Risk Factors and Postoperative Complications of Lobectomy for Non-Small Cell Lung Cancer: An Exploratory Analysis of Premedication and Clinical Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Perioperative Care and Surgical Technique
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Tumour Characteristics
3.2. Premedication
3.3. Postoperative Pneumonia and Other Complications
4. Discussion
4.1. Central Findings and Clinical Implications
4.2. Discrepancies with the Literature
4.3. Strengths, Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McIntyre, A.; Ganti, A.K. Lung cancer-A global perspective. J. Surg. Oncol. 2017, 115, 550–554. [Google Scholar] [CrossRef]
- Cancer Today. Available online: https://gco.iarc.fr/ (accessed on 21 March 2024).
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020. Clin. Chest. Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Hoy, H.; Lynch, T.; Beck, M. Surgical Treatment of Lung Cancer. Crit. Care Nurs. Clin. N. Am. 2019, 31, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Manerikar, A.; Querrey, M.; Cerier, E.; Kim, S.; Odell, D.D.; Pesce, L.L.; Bharat, A. Comparative Effectiveness of Surgical Approaches for Lung Cancer. J. Surg. Res. 2021, 263, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, M.; Jørgensen, O.D.; Kronborg, C.; Andersen, C.; Licht, P.B. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: A randomised controlled trial. Lancet Oncol. 2016, 17, 836–844. [Google Scholar] [CrossRef]
- Mercier, O. VATS versus open thoracotomy for lung cancer resection: Is the game still running? Eur. J. Cardiothorac. Surg. 2022, 62, ezac303. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; A Harris, R.; E McKeon, H.; Batchelor, T.J.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; et al. Impact of video-assisted thoracoscopic lobectomy versus open lobectomy for lung cancer on recovery assessed using self-reported physical function: VIOLET RCT. Health Technol. Assess. 2022, 26, 1–162. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-J.; Jiang, Z.-H.; Gong, L.; Ma, K.; Ren, P.; Yu, Z.-T.; Wei, Y.-C. Video-Assisted Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity Matched Analysis. Ann. Thorac. Surg. 2019, 108, 1072–1079. [Google Scholar] [CrossRef]
- Couñago, F.; Luna, J.; Guerrero, L.L.; Vaquero, B.; Guillén-Sacoto, M.C.; González-Merino, T.; Taboada, B.; Díaz, V.; Rubio-Viqueira, B.; Díaz-Gavela, A.A.; et al. Management of oligometastatic non-small cell lung cancer patients: Current controversies and future directions. World J. Clin. Oncol. 2019, 10, 318–339. [Google Scholar] [CrossRef]
