Abstract
Background and Objectives: Damage to the vocal folds frequently results in fibrosis, which can degrade vocal quality due to the buildup of collagen and modifications in the extracellular matrix (ECM). Conventional treatments have shown limited success in reversing fibrotic changes. Hepatocyte growth factor (HGF) and c-Met-targeting antibodies are promising due to their potential to inhibit fibrosis and promote regeneration. This research examines the effectiveness of injections containing c-Met agonistic antibodies relative to HGF in reducing fibrosis within a rat model of vocal fold injury. Materials and Methods: Forty-five Sprague Dawley rats were divided into three groups, which were HGF, c-Met agonistic antibody, and the control (PBS). The right vocal folds were injured and treated with HGF or c-Met agonistic antibody injections. RNA isolation and quantitative real-time PCR were performed to assess mRNA levels of fibrosis-related markers at 1 and 2 weeks post-injury. Histopathological analysis was conducted at 3 weeks to evaluate collagen and hyaluronic acid (HA) deposition. Results: Both the HGF and c-Met groups demonstrated reduced type III collagen mRNA expression compared to the PBS group. The c-Met group uniquely maintained fibronectin levels closer to normal. Additionally, the c-Met group showed significantly upregulated expression of hyaluronan synthase (HAS) 1 and HAS 3 at 2 weeks post-injury, indicating enhanced HA synthesis. Histological analysis showed significantly lower collagen deposition and higher HA in the c-Met group than in PBS, confirming superior anti-fibrotic effects and ECM restoration. Conclusions: c-Met agonistic antibody injections outperformed HGF in reducing fibrosis, upregulating HAS expression, and promoting HA deposition in injured vocal folds, highlighting its potential as a superior therapeutic approach for preventing fibrosis and enhancing ECM quality in vocal fold injuries. Further research on functional outcomes in larger models is recommended to validate these findings.
1. Introduction
Vocal fold vibration is essential for voice production and the quality of the produced voice depends on the composition and viscoelasticity of vocal fold tissue, particularly the extracellular matrix (ECM) of the lamina propria layer. Injury to the vocal fold often leads to scar formation. This scar, along with the changes in the ECM could cause serious voice problems. Therefore, minimizing scar formation is essential during the healing process.
Vocal fold scarring is difficult to treat effectively with current surgical methods and available tissue engineering materials [1]. The lack of appropriate treatments is largely due to the limitation of existing surgical options. which do not adequately address ECM biomechanics and tissue properties. Additionally current injectable materials fail to replicate the complex structure of the ECM [2].
Numerous attempts at vocal fold regeneration have been made, including the use of mesenchymal stem cells (MSCs) [3], human nasal inferior turbinate-derived mesenchymal stem cells (hTMSCs) [4], and conditioned media [5]. However, stem cell therapy presents several challenges, like an immunological response, cost concerns, the risks of malignant transformation, etc. [6]. While therapies using conditioned media or exosome therapy could potentially overcome some limitations of stem cell therapy, identifying the most effective therapeutic components remains difficult. Hepatocyte growth factor (HGF) has also been researched as a treatment candidate for vocal fold healing due to its anti-fibrotic properties [7,8]. HGF has been shown to contribute to prevent scar formation in the liver, kidneys, and lungs in animal models [9,10,11]. However, a short half-time (less than five min) is the limitation of its therapeutic use [12]. The c-Met protein is a transmembrane tyrosine kinase that binds with HGF. Research has shown that c-Met localizes in endothelial cells, where the interaction between HGF and c-Met facilitates multiple biological processes [13,14].
This study investigated whether injecting a c-Met agonistic antibody, which has a relative long half-time [15], can promote the regeneration of injured vocal folds, and it compared these effects to those of HGF. We used quantitative real-time polymerase chain reaction (PCR) and histologic examination to analyze the early phase of wound healing in rat vocal fold injury models [5].
