]
| Study Name | Design | Clinical Condition | EEG Band/Controls | Sample (n)/Age (Years) | Sessions/Minutes per Session | Outcome of Interest | |
|---|---|---|---|---|---|---|---|
| WM | EM | ||||||
| Alatorre–Cruz 2022 [20] | SBRCT | MCI | Theta (−) | 10/67.5 | 30/30 | WAIS-III-WMI | NEUROPSI—recall |
| Sham | 8/68.6 | ||||||
| Becerra 2012 [11] | OLRCT | MCI | Theta (−) | 7/65.8 | 30/30 | WAIS-III-WMI | NEUROPSI—memory |
| Sham | 7/67.0 | ||||||
| Campos da Paz 2018 [23] | OLRCT | Elderly | SMR (+) | 7/69.1 | 10/30 | DMST | |
| Sham | 6/69.1 | ||||||
| Gomez-Pilar 2016 [25] | OLRCT | Elderly | SMR (+) | 31/68.3 | 5/NA | Luria—AND immediate memory | Luria—AND logical memory |
| No NF | 32/68.0 | ||||||
| Jang 2019 [21] | OLPP | MCI | SMR (+) | 5/66.5 | 16/45 | CNSVS—composite memory | |
| Lecomte 2011 [26] | OLRCT | Elderly | Alpha (+), alpha/theta ratio (+) | 10/75.3 | 4/60 | SMB learning test | SMB recall test |
| Relaxation | 10/75.3 | ||||||
| Lavy 2021 [12] | SBRCT | MCI | Alpha (+) | 15/70.2 | 12/30 | NeuroTrax battery immediate verbal recall | NeuroTrax battery delayed verbal recall |
| Sham | 15/74.2 | ||||||
| Lavy 2019 [22] | OLPP | MCI | Alpha (+) | 11/70 | 10/30 | NeuroTrax battery delayed verbal recall | |
| Marlats 2020 [13] | OLPP | MCI | SMR (+)/theta (−) | 32/76.1 | 20/45 | Forward and backward digit span | Logic memory-recall |
| Marcos-Martínez 2021 [27] | OLRCT | Elderly | SMR (+) | 11/69.4 | 5/90 | Luria—AND immediately memory | Luria—AND logical memory |
| Reis 2016 [28] | OLRCT | Elderly | Alpha (+), theta (+) | 9/65.97 | 8/30 | M. Rot | |
| Sham | 6/65.97 | ||||||
| Staufenbeil 2014 [29] | DBRCT | Elderly | SMR (+) | 10/66.4 | 8/NA | Encoding memory delayed verbal recall | |
| Gamma (+) | 10/69.2 | ||||||
| van Eijk 2017 [24] | OLRCT | Elderly | SMR (+) | 10/77.9 | 10/21 | RAVLT immediate recall | RAVLT delayed recall |
| No NF | 6/79.2 | ||||||
| Wang 2013 [30] | OLRCT | Elderly | Theta (+) | 8/65.0 | 12/15 | Sternberg word recognition task | |
| Sham | 8/64.6 | ||||||
| Controlled Studies vs. Single Arm Pre–Post Studies | |||||||
|---|---|---|---|---|---|---|---|
| k | Hedge’s g | Lower Limit | Upper Limit | p-Value for Effect Size | I2 | p-Value for Heterogeneity | |
| WM–CT | 9 | 0.548 | 0.277 | 0.818 | <0.001 | 0 | 0.973 |
| WM–PP | 2 | 0.765 | 0.436 | 1.093 | <0.001 | 19.662 | 0.265 |
| EM–CT | 6 | 0.539 | 0.170 | 0.909 | 0.004 | 24.664 | 0.249 |
| EM–PP | 6 | 0.662 | 0.282 | 1.042 | 0.001 | 70.938 | 0.004 |
| MCI vs. Healthy elderly people | |||||||
| WM–MCI | 4 | 0.812 | 0.549 | 1.074 | <0.001 | 0 | 0.510 |
| WM–HE | 7 | 0.495 | 0.213 | 0.778 | 0.001 | 0 | 0.993 |
| EM–MCI | 6 | 0.503 | 0.088 | 0.919 | 0.018 | 61.028 | 0.022 |
| EM–HE | 6 | 0.729 | 0.483 | 0.976 | <0.001 | 0 | 0.472 |
| ≥10 sessions vs. <10 sessions | |||||||
