The Comparative Effects of Anakinra and Tocilizumab on Inflammation and Cerebral Vasospasm in an Experimental Subarachnoid Hemorrhage Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Experimental Protocol
2.3. Biomarker Analysis
2.4. Histopathologic Evaluation
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vivancos, J.; Gilo, F.; Frutos, R.; Maestre, J.; García-Pastor, A.; Quintana, F.; Roda, J.M.; Ximénez-Carrillo, A.; Díez Tejedor, E.; Fuentes, B.; et al. Clinical management guidelines for subarachnoid haemorrhage. Diagnosis and treatment. Neurologia 2014, 29, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Foudhaili, A.; Leclere, B.; Martinache, F.; Chauvin, A.; Vitiello, D.; Chousterman, B. Early mobilization in patients with aneurysmal subarachnoid haemorrhage may im-prove functional status and reduce cerebral vasospasm rate: A systematic review with meta-analysis. J. Rehabil. Med. 2024, 56, jrm41225. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Lan, J.X.; Si, Y.F.; Ren, Y.F.; Li, M.Y.; Guo, F.F.; Tang, G.; Bian, Y.; Wang, X.H.; Zhang, R.J.; et al. Epidemiological trends of subarachnoid hemorrhage at global, regional, and national level: A trend analysis study from 1990 to 2021. Mil. Med. Res. 2024, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Aladawi, M.; Elfil, M.; Ghozy, S.; Najdawi, Z.R.; Ghaith, H.; Alzayadneh, M.; Rabinstein, A.A.; Hawkes, M.A. The impact of systolic blood pressure reduction on aneurysm re-bleeding in subarachnoid hemorrhage: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2024, 33, 108084. [Google Scholar] [CrossRef]
- Xiao, Z.K.; Wang, B.; Liu, J.; Yang, Y.; Jie, N.; Mao, X.; Gong, X.; Liu, A.; Duan, Y.H. Risk factors for the development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. World Neurosurg. 2024, in press. [Google Scholar] [CrossRef]
- Al-Salihi, M.M.; Gillani, S.A.; Saha, R.; Abd Elazim, A.; Al-Jebur, M.S.; Al-Salihi, Y.; Ayyad, A.; Nattanmai, P.; Siddiq, F.; Gomez, C.R.; et al. Clinical Characteristics as Predictors of Early and Delayed Cerebral Infarction in Aneurysmal Subarachnoid Hemorrhage Patients: A Meta-Analysis of 4527 Cases. World Neurosurg. 2024, 189, 373–380.e373. [Google Scholar] [CrossRef]