- Freeman, B.S.; Berger, J.S. Anesthesiology Core Review. Part 1, Basic Exam; Mcgraw-Hill Education Medical: New York, NY, USA, 2014. [Google Scholar]
- McDonald, C.L.; Cohen, B.H.; Medina Pérez, G.; Modest, J.M.; Kuris, E.O.; Born, C. Pre-Operative Medications as a Predictor for Post-Operative Complications Following Geriatric Hip Fracture Surgery. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 215145932210910. [Google Scholar] [CrossRef]
- Choi, K.S.; Jeong, Y.M.; Lee, E.; Kim, K.I.; Yee, J.; Lee, B.K.; Chung, J.E.; Rhie, S.J.; Gwak, H.S. Association of pre-operative medication use with post-surgery mortality and morbidity in oncology patients receiving comprehensive geriatric assessment. Aging Clin. Exp. Res. 2018, 30, 1177–1185. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Cheng, Y.-D.; Cheng, W.-Y.; Tsai, T.-H.; Huang, K.-H. The Prevalence of Anticholinergic Drugs and Correlation with Pneumonia in Elderly Patients: A Population-Based Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 6260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-X.; Shen, Y.; Chen, J.; Zhang, L.; Lin, W. Risk Factors of Pulmonary Complications After Minimally Invasive Surgery for Elderly Patients with Vertebral Compression Fractures. Ther. Clin. Risk Manag. 2020, 16, 7–15. [Google Scholar] [CrossRef]
- Seely, A.J.; Ivanovic, J.; Threader, J.; Al-Hussaini, A.; Al-Shehab, D.; Ramsay, T.; Gilbert, S.; Maziak, D.E.; Shamji, F.M.; Sundaresan, R.S. Systematic classification of morbidity and mortality after thoracic surgery. Ann. Thorac. Surg. 2010, 90, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Schussler, O.; Alifano, M.; Dermine, H.; Strano, S.; Casetta, A.; Sepulveda, S.; Chafik, A.; Coignard, S.; Rabbat, A.; Regnard, J.-F. Postoperative Pneumonia after Major Lung Resection. Am. J. Respir. Crit. Care Med. 2006, 173, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Detillon, D.D.E.M.A.; Veen, E.J. Postoperative Outcome After Pulmonary Surgery for Non-Small Cell Lung Cancer in Elderly Patients. Ann. Thorac. Surg. 2018, 105, 287–293. [Google Scholar] [CrossRef]
- Yao, L.; Luo, J.; Liu, L.; Wu, Q.; Zhou, R.; Li, L.; Zhang, C. Risk factors for postoperative pneumonia and prognosis in lung cancer patients after surgery. Medicine 2021, 100, e25295. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameri, M.; Bergman, P.; Franco-Cereceda, A.; Sartipy, U. Video-assisted thoracoscopic versus open thoracotomy lobectomy: A Swedish nationwide cohort study. J. Thorac. Dis. 2018, 10, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, F.; Lyberis, P.; Lausi, P.O.; Cristofori, R.C.; Giobbe, R.; Molinatti, M.; Filosso, P.L.; Curcio, C.; Crisci, R.; Ruffini, E. Does morbid obesity influence perioperative outcomes after video-assisted thoracic surgery (VATS) lobectomy for non-small cell lung cancer? Analysis of the Italian VATS group registry. Surg. Endosc. 