2. Materials and Methods
2.1. Animal Experiments
Approval for this study was granted by the Animal Ethics Committee of the Catholic University of Korea (permit no. CMCDJ-AP-2022-001), and all procedures followed institutional care guidelines. Forty-five Sprague Dawley rats were divided among three groups, with 12 each in the HGF and c-Met experimental groups and 12 in the PBS control group, with three animals from each group reserved for histological analysis. The animals were anesthetized with ketamine hydrochloride (100 mg/kg) and xylazine hydrochloride (10 mg/kg), positioned semi-vertically on a custom platform and visualized using a pediatric endoscope. Microscissors were used to expose the thyroarytenoid muscle on the right vocal folds, while the left vocal fold served as an untreated control. Immediately following injury, 100 ng of HGF (Millipore, Bedford, MA, USA) in 50 µL PBS or 100 ng of c-Met agonistic antibody (Helixmith, Seoul, Republic of Korea) in 50 µL PBS was injected into the injury site for experimental groups. The PBS control group received an injection of 50 µL PBS alone. All injections were administered under direct visualization using a 30-gauge needle and pediatric laryngoscope.
2.2. RNA Isolation and Real-Time PCR of Rat Vocal Fold Tissues
The rat vocal folds were collected at 1 and 2 weeks post-injury and homogenized using a TissueLyser II (Qiagen, Valencia, CA, USA). RNA extraction was carried out with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), then ribonuclease-free deoxyribonuclease I (Qiagen) was used to minimize contamination from genomic DNA. Complementary DNA (cDNA) was synthesized using a Reverse Transcriptase Premix Kit (Elpis Biotech, Daejeon, Republic of Korea) with 1 μg of RNA. Real-time PCR was conducted on an ABI 7500 FAST system (Applied Biosystems, Foster City, CA, USA) in a 20 μL reaction volume, containing Power SYBR Green PCR Master Mix (Applied Biosystems), 500 nM forward and reverse primers, and 1 μL of cDNA. mRNA expression levels were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Primer sets used in this study are shown in Table 1.

Table 1.
Primer information.
2.3. Histopathological Analysis of Rat Vocal Fold Tissue
Three larynges from each group were collected for histological examination following euthanasia, three weeks after injury. The tissues were embedded in paraffin, sectioned coronally at a thickness of 4 µm using a microtome, and stained with hematoxylin and eosin. Verhoeff–Van Gieson and Masson’s trichrome were used for the detection of collagen fiber and elastic fiber, following standard pathology protocols. Prepared slides were examined under a light microscope (Eclipse TE300; Nikon, Tokyo, Japan). Densitometric analysis was conducted to quantify extracellular matrix staining, with areas measured using ImageJ software (Version 1.53e, National Institutes of Health, Bethesda, MD, USA).
2.4. Statistics
Results are presented as means with standard error of the mean (SEM). GraphPad Prism 5 (GraphPad, Inc., La Jolla, CA, USA) was used for conducting statistical analyses. To compare multiple groups, a one-way ANOVA was followed by Tukey’s post hoc test. A p-value of less than 0.05 was used to determine statistical significance.
3. Results
3.1. Gene Expression at 1 Week Post-Injury
The expression levels of mRNAs encoding hyaluronan synthase (HAS) 1, HAS 2, and HAS 3 did not differ among the normal, PBS, HGF, and c-Met groups. The mRNA expression level of procollagen type III (COL III) was significantly downregulated in both the c-Met and HGF groups compared with the PBS group, while the gene encoding fibronectin (FN) was upregulated in the PBS and HGF groups compared with the normal group. However, in the c-Met group, the mRNA level of FN expression did not increase as much as in the PBS and HGF groups, showing no significant difference compared to the normal group (Figure 1).