- Pluta, R.M.; Hansen-Schwartz, J.; Dreier, J.; Vajkoczy, P.; Macdonald, R.L.; Nishizawa, S.; Kasuya, H.; Wellman, G.; Keller, E.; Zauner, A.; et al. Cerebral vasospasm following subarachnoid hemorrhage: Time for a new world of thought. Neurol. Res. 2009, 31, 151–158. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Logsdon, A.F.; Manoranjan, B.; Turner, R.C.; McConnell, E.; Vates, G.E.; Huber, J.D.; Rosen, C.L.; Simard, J.M. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int. J. Mol. Sci. 2016, 17, 497. [Google Scholar] [CrossRef]
- Qi, X.; Chiavaroli, L.; Lee, D.; Ayoub-Charette, S.; Khan, T.A.; Au-Yeung, F.; Ahmed, A.; Cheung, A.; Liu, Q.; Blanco Mejia, S.; et al. Effect of Important Food Sources of Fructose-Containing Sugars on Inflammatory Biomarkers: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. Nutrients 2022, 14, 3986. [Google Scholar] [CrossRef]
- Li, S.; Yu, C.; Xiao, H.; Xu, Q.; Gao, B.; Guo, L.; Sun, Z.; Liu, J. NOSTRIN is involved in benign prostatic hyperplasia via inhibition of proliferation, oxidative stress, and inflammation in prostate epithelial cells. Transl. Androl. Urol. 2024, 13, 2055–2069. [Google Scholar] [CrossRef]
- Konsman, J.P. Cytokines in the Brain and Neuroinflammation: We Didn’t Starve the Fire! Pharmaceuticals 2022, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, Z.; Li, G.; Zhou, L.; Huang, K.; Wu, Z.; Zhan, R.; Shen, J. Inflammation and Oxidative Stress: Potential Targets for Improving Prognosis After Subarachnoid Hemorrhage. Front. Cell Neurosci. 2021, 15, 739506. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.M.; Sivanrupan, S.; Wanderer, S.; Agnoletto, G.J.; Chiappini, A.; Grüter, B.E.; Andereggen, L.; Mariani, L.; Taussky, P.; Marbacher, S. Preclinical and clinical role of interleukin-6 in the development of delayed cerebral vasospasm and neuronal cell death after subarachnoid hemorrhage: Towards a potential target therapy? Neurosurg. Rev. 2022, 45, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.M.; Wanderer, S.; Strange, F.; Grüter, B.E.; Sivanrupan, S.; Andereggen, L.; Casoni, D.; von Gunten, M.; Widmer, H.R.; Di Santo, S.; et al. Tocilizumab Reduces Vasospasms, Neuronal Cell Death, and Microclot Formation in a Rabbit Model of Subarachnoid Hemorrhage. Transl. Stroke Res. 2021, 12, 894–904. [Google Scholar] [CrossRef]