| WM–≥10 | 7 | 0.728 | 0.491 | 0.966 | <0.001 | 0 | 0.603 |
| WM–<10 | 4 | 0.546 | 0.219 | 0.873 | 0.001 | 0 | 0.951 |
| EM–≥10 | 7 | 0.534 | 0.148 | 0.920 | 0.007 | 57.847 | 0.027 |
| EM–<10 | 5 | 0.716 | 0.445 | 0.986 | <0.001 | 10.813 | 0.344 |
| ≥300 total minutes vs. <300 total minutes | |||||||
| WM–≥300 | 6 | 0.743 | 0.510 | 0.976 | <0.001 | 0 | 0.612 |
| WM–<300 | 4 | 0.388 | −0.077 | 0.853 | 0.102 | 0 | 0.962 |
| EM–≥300 | 7 | 0.516 | 0.156 | 0.876 | 0.005 | 57.381 | 0.027 |
| EM–<300 | 2 | 0.386 | −0.454 | 1.225 | 0.368 | 40.066 | 0.196 |
| SMR vs. alpha vs. others | |||||||
| WM–SMR | 5 | 0.710 | 0.483 | 0.937 | <0.001 | 0 | 0.624 |
| WM–alpha | 3 | 0.721 | 0.232 | 1.209 | 0.004 | 0 | 0.475 |
| WM–others | 3 | 0.346 | −0.195 | 0.887 | 0.210 | 0 | 0.981 |
| EM–SMR | 6 | 0.731 | 0.294 | 1.169 | 0.001 | 72.652 | 0.003 |
| EM–alpha | 3 | 0.339 | −0.009 | 0.687 | 0.056 | 0 | 0.383 |
| EM–others | 3 | 0.802 | 0.379 | 1.225 | <0.001 | 0 | 0.561 |
| k | Coefficient | Standard Error | Lower Limit | Upper Limit | p-Value | |
|---|---|---|---|---|---|---|
| Mean age–study level | ||||||
| WM | 11 | 0.029 | 0.024 | −0.017 | 0.075 | 0.221 |
| EM | 12 | −0.069 | 0.025 | −0.118 | −0.019 | 0.006 |
| Total sessions | ||||||
| WM | 11 | 0.008 | 0.012 | −0.016 | 0.032 | 0.514 |
| EM | 12 | −0.009 | 0.017 | −0.041 | 0.023 | 0.581 |
| Minutes/sessions | ||||||
| WM | 10 | −0.000 | 0.005 | −0.011 | 0.010 | 0.938 |
| EM | 9 | −0.000 | 0.008 | −0.016 | 0.016 | 0.987 |
| Total training time | ||||||
| WM | 10 | 0.000 | 0.000 | −0.000 | 0.001 | 0.223 |
| EM | 9 | 0.000 | 0.000 | −0.001 | 0.001 | 0.896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-R.; Hsu, T.-W.; Hsu, C.-W.; Chen, P.-Y.; Tseng, P.-T.; Liang, C.-S. Effectiveness of Electroencephalography Neurofeedback for Improving Working Memory and Episodic Memory in the Elderly: A Meta-Analysis. Medicina 2024, 60, 369. https://doi.org/10.3390/medicina60030369
Lin Y-R, Hsu T-W, Hsu C-W, Chen P-Y, Tseng P-T, Liang C-S. Effectiveness of Electroencephalography Neurofeedback for Improving Working Memory and Episodic Memory in the Elderly: A Meta-Analysis. Medicina. 2024; 60(3):369. https://doi.org/10.3390/medicina60030369
Chicago/Turabian StyleLin, Yu-Ru, Tien-Wei Hsu, Che-Wei Hsu, Peng-Yu Chen, Ping-Tao Tseng, and Chih-Sung Liang. 2024. "Effectiveness of Electroencephalography Neurofeedback for Improving Working Memory and Episodic Memory in the Elderly: A Meta-Analysis" Medicina 60, no. 3: 369. https://doi.org/10.3390/medicina60030369
APA StyleLin, Y.-R., Hsu, T.-W., Hsu, C.-W., Chen, P.-Y., Tseng, P.-T., & Liang, C.-S. (2024). Effectiveness of Electroencephalography Neurofeedback for Improving Working Memory and Episodic Memory in the Elderly: A Meta-Analysis. Medicina, 60(3), 369. https://doi.org/10.3390/medicina60030369






