Comparison of Fusion Rates among Various Demineralized Bone Matrices in Posterior Lumbar Interbody Fusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Outcome Measurements
2.3. Statistical Analysis
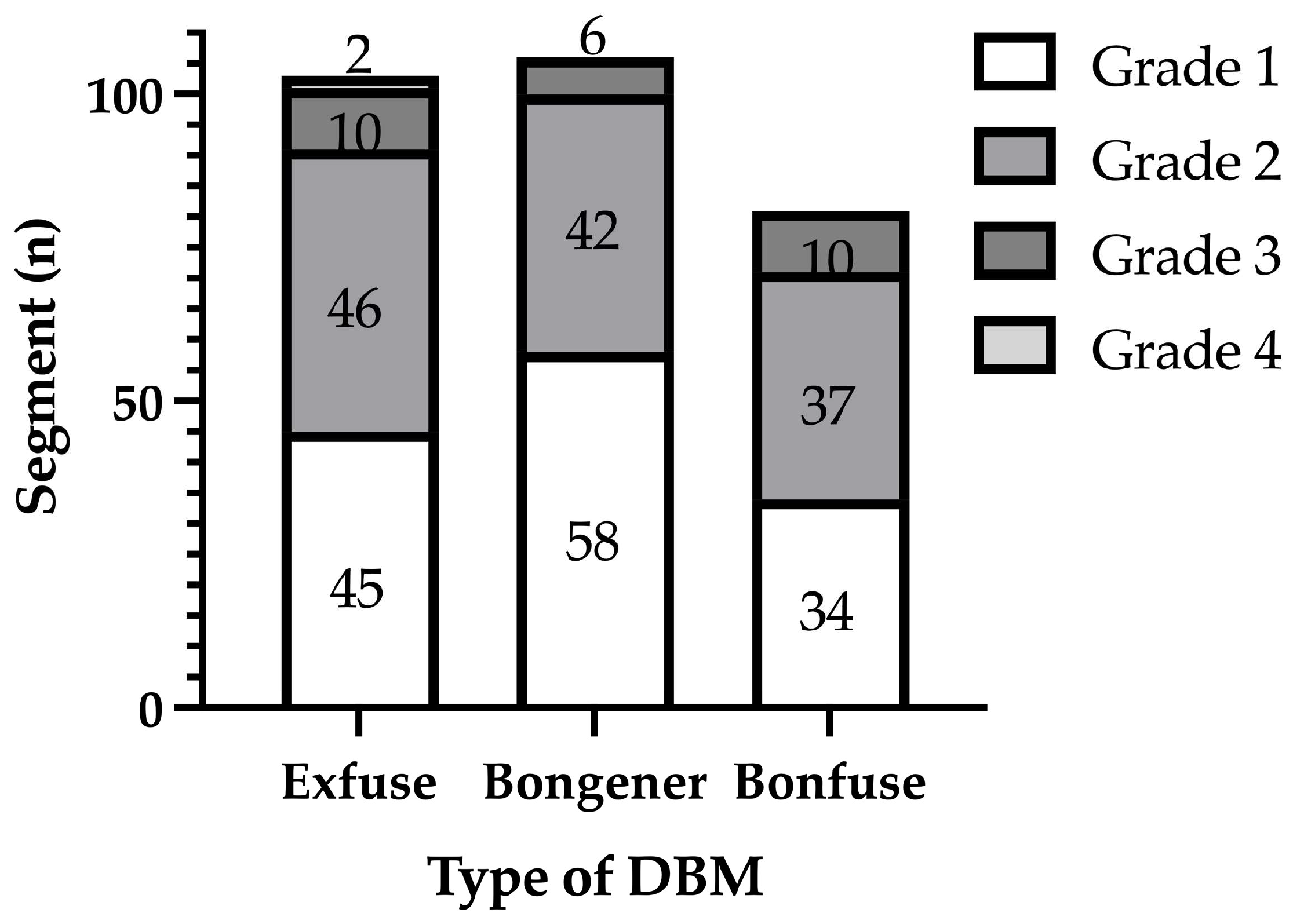
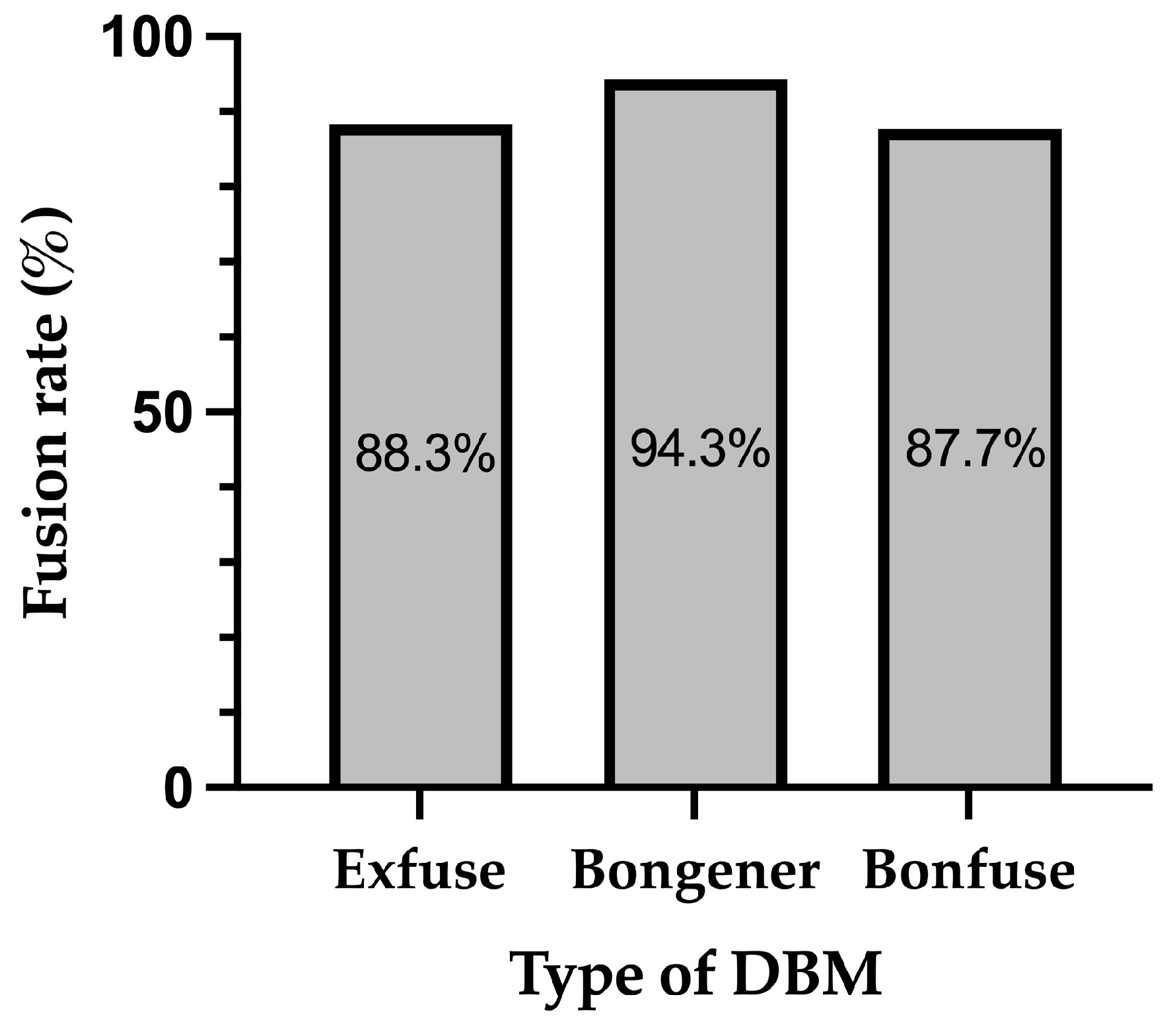
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidhu, G.S.; Henkelman, E.; Vaccaro, A.R.; Albert, T.J.; Hilibrand, A.; Anderson, D.G.; Rihn, J.A. Minimally invasive versus open posterior lumbar interbody fusion: A systematic review. Clin. Orthop. Relat. Res. 2014, 472, 1792–1799. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, S.T.; Lee, S.S. Posterior lumbar interbody fusion using a unilateral single cage and a local morselized bone graft in the degenerative lumbar spine. Clin. Orthop. Surg. 2009, 1, 214–221. [Google Scholar] [CrossRef]
- McLaughlin, M.R.; Haid, R.W., Jr.; Rodts, G.E., Jr.; Subach, B.R. Posterior lumbar interbody fusion: Indications, techniques, and results. Clin. Neurosurg. 2000, 47, 514–527. [Google Scholar] [PubMed]
- Steinmann, J.C.; Herkowitz, H.N. Pseudarthrosis of the spine. Clin. Orthop. Relat. Res. 1992, 284, 80–90. [Google Scholar] [CrossRef]
- How, N.E.; Street, J.T.; Dvorak, M.F.; Fisher, C.G.; Kwon, B.K.; Paquette, S.; Smith, J.S.; Shaffrey, C.I.; Ailon, T. Pseudarthrosis in adult and pediatric spinal deformity surgery: A systematic review of the literature and meta-analysis of incidence, characteristics, and risk factors. Neurosurg. Rev. 2019, 42, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.S.; Wang, I.C.; Lu, M.L.; Hsieh, M.K.; Chen, L.H.; Chen, W.J. The fusion rate of demineralized bone matrix compared with autogenous iliac bone graft for long multi-segment posterolateral spinal fusion. BMC Musculoskelet. Disord. 2016, 17, 3. [Google Scholar] [CrossRef]
- Edwards, J.T.; Diegmann, M.H.; Scarborough, N.L. Osteoinduction of human demineralized bone: Characterization in a rat model. Clin. Orthop. Relat. Res. 1998, 357, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.L.; Block, J.E. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: Impact of processing techniques and study methodology. Orthopedics 1999, 22, 524–531. [Google Scholar]