- Croci, D.; Nevzati, E.; Muroi, C.; Schöpf, S.; Hornemann, T.; Widmer, H.R.; Danura, H.; Fandino, J.; Marbacher, S. Changes in the cerebrospinal fluid lipid profile following subarachnoid hemorrhage in a closed cranium model: Correlations to cerebral vasospasm, neuronal cell death and Interleukin-6 synthesis. A pilot study. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 105054. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Anakinra Therapy for Non-cancer Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1157. [Google Scholar] [CrossRef]
- Charlesworth, J.E.G.; Kavirayani, A. Intravenous anakinra for the treatment of haemophagocytic lymphohistiocytosis/macrophage activation syndrome: A systematic review. Eur. J. Haematol. 2023, 111, 458–476. [Google Scholar] [CrossRef]
- Lokau, J.; Kleinegger, F.; Garbers, Y.; Waetzig, G.H.; Grötzinger, J.; Rose-John, S.; Haybaeck, J.; Garbers, C. Tocilizumab does not block interleukin-6 (IL-6) signaling in murine cells. PLoS ONE 2020, 15, e0232612. [Google Scholar] [CrossRef]
- Antonio, A.A.; Santos, R.N.; Abariga, S.A. Tocilizumab for giant cell arteritis. Cochrane Database Syst. Rev. 2022, 5, Cd013484. [Google Scholar] [CrossRef]
- Ozkan, F.; Sari, S. Comparison of Anakinra and Tocilizumab in Anticytokine Therapy in the Treatment of Coronavirus Disease-2019. Indian. J. Crit. Care Med. 2022, 26, 1091–1098. [Google Scholar] [CrossRef]
- Aledo-Serrano, A.; Hariramani, R.; Gonzalez-Martinez, A.; Álvarez-Troncoso, J.; Toledano, R.; Bayat, A.; Garcia-Morales, I.; Becerra, J.L.; Villegas-Martínez, I.; Beltran-Corbellini, A.; et al. Anakinra and tocilizumab in the chronic phase of febrile infection-related epilepsy syndrome (FIRES): Effectiveness and safety from a case-series. Seizure 2022, 100, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Bicer, S.; Suleyman, B.; Mammadov, R.; Yavuzer, B.; Cicek, B.; Altuner, D.; Coban, T.A.; Suleyman, H. Effect of anakinra, tocilizumab, and the combination thereof on bladder ischemia-reperfusion damage in albino Wistar-type rats. Investig. Clínica 2023, 64, 368–378. [Google Scholar] [CrossRef]