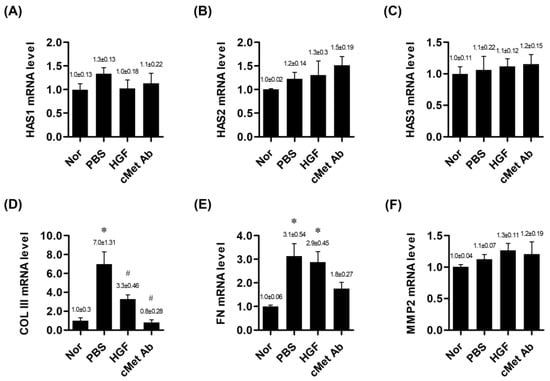
Figure 1.
mRNA expression ratios of genes related to vocal fold tissue regeneration in the normal, PBS, and c-Met Ab groups on post-injury day 7. * p < 0.05 vs. Nor (normal), # p < 0.05 vs. PBS, p < 0.05 vs. HGF. (A–C) Expression levels of HAS1, HAS2, and HAS3 mRNA, respectively. (D) mRNA expression of COL III. (E) mRNA expression of FN. (F) mRNA expression of MMP2. Normal (Nor) tissues were compared to injured tissues treated with phosphate-buffered saline (PBS), hepatocyte growth factor (HGF), or c-Met agonistic antibody (c-Met Ab).
3.2. Gene Expression at 2 Weeks Post-Injury
At two weeks post-injury, the mRNA expression levels of HAS 1 and HAS 3 were significantly upregulated in the c-Met group compared to the normal group. HAS 2 expression in the c-Met group showed an increasing trend, although it did not reach statistical significance. In the HGF group, only HAS 3 mRNA expression was significantly upregulated compared to the normal group. COL III mRNA expression was significantly upregulated in the PBS group compared to the normal group and was significantly downregulated in both the HGF and c-Met groups. FN was upregulated in the PBS, HGF, and c-Met groups compared with the normal group, but FN was downregulated in the c-Met group compared to the PBS group (Figure 2).
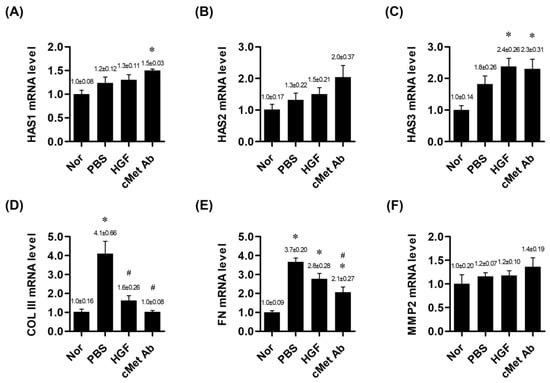
Figure 2.
mRNA expression ratios of genes related to vocal fold tissue regeneration in the normal, PBS, and c-Met Ab groups on post-injury day 14. * p < 0.05 vs. Nor (normal), # p < 0.05 vs. PBS, p < 0.05 vs. HGF. (A–C) Expression levels of HAS1, HAS2, and HAS3 mRNA, respectively. (D) mRNA expression of COL III. (E) mRNA expression of FN. (F) mRNA expression of MMP2. Normal (Nor) tissues were compared to injured tissues treated with phosphate-buffered saline (PBS), hepatocyte growth factor (HGF), or c-Met agonistic antibody (c-Met Ab).
3.3. Histological Examination
Histological analysis was conducted at 3 weeks post-injury. A densitometric analysis of histological samples showed that the c-Met groups exhibited stronger anti-fibrotic effects than the PBS group. Masson’s trichrome staining indicated significantly reduced collagen deposition in the c-Met groups compared to the PBS group. Although the HGF group also showed reduced collagen deposition compared to the PBS group, the difference was not statistically significant. HA was detected by alcian blue staining. The c-Met group exhibited significantly increased HA deposition compared to the PBS group. The HGF group also showed increased HA deposition compared to the PBS group, though this difference did not reach statistical significance (Figure 3).