2021, 36, 3567–3573. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.J.; Walker, R.L.; Dublin, S. Anticholinergic Medications and Risk of Community-Acquired Pneumonia in Elderly Adults: A Population-Based Case-Control Study. J. Am. Geriatr. Soc. 2015, 63, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Batihan, G.; Ceylan, K.C.; Usluer, O.; Kaya, Ş.Ö. Video-Assisted Thoracoscopic Surgery vs Thoracotomy for Non-Small Cell Lung Cancer Greater Than 5 cm: Is VATS a feasible approach for large tumors? J. Cardiothorac. Surg. 2020, 15, 261. [Google Scholar] [CrossRef]
- Simonsen, D.F.; Søgaard, M.; Bozi, I.; Horsburgh, C.R.; Thomsen, R.W. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir. Med. 2015, 109, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jin, S.-M.; Lee, C.-H.; Lee, B.J.; Kang, C.-H.; Yim, J.-J.; Yang, S.-C.; Yoo, C.-G.; Han, S.K.; Kim, J.H.; et al. Risk factors of postoperative pneumonia after lung cancer surgery. J. Korean Med. Sci. 2011, 26, 979–984. [Google Scholar] [CrossRef]
- Ogawa, F.; Wang, G.; Matsui, Y.; Hara, H.; Iyoda, A.; Satoh, Y. Risk factors for postoperative complications in the elderly with lung cancer. Asian Cardiovasc. Thorac. Ann. 2013, 21, 313–318. [Google Scholar] [CrossRef]
- Tulinský, L.; Sengul, I.; Ihnát, P.; Ostruszka, P.; Toman, D.; Guňková, P.; Pelikán, A.; Sengul, D. Obesity in cases undergoing the surgical procedure of lung lobectomy: Risk or benefit? Rev. Assoc. Med. Bras. 2022, 68, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Tulinský, L.; Mitták, M.; Tomášková, H.; Ostruszka, P.; Penka, I.; Ihnát, P. Obesity paradox in patients undergoing lung lobectomy—Myth or reality? BMC Surg. 2018, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, P.; Tan, K.S.; Chudgar, N.P.; Dycoco, J.; Adusumilli, P.S.; Bains, M.S.; Bott, M.J.; Downey, R.J.; Huang, J.; Isbell, J.M.; et al. Operative Time Is Associated with Postoperative Complications After Pulmonary Lobectomy. Ann. Surg. 2022, 278, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, M.T.; Forcada, C.; Varela, G.; Jiménez, M.F. Operating time: An independent and modifiable risk factor for short-term complications after video-thoracoscopic pulmonary lobectomy. Eur. J. Cardiothorac. Surg. 2022, 62, ezac503. [Google Scholar] [CrossRef] [PubMed]
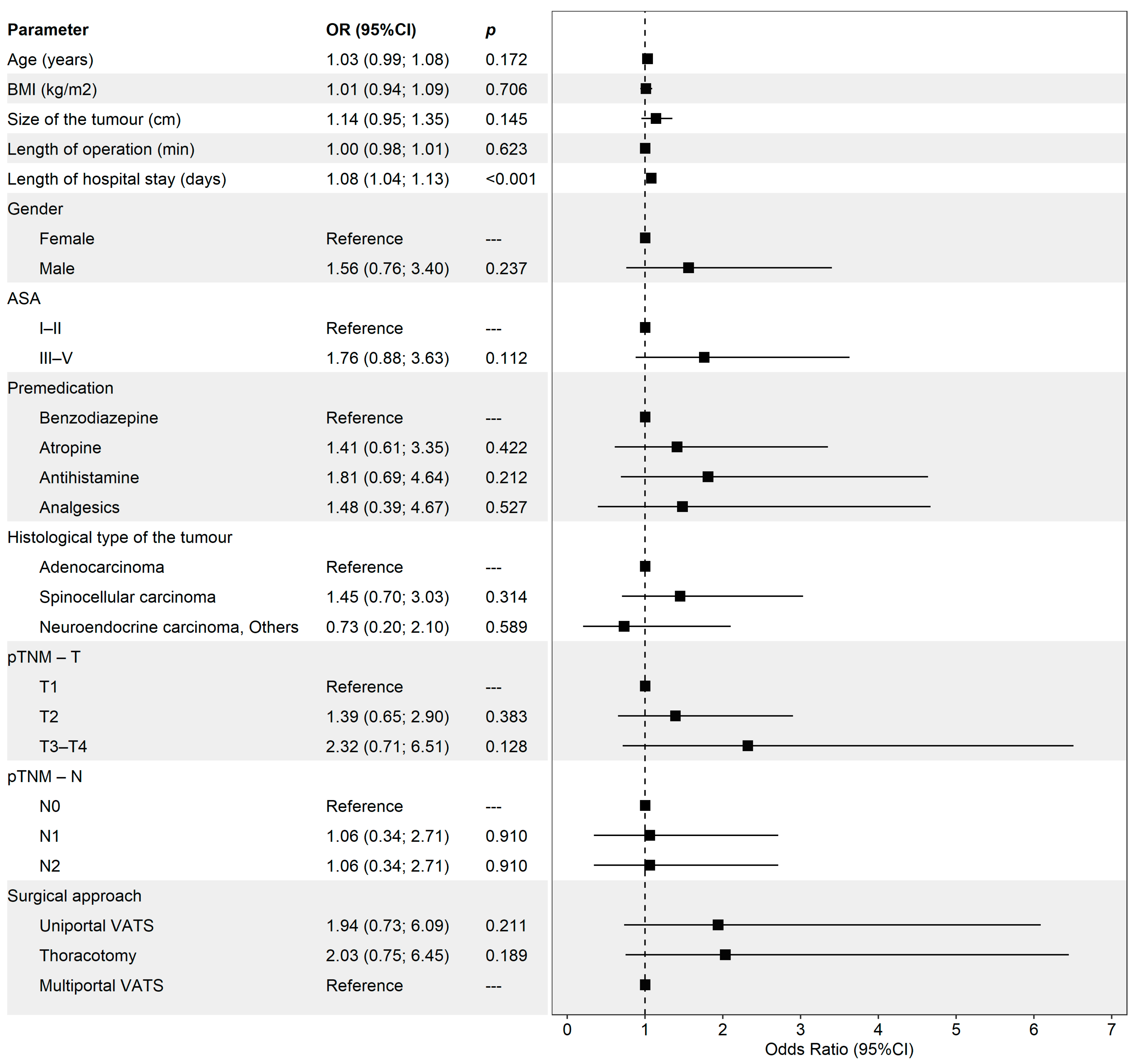

| Total, n = 346 | |
|---|---|
| Age, years, median (IQR) | 69 (63; 73) |