- Frenkel, S.R.; Moskovich, R.; Spivak, J.; Zhang, Z.H.; Prewett, A.B. Demineralized bone matrix. Enhancement of spinal fusion. Spine 1993, 18, 1634–1639. [Google Scholar] [CrossRef]
- Ragni, P.; Lindholm, T.S. Interaction of allogeneic demineralized bone matrix and porous hydroxyapatite bioceramics in lumbar interbody fusion in rabbits. Clin. Orthop. Relat. Res. 1991, 272, 292–299. [Google Scholar] [CrossRef]
- Ko, S.; Jun, C.; Nam, J. Comparison of Fusion Rate and Functional Outcome between Local Cancellous Bone Plus Demineralized Bone Matrix and Local Bone in 1-Level Posterior Lumbar Interbody Fusion. Clin. Spine Surg. 2022, 35, E621–E626. [Google Scholar] [CrossRef]
- Ahn, D.K.; Moon, S.H.; Kim, T.W.; Boo, K.H.; Hong, S.W. Demineralized bone matrix, as a graft enhancer of auto-local bone in posterior lumbar interbody fusion. Asian Spine J. 2014, 8, 129–137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.H.; Ha, K.Y.; Rhyu, K.W.; Park, H.Y.; Cho, C.H.; Kim, H.C.; Lee, H.J.; Kim, S.I. Lumbar Interbody Fusion: Techniques, Pearls and Pitfalls. Asian Spine J. 2020, 14, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Ito, Z.; Imagama, S.; Kanemura, T.; Hachiya, Y.; Miura, Y.; Kamiya, M.; Yukawa, Y.; Sakai, Y.; Katayama, Y.; Wakao, N.; et al. Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion (PLIF): A multicenter study. Eur. Spine J. 2013, 22, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, D.W.; Lee, S.J.; Chang, B.S.; Lee, C.K. Fusion rates and subsidence of morselized local bone grafted in titanium cages in posterior lumbar interbody fusion using quantitative three-dimensional computed tomography scans. Spine 2010, 35, 1460–1465. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, S.; Reverdin, A.; May, D. Posterior lumbar interbody fusion with cages: An independent review of 71 cases. J. Neurosurg. 1999, 91, 186–192. [Google Scholar] [CrossRef]
- Suh, K.T.; Park, W.W.; Kim, S.J.; Cho, H.M.; Lee, J.S.; Lee, J.S. Posterior lumbar interbody fusion for adult isthmic spondylolisthesis: A comparison of fusion with one or two cages. J. Bone Jt. Surg. Br. 2008, 90, 1352–1356. [Google Scholar] [CrossRef]