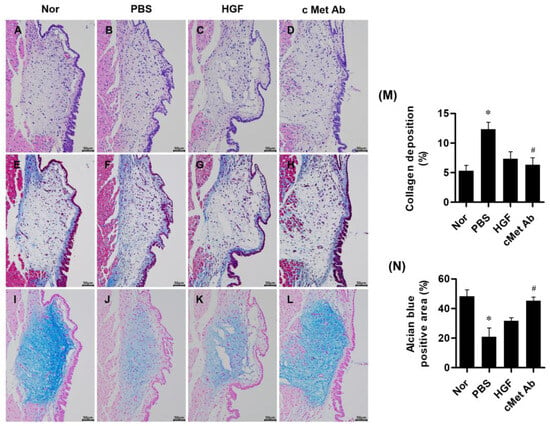
Figure 3.
Representative images of stained vocal fold tissues at 3 weeks post-injury: hematoxylin and eosin staining (A–D), Masson’s trichrome staining for collagen (E–H), and alcian blue staining for hyaluronic acid (I–L) from the normal (Nor), PBS, HGF, and c-Met Ab groups. Scale bars = 50 µm. Quantitative analysis of collagen deposition (blue-stained collagen) (M) and alcian blue-positive area (blue-colored hyaluronic acids) (N) are shown. Original magnification: 200×; * p < 0.05 compared to Nor (normal), # p < 0.05 compared to PBS, and p < 0.05 compared to HGF.
4. Discussion
Scarring after vocal fold injuries is a prevalent cause of severe voice impairment. The scarring process includes three main phases, namely the inflammatory response, cellular proliferation, and tissue remodeling [16].
Inflammatory factors are produced within 4 to 8 h post-injury, after which there is an upregulation of hyaluronan synthases I and II and procollagens I and III, followed by a substantial recruitment of cells, mainly with fibroblastic characteristics, originating from the macula flava. On days 5–7 post-injury, the fibroblasts undergo differentiation myofibroblasts, leading to a disorganization of collagen and elastin bundles and a reduction in the density of elastin and hyaluronic acid. Subsequently, type III collagen is replaced by type I collagen, and the density of fibronectin increases [17,18]. Vocal fold scarring is characterized by excessive collagen deposition. Thus, reducing collagen production is crucial to decreasing scar formation. While fibronectin is necessary for wound healing, prolonged upregulated fibronectin expression after injury causes fibrosis and impairs the viscoelastic properties of the vocal fold [19]. The key component of vocal fold viscoelastic properties is hyaluronic acid; therefore, HA synthesis is important [20,21]. HAS genes have three subtypes, with HAS2 and HAS3 exhibiting higher enzymatic activity compared to HAS1 [22].
The key focus in the wound healing process is to reduce scarring and restore the normal composition of the lamina propria, which is distinct from simply accelerating wound closure. A recent study, although conducted on skin tissue, reported that blocking potassium channels promotes wound healing [23]. However, this study did not include histological analysis, making it difficult to determine the extent of scar formation compared to normal tissue. The researchers hypothesized that fibroblast migration and activation at the wound site accelerate wound healing. However, based on previous studies, such mechanisms are known to potentially exacerbate scarring [24,25].
In this study, both the c-Met and HGF groups exhibited reduced COL III mRNA expression at 1 or 2 weeks post-injury. However, in the c-Met group, FN mRNA expression did not increase relative to the normal group at 1 week post-injury and decreased compared to the PBS group at 2 weeks post-injury. Regarding HA, no differences were observed in the RNA expression levels of HAS1, HAS2, or HAS3 at 1 week post-injury. However, by 2 weeks post-injury, a significant increase in HAS3 mRNA expression was detected. This finding aligns with previous studies, which report an initial rise in HAS1 and HAS2—especially HAS2—within three days post-injury, followed by a subsequent increase in HAS3 [26]. Furthermore, the observed significant upregulation of HAS3 mRNA expression in both the HGF and c-Met groups is consistent with earlier research suggesting that HGF negatively regulates TGF-β, which is known to suppress HAS3 expression during tissue injury. After 3 weeks, the histologic examination confirmed that mRNA expression correlated with histological change. The c-Met group had less collagen and more HA deposition, demonstrating a more pronounced anti-fibrotic effect than the PBS group with significance. However, there was no statistical difference from the HGF group. In this report, we demonstrated that the c-Met antagonistic antibody performs better than the HGF and PBS group in the anti-fibrotic effects of vocal fold injury.