| BMI (kg/m2), median (IQR) | 27.9 (24.8; 31.2) |
| Size of the tumour, cm, median (IQR) | 2.5 (2.0; 4.0) |
| Length of operation, min, median (IQR) | 90 (70; 110) |
| Length of hospital stay, days, median (IQR) | 11 (8; 13) |
| Gender, n (%) | |
| Male | 212 (61.3) |
| Female | 134 (38.7) |
| ASA, n (%) | |
| I–II | 174 (50.3) |
| III | 169 (48.8) |
| IV–V | 3 (0.9) |
| Premedication, n (%) | |
| Benzodiazepine | 133 (38.5) |
| Atropine | 115 (33.2) |
| Antihistamine | 64 (18.5) |
| Analgesics | 34 (9.8) |
| Histological type of the tumour, n (%) | |
| Adenocarcinoma | 165 (47.8) |
| Spinocellular carcinoma | 126 (36.4) |
| Neuroendocrine carcinoma | 32 (9.2) |
| Others | 23 (6.6) |
| pTNM—T, n (%) | |
| T1 | 202 (58.4) |
| T2 | 117 (33.8) |
| T3 | 22 (6.4) |
| T4 | 5 (1.4) |
| pTNM—N, n (%) | |
| N0 | 256 (74.0) |
| N1 | 45 (13.0) |
| N2 | 45 (13.0) |
| Surgical approach, n (%) | |
| Uniportal VATS | 145 (42.0) |
| Thoracotomy | 123 (35.5) |
| Multiportal VATS | 78 (22.5) |
| Pneumonia, n (%) | |
| Yes | 37 (10.7) |
| No | 309 (89.3) |
| Clavien–Dindo classification, n (%) | |
| Grade 0 | 256 (74.0) |
| Grade I–II | 55 (15.9) |
| Grade III–IV | 23 (6.6) |
| Grade V | 12 (3.5) |
| Benzodiazepine, n = 133 | Atropine, n = 115 | Antihistamine, n = 64 | Analgesics, n = 34 | p a | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 67 (61; 71) | 68 (63; 73) | 73 (68; 76) | 68 (64; 73) | <0.001 b |
| BMI (kg/m2), median (IQR) | 27.8 (24.4; 31.1) | 27.5 (24.4; 31.0) | 28.4 (26.4; 31.8) | 29.1 (26.8; 31.1) | 0.194 |
| Size of the tumour, cm, median (IQR) | 3.0 (1.6; 4.0) | 2.5 (2.0; 3.8) | 2.5 (2.0; 3.6) | 2.8 (2.0; 3.4) | 0.961 |
| Gender, n (%) | 0.044 | ||||
| Male | 70 (52.6) | 79 (68.7) | 39 (60.9) | 24 (70.6) | |
| Female | 63 (47.4) | 36 (31.3) | 25 (39.1) | 10 (29.4) | |
| ASA, n (%) | 0.100 | ||||
| I–II | 69 (51.9) | 65 (56.5) | 24 (37.4) | 16 (47.1) | |
| III | 63 (47.4) | 50 (43.5) | 39 (60.9) | 17 (50.0) | |
| IV–V | 1 (0.7) | --- | 1 (1.7) | 1 (2.9) | |
| Histological type of the tumour, n (%) | 0.197 | ||||
| Adenocarcinoma | 73 (54.9) | 46 (40.0) | 31 (48.4) | 15 (44.2) | |
| Spinocellular carcinoma | 39 (29.3) | 53 (46.1) | 22 (34.5) | 12 (35.3) | |
| Neuroendocrine carcinoma | 12 (9.0) | 11 (9.6) | 4 (6.2) | 5 (14.7) | |
| Others | 9 (6.8) | 5 (4.3) | 7 (10.9) | 2 (5.8) | |
| pTNM—T, n (%) | 0.674 | ||||
| T1 | 73 (54.9) | 66 (57.4) | 39 (60.9) | 24 (70.6) | |
| T2 | 47 (35.3) | 42 (36.5) | 20 (31.2) | 8 (23.5) | |
| T3 | 9 (6.8) | 7 (6.1) | 4 (6.2) | 2 (5.9) | |
| T4 | 4 (3.0) | --- | 1 (1.7) | --- | |
| pTNM—N, n (%) | 0.984 | ||||