- Brodano, G.B.; Giavaresi, G.; Lolli, F.; Salamanna, F.; Parrilli, A.; Martini, L.; Griffoni, C.; Greggi, T.; Arcangeli, E.; Pressato, D.; et al. Hydroxyapatite-Based Biomaterials Versus Autologous Bone Graft in Spinal Fusion: An In Vivo Animal Study. Spine 2014, 39, E661–E668. [Google Scholar] [CrossRef]
- Ohtori, S.; Suzuki, M.; Koshi, T.; Takaso, M.; Yamashita, M.; Yamauchi, K.; Inoue, G.; Suzuki, M.; Orita, S.; Eguchi, Y.; et al. Single-level instrumented posterolateral fusion of the lumbar spine with a local bone graft versus an iliac crest bone graft: A prospective, randomized study with a 2-year follow-up. Eur. Spine J. 2011, 20, 635–639. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Kwon, B.; Jenis, L.G. Carrier materials for spinal fusion. Spine J. 2005, 5, 224S–230S. [Google Scholar] [CrossRef] [PubMed]
- Nam, W.D.; Yi, J. Bone Union Rate Following Instrumented Posterolateral Lumbar Fusion: Comparison between Demineralized Bone Matrix versus Hydroxyapatite. Asian Spine J. 2016, 10, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.J., Jr.; Boden, S.D.; Titus, L.; Scarborough, N.L. New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine 1999, 24, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Shepard, N.A.; Rush, A.J., III; Scarborough, N.L.; Carter, A.J.; Phillips, F.M. Demineralized Bone Matrix in Spine Surgery: A Review of Current Applications and Future Trends. Int. J. Spine Surg. 2021, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, N.; Shin, D.A.; Yi, S.; Kim, K.N.; Ha, Y. Matched Comparison of Fusion Rates between Hydroxyapatite Demineralized Bone Matrix and Autograft in Lumbar Interbody Fusion. J. Korean Neurosurg. Soc. 2016, 59, 363–367. [Google Scholar] [CrossRef]
- Glassman, S.D.; Carreon, L.Y.; Djurasovic, M.; Campbell, M.J.; Puno, R.M.; Johnson, J.R.; Dimar, J.R. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: A randomized, controlled trial in patients over sixty years of age. Spine 2008, 33, 2843–2849. [Google Scholar] [CrossRef]
- Michielsen, J.; Sys, J.; Rigaux, A.; Bertrand, C. The effect of recombinant human bone morphogenetic protein-2 in single-level posterior lumbar interbody arthrodesis. J. Bone Jt. Surg. Am. 2013, 95, 873–880. [Google Scholar] [CrossRef]