Currently used injectable materials for the vocal folds primarily aim to improve glottic closure, with Radiesse (Merz North America, Inc., Raleigh, NC, USA) providing long-term effects and hyaluronic acid (HA) offering short-term effects [27,28]. These materials are typically injected either into or outside the thyroarytenoid muscle to facilitate vocal fold adduction with a space-occupying effect [29]. However, their purpose differs from regenerating the lamina propria during the wound healing process of vocal fold injuries
The restoration of normal vocal fold properties is essential to the treatment of vocal fold scars.
Many attempts have been made to reduce vocal fold fibrosis following injury.
In tissue engineering, the regeneration of tissues or organs can be achieved by the combination of scaffolds, cells, and regulators under proper conditions. Growth factor therapy is one of the therapeutic strategies in regenerative medicine. Multiple studies have highlighted HGF’s efficacy as a promising growth factor therapy for vocal fold scarring, demonstrating its capacity to regulate ECM production in fibroblasts [7,14,30,31].
In vocal fold regeneration, HGF and c-Met expression, known for their anti-fibrotic effects, decrease immediately following vocal fold injury but increase again after 14 days [32]. This period is considered critical for vocal fold scar remodeling. Supplementing the diminished HGF effect, such as through external administration, may aid in preventing scar formation in the vocal folds [30,32]. However, the effectiveness of HGF might be limited by its short half-life, necessitating repeated injection or the use of slow-release scaffolds [33,34,35].
Repeated injections may damage the vocal folds, potentially hindering the healing process [36]. Slow-release scaffolds, although designed for sustained release, do not replicate the precise biomechanical properties of the native ECM, which may interfere with vocal fold vibration until fully degraded [29,37]. Additionally, scaffold degradation may trigger inflammatory responses, posing further risks [38]. Therefore, a single, long-acting injection without a scaffold would be more suitable for effective treatment. The c-Met agonistic antibody is administered in a single injection with PBS, eliminating the need for scaffold use.
Recently, studies have explored the use of HGF-transfected stem cells via viral vectors to extend the effects of HGF [39]. However, the use of stem cells poses challenges, such as immunological responses and the risk of malignant transformation, as previously noted. While autologous or homologous stem cells may reduce these risks, they present issues related to consistency and cost. Additionally, viral transfection techniques are limited by concerns over mutations and stability [40,41]. In contrast, the c-Met agonistic antibody offers a cell-free, virus-free therapeutic approach, potentially circumventing these issues.
The serum half-life of the c-Met agonistic antibody used in this study had a half-life of approximately 3 days, with persistence in the body for up to 6 days following intravenous injection [15]. Therefore, this agonistic antibody has the potential to compensate for the short half-life of HGF.
In this report, we demonstrated that the c-Met antagonistic antibody performs better than the HGF and PBS group in anti-fibrotic effects during the remodeling of injured vocal folds. However, there were some limitations; first, while the c-Met group was more effective in anti-fibrosis than the HGF group, the difference was smaller than expected. To clarify this daily examination of the activated c-Met receptor, mRNA expression of CoLIII, FN, and HAS following injection may be beneficial. Additionally, conducting histological examinations beyond three weeks post-injury would further enhance our understanding of the findings. But conducting such experiments would require a significantly larger number of experimental animals compared to the current study. Second, the regeneration of vocal fold tissue does not necessarily guarantee functional restoration of vocal fold abilities. Therefore, to evaluate the function of the recovered vocal folds (such as the symmetrical mucosa waves during phonation between control and study group vocal folds), additional experiments involving larger animal models, such as rabbits, and tools like high-speed video cameras are required. If additional experiments are conducted considering these limitations, it is believed that the c-Met agonist antibody can be used in human research.