- Miller, B.A.; Turan, N.; Chau, M.; Pradilla, G. Inflammation, vasospasm, and brain injury after subarachnoid hemorrhage. Biomed. Res. Int. 2014, 2014, 384342. [Google Scholar] [CrossRef] [PubMed]
- Akturk, U.D.; Tuncer, C.; Bozkurt, H.; Sahin, O.S.; Bulut, H.; Arikok, A.; Dinc, C.; Gurer, B.; Turkoglu, E. Blocking VEGF by Bevacizumab Attenuates VEGF-Induced Vasospasm After Experimental Subarachnoid Hemorrhage in Rabbits. World Neurosurg. 2020, 139, e136–e143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Geng, G.; Zhu, T.; Chen, W.; Li, X.; Gu, J.; Jiang, E. Progress in Research on TLR4-Mediated Inflammatory Response Mechanisms in Brain Injury after Subarachnoid Hemorrhage. Cells 2022, 11, 3781. [Google Scholar] [CrossRef]
- Millán Solano, M.V.; Salinas Lara, C.; Sánchez-Garibay, C.; Soto-Rojas, L.O.; Escobedo-Ávila, I.; Tena-Suck, M.L.; Ortíz-Butrón, R.; Choreño-Parra, J.A.; Romero-López, J.P.; Meléndez Camargo, M.E. Effect of Systemic Inflammation in the CNS: A Silent History of Neuronal Damage. Int. J. Mol. Sci. 2023, 24, 11902. [Google Scholar] [CrossRef]
- Romoli, M.; Giammello, F.; Mosconi, M.G.; De Mase, A.; De Marco, G.; Digiovanni, A.; Ciacciarelli, A.; Ornello, R.; Storti, B. Immunological Profile of Vasospasm after Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2023, 24, 8856. [Google Scholar] [CrossRef]
- Ahn, S.H.; Savarraj, J.P.J.; Parsha, K.; Hergenroeder, G.W.; Chang, T.R.; Kim, D.H.; Kitagawa, R.S.; Blackburn, S.L.; Choi, H.A. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J. Neuroinflammation 2019, 16, 213. [Google Scholar] [CrossRef]
- Ku, E.J.; Kim, B.R.; Lee, J.I.; Lee, Y.K.; Oh, T.J.; Jang, H.C.; Choi, S.H. The Anti-Atherosclerosis Effect of Anakinra, a Recombinant Human Interleukin-1 Receptor Antagonist, in Apolipoprotein E Knockout Mice. Int. J. Mol. Sci. 2022, 23, 4906. [Google Scholar] [CrossRef]