| N0 | 101 (75.9) | 83 (72.2) | 48 (75.0) | 24 (70.6) | |
| N1 | 17 (12.8) | 16 (13.9) | 7 (10.9) | 5 (14.7) | |
| N2 | 15 (11.3) | 16 (13.9) | 9 (14.1) | 5 (14.7) | |
| Surgical approach, n (%) | 0.414 | ||||
| Uniportal VATS | 52 (39.1) | 55 (47.8) | 23 (36.0) | 15 (44.2) | |
| Thoracotomy | 49 (36.8) | 40 (34.8) | 21 (32.8) | 13 (38.2) | |
| Multiportal VATS | 32 (24.1) | 20 (17.4) | 20 (31.2) | 6 (17.6) |
| Benzodiazepine, n = 133 | Atropine, n = 115 | Antihistamine, n = 64 | Analgesics, n = 34 | p a | |
|---|---|---|---|---|---|
| Length of operation, min, median (IQR) | 90 (70; 105) | 90 (75; 110) | 90 (74; 110) | 90 (70; 110) | 0.572 |
| Length of hospital stay, days, median (IQR) | 10 (8; 13) | 11 (8; 13) | 11 (9; 13) | 11 (8; 13) | 0.233 |
| Pneumonia, n (%) | 11 (8.3) | 13 (11.3) | 9 (14.1) | 4 (11.8) | 0.645 |
| Postoperative complications, n (%) | 32 (24.1) | 30 (26.1) | 17 (26.6) | 11 (32.4) | 0.805 |
| Clavien–Dindo classification, n (%) | 0.893 | ||||
| Grade 0 | 101 (75.9) | 85 (73.9) | 47 (73.4) | 23 (67.6) | |
| Grade I–II | 19 (14.3) | 21 (18.3) | 10 (15.6) | 5 (14.7) | |
| Grade III–IV | 8 (6.0) | 7 (6.1) | 4 (6.3) | 4 (11.8) | |
| Grade V | 5 (3.8) | 2 (1.7) | 3 (4.7) | 2 (5.9) | 0.430 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kepičová, M.; Tulinský, L.; Kondé, A.; Dzurňáková, P.; Ihnát, P.; Adamica, D.; Neoral, Č.; Martínek, L. Risk Factors and Postoperative Complications of Lobectomy for Non-Small Cell Lung Cancer: An Exploratory Analysis of Premedication and Clinical Variables. Medicina 2024, 60, 2088. https://doi.org/10.3390/medicina60122088
Kepičová M, Tulinský L, Kondé A, Dzurňáková P, Ihnát P, Adamica D, Neoral Č, Martínek L. Risk Factors and Postoperative Complications of Lobectomy for Non-Small Cell Lung Cancer: An Exploratory Analysis of Premedication and Clinical Variables. Medicina. 2024; 60(12):2088. https://doi.org/10.3390/medicina60122088
Chicago/Turabian StyleKepičová, Markéta, Lubomír Tulinský, Adéla Kondé, Paula Dzurňáková, Peter Ihnát, Dávid Adamica, Čestmír Neoral, and Lubomír Martínek. 2024. "Risk Factors and Postoperative Complications of Lobectomy for Non-Small Cell Lung Cancer: An Exploratory Analysis of Premedication and Clinical Variables" Medicina 60, no. 12: 2088. https://doi.org/10.3390/medicina60122088
APA StyleKepičová, M., Tulinský, L., Kondé, A., Dzurňáková, P., Ihnát, P., Adamica, D., Neoral, Č., & Martínek, L. (2024). Risk Factors and Postoperative Complications of Lobectomy for Non-Small Cell Lung Cancer: An Exploratory Analysis of Premedication and Clinical Variables. Medicina, 60(12), 2088. https://doi.org/10.3390/medicina60122088