5. Conclusions
The injection of a c-Met agonistic antibody into injured vocal folds showed an anti-fibrotic effect in the early phase of wound healing, demonstrating superiority over HGF injection. These findings highlight the potential of c-Met agonistic antibodies for regenerative therapy in vocal fold injuries.
Author Contributions
C.-S.K. designed the study. H.C. analyzed and interpreted the data. J.-K.J. provided technical support and the c-Met antibody. C.-S.K. and H.C. conducted the experiments. C.-S.K. and H.-I.S. prepared the manuscript. H.-I.S. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Clinical Research Institute grant funded by The Catholic University of Korea Daejeon St. Mary’s Hospital (CMCDJ-P-2023-001) and The E.N.T. Fund of the Catholic University of Korea made in the program year of 2023.
Institutional Review Board Statement
The study was approved by the Animal Ethics Committee of the Catholic University of Korea (permit no. CMCDJ-AP-2022-001). Approval date: 24 June 2022.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Helixmith Co., Ltd., Seoul, Republic of Korea, provided the c-Met-agonistic antibody (c-Met Ab (VM507)) for the experiments. Choung-Soo Kim wishes to acknowledge the financial support of the Catholic Medical Center Research Foundation made in the program year of 2023.
Conflicts of Interest
Author Jae-Kyun Jung was employed by the company Helixmith Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
List of abbreviations used in this manuscript.
| Abbreviations | Definition |
| ECM | Extracellular matrix |
| HGF | Hepatocyte growth factor |
| PBS | Phosphate buffered saline |
| RNA | Ribonucleic Acid |
| HA | Hyaluronic acid |
| HAS | Hyaluronan synthase |
| MSC | Mesenchymal stem cells |
| PCR | Polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| COLIII | Procollagen type III |
| FN | Fibronectin |
| TGF-β | Transforming growth factors-beta superfamily |
References
- Thibeault, S.L.; Klemuk, S.A.; Chen, X.; Quinchia Johnson, B.H. In Vivo engineering of the vocal fold ECM with injectable HA hydrogels-late effects on tissue repair and biomechanics in a rabbit model. J. Voice 2011, 25, 249–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagubothu, S.R.; Sugars, R.V.; Tudzarovski, N.; Andrén, A.T.; Bottai, M.; Davies, L.C.; Hertegård, S.; Le Blanc, K. Mesenchymal stromal cells modulate tissue repair responses within the injured vocal fold. Laryngoscope 2020, 130, E21–E29. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, J.; Ahrlund-Richter, L.; Svensson, B.; Forsgren, K.; Maurer, F.H.; Vidovska, D.; Hertegård, S. Injection of embryonic stem cells into scarred rabbit vocal folds enhances healing and improves viscoelasticity: Short-term results. Laryngoscope 2007, 117, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Choi, H.; Park, K.C.; Kim, S.W.; Sun, D.I. The Ability of Human Nasal Inferior Turbinate-Derived Mesenchymal Stem Cells to Repair Vocal Fold Injuries. Otolaryngol. Head Neck Surg. 2018, 159, 335–342. [Google Scholar] [CrossRef]
- Kim, C.S.; Choi, H.; Kim, S.W.; Sun, D.I. The Ability of Conditioned Media from Stem Cells to Repair Vocal Fold Injuries. Laryngoscope 2019, 129, 1867–1875. [Google Scholar] [CrossRef]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Hirano, S.; Suehiro, A.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J. Effect of exogenous hepatocyte growth factor on vocal fold fibroblasts. Ann. Otol. Rhinol. Laryngol. 2009, 118, 606–611. [Google Scholar] [CrossRef]