- Roh, Y.H.; Lee, J.C.; Cho, H.K.; Jang, H.D.; Choi, S.W.; Shin, B.J. Comparative Study of Radiological and Clinical Outcomes in Patients Undergoing Minimally Invasive Lateral Lumbar Interbody Fusion Using Demineralized Bone Matrix Alone or with Low-Dose Escherichia coli-Derived rhBMP-2. World Neurosurg. 2022, 158, e557–e565. [Google Scholar] [CrossRef]
- Fischgrund, J.S.; Mackay, M.; Herkowitz, H.N.; Brower, R.; Montgomery, D.M.; Kurz, L.T. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine 1997, 22, 2807–2812. [Google Scholar] [CrossRef]
- Herkowitz, H.N.; Kurz, L.T. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J. Bone Jt. Surg. Am. 1991, 73, 802–808. [Google Scholar] [CrossRef]
- Park, Y.; Ha, J.W.; Lee, Y.T.; Sung, N.Y. The effect of a radiographic solid fusion on clinical outcomes after minimally invasive transforaminal lumbar interbody fusion. Spine J. 2011, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
| Exfuse n = 98 | Bongener n = 72 | Bonfuse n = 66 | |
|---|---|---|---|
| Age (years) | 67.3 ± 7.1 | 69.9 ± 8.5 | 69.4 ± 7.7 |
| Sex (Male/Female) | 35/63 | 25/47 | 16/50 |
| Height, cm | 158.72 ± 8.44 | 156.43 ± 8.60 | 155.32 ± 8.29 |
| Weight, kg | 63.06 ± 10.17 | 60.45 ± 11.25 | 60.38 ± 11.54 |
| BMI, kg/m2 | 24.96 ± 3.09 | 24.62 ± 3.35 | 24.94 ± 3.80 |
| Mean number of levels fused (n) | 1.05 | 1.47 | 1.23 |
| VAS a for back pain | 6.05 ± 2.46 | 6.41 ± 2.55 | 6.40 ± 2.50 |
| VAS for leg pain | 6.74 ± 2.34 | 6.74 ± 2.60 | 7.02 ± 2.69 |
| ODI score b | 20.9.3 ± 7.88 | 22.67 ± 8.64 | 22.83 ± 7.36 |
| EQ-5D c | 0.338 ± 0.266 | 0.303 ± 0.300 | 0.348 ± 0.279 |
| Exfuse | Bongener | Bonfuse | p-Value | |
|---|---|---|---|---|
| VAS a for back pain | 0.307 | |||
| Baseline | 6.05 ± 2.46 | 6.41 ± 2.55 | 6.40 ± 2.50 | |
| At 12 months | 4.70 ± 2.89 | 4.45 ± 2.40 | 5.07 ± 3.33 | |
| VAS for leg pain | 0.065 | |||
| Baseline | 6.74 ± 2.34 | 6.74 ± 2.60 | 7.02 ± 2.69 | |
| At 12 months | 3.49 ± 3.19 | 4.49 ± 2.21 | 4.81 ± 3.64 | |
| ODI b | 0.891 | |||
| Baseline | 20.93 ± 7.88 | 22.67 ± 8.64 | 22.83 ± 7.36 | |