- Choi, J.S.; Heang Oh, S.; Kim, Y.M.; Lim, J.Y. Hyaluronic Acid/Alginate Hydrogel Containing Hepatocyte Growth Factor and Promotion of Vocal Fold Wound Healing. Tissue Eng. Regen. Med. 2020, 17, 651–658. [Google Scholar] [CrossRef]
- Schievenbusch, S.; Strack, I.; Scheffler, M.; Wennhold, K.; Maurer, J.; Nischt, R.; Dienes, H.P.; Odenthal, M. Profiling of anti-fibrotic signaling by hepatocyte growth factor in renal fibroblasts. Biochem. Biophys. Res. Commun. 2009, 385, 55–61. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chopra, P.; Hak, A.; Dastidar, S.G.; Ray, A. Hepatocyte growth factor is an attractive target for the treatment of pulmonary fibrosis. Expert Opin. Investig. Drugs 2013, 22, 499–515. [Google Scholar] [CrossRef]
- Ishikawa, H.; Jo, J.; Tabata, Y. Liver Anti-Fibrosis Therapy with Mesenchymal Stem Cells Secreting Hepatocyte Growth Factor. J. Biomater. Sci. Polym. Ed. 2012, 23, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.; Meneu, J.C.; Calvo, J.; Pérez, B.; Sesma, A.G.; Manrique, A.; Vegh, I.; Aragón, A.M.; Grau, M.; Gimeno, A.; et al. Modulation of hepatocyte growth factor plasma levels in relation to the dose of exogenous heparin administered: An experimental study in rats. Transplant. Proc. 2005, 37, 3943–3947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Thibeault, S.; Bless, D.M.; Ford, C.N.; Kanemaru, S. Hepatocyte growth factor and its receptor c-met in rat and rabbit vocal folds. Ann. Otol. Rhinol. Laryngol. 2002, 111, 661–666. [Google Scholar] [CrossRef]
- An, J.N.; Li, L.; Lee, J.; Yu, S.S.; Lee, J.; Kim, Y.C.; Kim, D.K.; Oh, Y.K.; Lim, C.S.; Kim, Y.S.; et al. cMet agonistic antibody attenuates apoptosis in ischaemia-reperfusion-induced kidney injury. J. Cell. Mol. Med. 2020, 24, 5640–5651. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of wound healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef]
- Mattei, A.; Magalon, J.; Bertrand, B.; Philandrianos, C.; Veran, J.; Giovanni, A. Cell therapy and vocal fold scarring. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2017, 134, 339–345. [Google Scholar] [CrossRef]
- Rousseau, B.; Ge, P.J.; Ohno, T.; French, L.C.; Thibeault, S.L. Extracellular matrix gene expression after vocal fold injury in a rabbit model. Ann. Otol. Rhinol. Laryngol. 2008, 117, 598–603. [Google Scholar] [CrossRef]
- Kazemirad, S.; Heris, H.K.; Mongeau, L. Viscoelasticity of hyaluronic acid-gelatin hydrogels for vocal fold tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 283–290. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A.; Sivasankar, P.M. A Review of Hyaluronic Acid and Hyaluronic Acid-based Hydrogels for Vocal Fold Tissue Engineering. J. Voice 2017, 31, 416–423. [Google Scholar] [CrossRef]
- Siiskonen, H.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K. Hyaluronan synthase 1: A mysterious enzyme with unexpected functions. Front. Immunol. 2015, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.; Vatasescu-Balcan, A.; Stoleru, S.; Zugravu, A.; Poenaru, E.; Engi, M.; Coman, O.A.; Fulga, I. Experimental Research on the Influence of Ion Channels on the Healing of Skin Wounds in Rats. Processes 2024, 12, 109. [Google Scholar] [CrossRef]
- McDougall, S.; Dallon, J.; Sherratt, J.; Maini, P. Fibroblast migration and collagen deposition during dermal wound healing: Mathematical modelling and clinical implications. Philos. Trans. A Math. Phys. Eng. Sci. 2006, 364, 1385–1405. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Minn, K.W. The effect of myofibroblast on contracture of hypertrophic scar. Plast. Reconstr. Surg. 2004, 113, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.J.; Spicer, A.P.; Mascarenhas, M.M.; Yu, L.; Ochoa, C.D.; Garg, H.G.; Quinn, D.A. The role of hyaluronan synthase 3 in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2005, 172, 92–98. [Google Scholar] [CrossRef]
- Halderman, A.A.; Bryson, P.C.; Benninger, M.S.; Chota, R. Safety and length of benefit of restylane for office-based injection medialization-a retrospective review of one institution’s experience. J. Voice 2014, 28, 631–635. [Google Scholar] [CrossRef]
- Kwon, T.K.; Lee, J.E.; Cha, W.J.; Song, C.M.; Sung, M.W.; Kim, K.H. Serial computed tomographic and positron emission tomographic properties of injection material used for vocal fold augmentation. Laryngoscope 2013, 123, 2491–2496. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, H.; Park, C.; Choi, S.; Kim, E.; Kim, S.W.; Kim, C.-S.; Koo, H. In vivo vocal fold augmentation using an injectable polyethylene glycol hydrogel based on click chemistry. Biomater. Sci. 2021, 9, 108–115. [Google Scholar] [CrossRef]
- Chen, H.; Erndt-Marino, J.; Diaz-Rodriguez, P.; Kulwatno, J.; Jimenez-Vergara, A.C.; Thibeault, S.L.; Hahn, M.S. In vitro evaluation of anti-fibrotic effects of select cytokines for vocal fold scar treatment. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1056–1067. [Google Scholar] [CrossRef]
- Dias Garcia, R.I.; Tsuji, D.H.; Imamura, R.; Mauad, T.; Ferraz da Silva, L.F. Effects of hepatocyte growth factor injection and reinjection on healing in the rabbit vocal fold. J. Voice 2012, 26, 667.e7–667.e12. [Google Scholar] [CrossRef]
- Xu, H.; Fan, G.K. The Role of Cytokines in Modulating Vocal Fold Fibrosis: A Contemporary Review. Laryngoscope 2021, 131, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Hirano, S.; Kishimoto, Y.; Hiwatashi, N.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J. Pharmacokinetics and safety of human recombinant hepatocyte growth factor administered to vocal folds. Laryngoscope 2014, 124, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, S.; Kim, D.Y.; Kim, Y.M.; Kim, M.S.; Lim, J.Y. Functional remodeling after vocal fold injury by small intestinal submucosa gel containing hepatocyte growth factor. Biomaterials 2015, 40, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Ahn, H.J.; Park, M.R.; Han, M.J.; Lee, J.H.; Kwon, S.K. Dual growth factor-immobilized bioactive injection material for enhanced treatment of glottal insufficiency. Acta Biomater. 2019, 86, 269–279. [Google Scholar] [CrossRef]
- Shi, L.L.; Giraldez-Rodriguez, L.A.; Johns, M.M., 3rd. The Risk of Vocal Fold Atrophy after Serial Corticosteroid Injections of the Vocal Fold. J. Voice 2016, 30, 762.e11–762.e13. [Google Scholar] [CrossRef]
- Klemuk, S.A.; Titze, I.R. Viscoelastic properties of three vocal-fold injectable biomaterials at low audio frequencies. Laryngoscope 2004, 114, 1597–1603. [Google Scholar] [CrossRef]
- Li, L.; Stiadle, J.M.; Lau, H.K.; Zerdoum, A.B.; Jia, X.; Thibeault, S.L.; Kiick, K.L. Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration. Biomaterials 2016, 108, 91–110. [Google Scholar] [CrossRef]
- Xie, X.; Li, X.; Lin, X.; Chen, X.; Zhang, C.; Sun, G. Effects of hepatocyte growth factor-transfected mesenchymal stem cell transplantation in canine injured vocal folds. Growth Factors 2023, 41, 130–139. [Google Scholar] [CrossRef]
- Fang, Y.; Gong, X.; Xu, M.; Zeng, F.; Zhang, J. A self-deletion lentiviral vector to reduce the risk of replication-competent virus formation. J. Gene Med. 2013, 15, 102–112. [Google Scholar] [CrossRef]
- Schambach, A.; Zychlinski, D.; Ehrnstroem, B.; Baum, C. Biosafety features of lentiviral vectors. Hum. Gene Ther. 2013, 24, 132–142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).