| At 12 months | 14.29 ± 9.26 | 16.42 ± 7.60 | 17.07 ± 10.34 | |
| EQ-5D c | 0.573 | |||
| Baseline | 0.338 ± 0.266 | 0.303 ± 0.300 | 0.348 ± 0.279 | |
| At 12 months | 0.504 ± 0.328 | 0.494 ± 0.264 | 0.416 ± 0.350 |
| Fusion | Non-Fusion | p-Value | |
|---|---|---|---|
| VAS a for back pain | |||
| Exfuse | 4.64 ± 2.92 | 5.08 ± 2.75 | 0.620 |
| Bongener | 4.53 ± 2.41 | 3.40 ± 2.30 | 0.314 |
| Bonfuse | 4.87 ± 3.30 | 6.00 ± 3.50 | 0.336 |
| Total | 4.66 ± 3.02 | 5.11 ± 3.02 | 0.448 |
| VAS for leg pain | |||
| Exfuse | 3.26 ± 3.13 | 5.00 ± 3.33 | 0.079 |
| Bongener | 4.35 ± 2.69 | 6.20 ± 2.68 | 0.145 |
| Bonfuse | 4.85 ± 3.63 | 4.60 ± 3.86 | 0.845 |
| Total | 4.03 ± 3.18 | 5.07 ± 3.36 | 0.114 |
| ODI b | |||
| Exfuse | 13.77 ± 8.93 | 17.58 ± 11.04 | 0.187 |
| Bongener | 16.34 ± 7.75 | 17.40 ± 6.07 | 0.766 |
| Bonfuse | 17.40 ± 10.76 | 15.60 ± 8.55 | 0.623 |
| Total | 15.53 ± 9.13 | 16.81 ± 9.14 | 0.497 |
| EQ-5D c | |||
| Exfuse | 0.505 ± 0.334 | 0.500 ± 0.307 | 0.961 |
| Bongener | 0.502 ± 0.267 | 0.400 ± 0.234 | 0.414 |
| Bonfuse | 0.408 ± 0.354 | 0.454 ± 0.344 | 0.710 |
| Total | 0.480 ± 0.319 | 0.464 ± 0.301 | 0.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Ham, D.-W.; Kwon, O.; Park, J.-H.; Yoon, Y.; Kim, H.-J. Comparison of Fusion Rates among Various Demineralized Bone Matrices in Posterior Lumbar Interbody Fusion. Medicina 2024, 60, 265. https://doi.org/10.3390/medicina60020265
Lee S, Ham D-W, Kwon O, Park J-H, Yoon Y, Kim H-J. Comparison of Fusion Rates among Various Demineralized Bone Matrices in Posterior Lumbar Interbody Fusion. Medicina. 2024; 60(2):265. https://doi.org/10.3390/medicina60020265
Chicago/Turabian StyleLee, Sanghoon, Dae-Woong Ham, Ohsang Kwon, Joon-Hee Park, Youngsang Yoon, and Ho-Joong Kim. 2024. "Comparison of Fusion Rates among Various Demineralized Bone Matrices in Posterior Lumbar Interbody Fusion" Medicina 60, no. 2: 265. https://doi.org/10.3390/medicina60020265
APA StyleLee, S., Ham, D.-W., Kwon, O., Park, J.-H., Yoon, Y., & Kim, H.-J. (2024). Comparison of Fusion Rates among Various Demineralized Bone Matrices in Posterior Lumbar Interbody Fusion. Medicina, 60(2), 265. https://doi.org/10.3390/medicina60020265