- Singh, N.; Hopkins, S.J.; Hulme, S.; Galea, J.P.; Hoadley, M.; Vail, A.; Hutchinson, P.J.; Grainger, S.; Rothwell, N.J.; King, A.T.; et al. The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: A phase II randomised controlled trial. J. Neuroinflammation 2014, 11, 1. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; Brough, D.; Robinson, E.M.; Girard, S.; Rothwell, N.J.; Allan, S.M. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis. Models Mech. 2012, 5, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.; Rasool, H.F.; Javed, I.; Raza, S.; Abang, L.; Hashim, M.M.A.; Saleem, Z.; Abdullah, R.M.; Faraz, M.A.; Hassan, K.M.; et al. Comparative Roles of IL-1, IL-6, IL-10, IL-17, IL-18, 1L-22, IL-33, and IL-37 in Various Cardiovascular Diseases With Potential Insights for Targeted Immunotherapy. Cureus 2023, 15, e42494. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Rigoard, P.; Billot, M.; Moens, M.; Goudman, L.; El-Hajj, H.; Ingrand, P.; Ounajim, A.; Roulaud, M.; Page, P.; Babin, E.; et al. Evaluation of External Trigeminal Nerve Stimulation to Prevent Cerebral Vasospasm after Subarachnoid Hemorrhage Due to Aneurysmal Rupture: A Randomized, Double-Blind Proof-of-Concept Pilot Trial (TRIVASOSTIM Study). Int. J. Environ. Res. Public Health 2023, 20, 5836. [Google Scholar] [CrossRef]
- Siasios, I.; Kapsalaki, E.Z.; Fountas, K.N. Cerebral vasospasm pharmacological treatment: An update. Neurol. Res. Int. 2013, 2013, 571328. [Google Scholar] [CrossRef]
- Lucke-Wold, B.; Dodd, W.; Motwani, K.; Hosaka, K.; Laurent, D.; Martinez, M.; Dugan, V.; Chalouhi, N.; Lucke-Wold, N.; Barpujari, A.; et al. Investigation and modulation of interleukin-6 following subarachnoid hemorrhage: Targeting inflammatory activation for cerebral vasospasm. J. Neuroinflammation 2022, 19, 228. [Google Scholar] [CrossRef]
- Bonaventura, A.; Liberale, L.; Vecchié, A.; Casula, M.; Carbone, F.; Dallegri, F.; Montecucco, F. Update on Inflammatory Biomarkers and Treatments in Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 1967. [Google Scholar] [CrossRef]
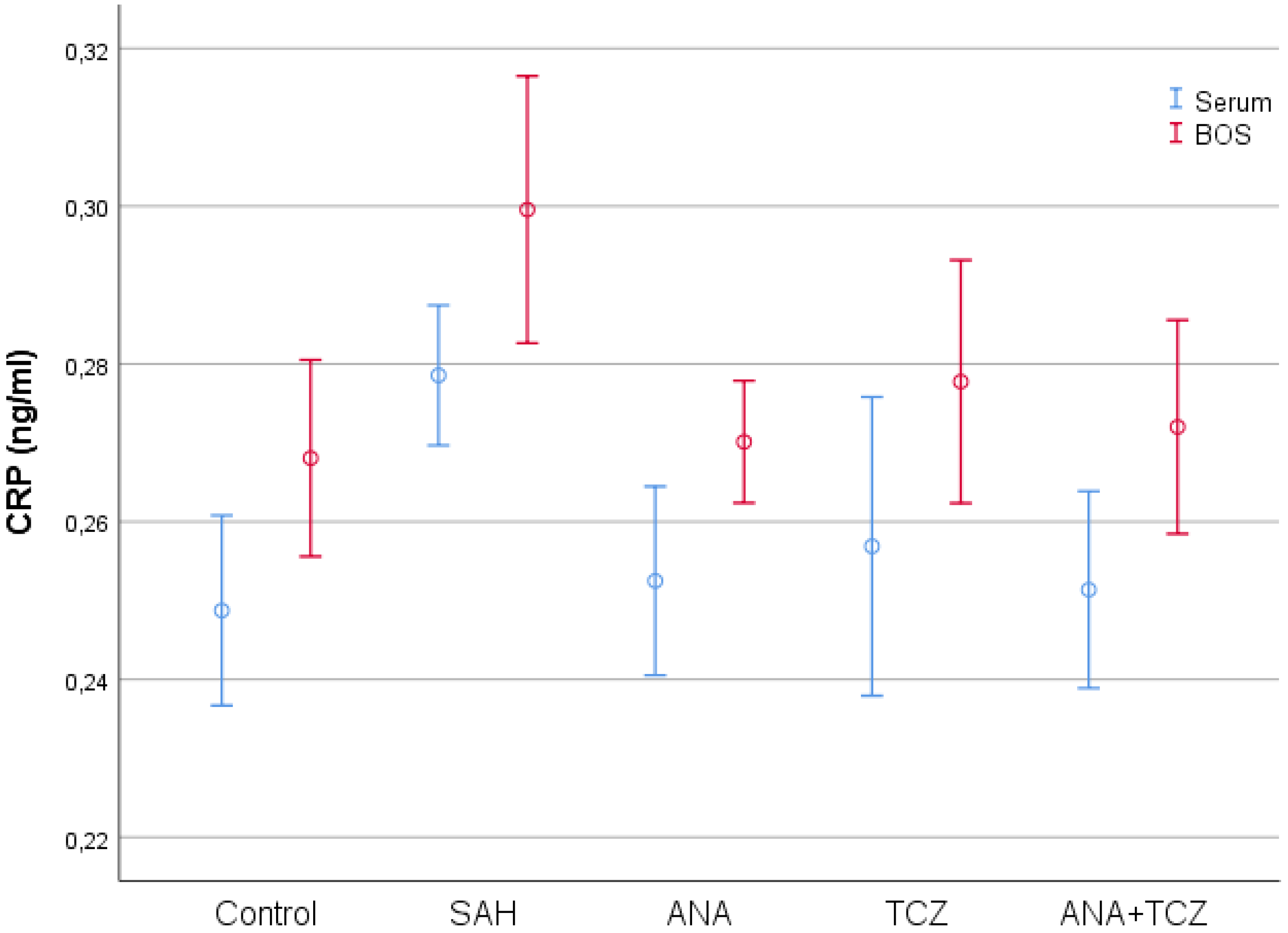
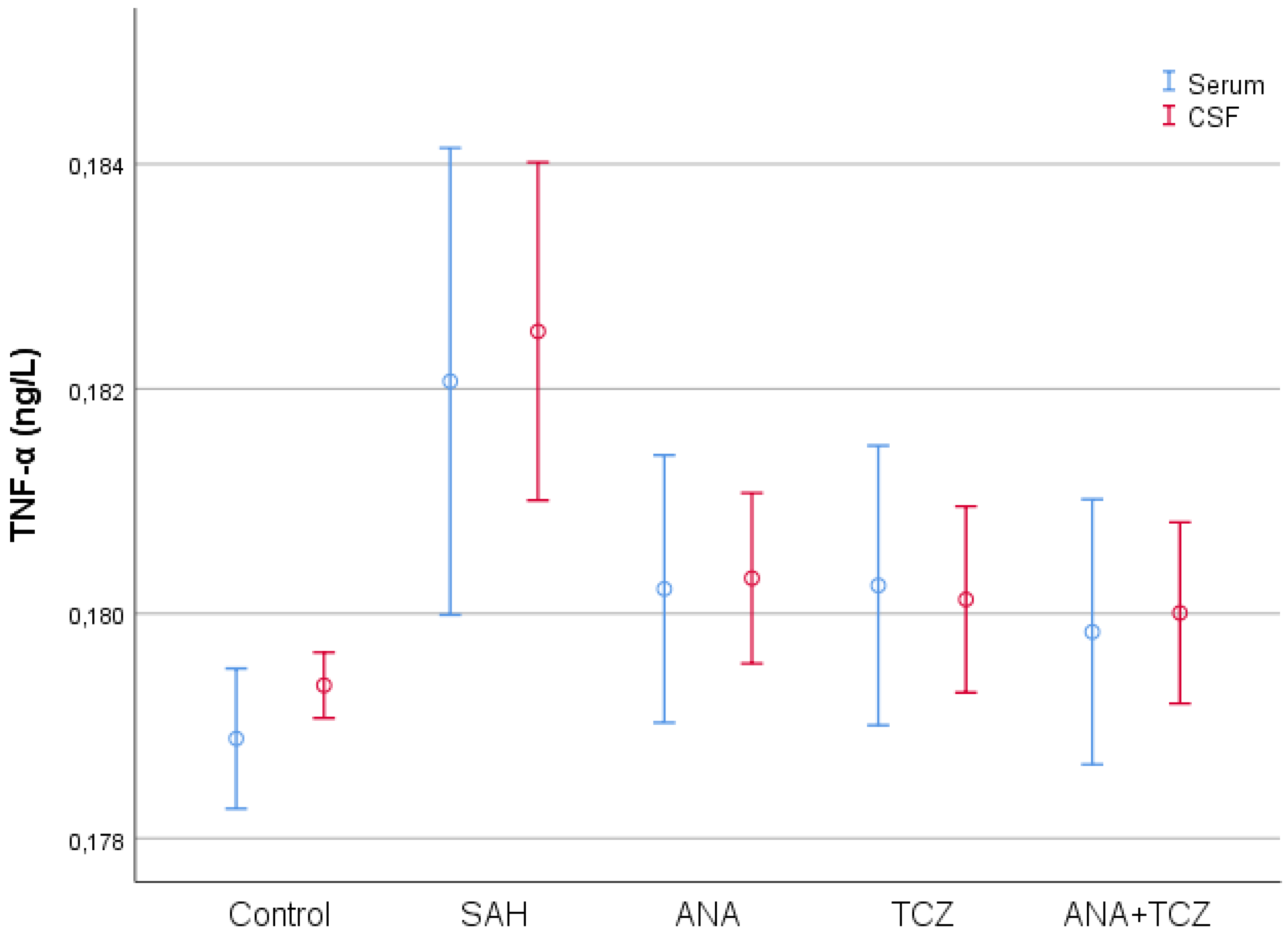
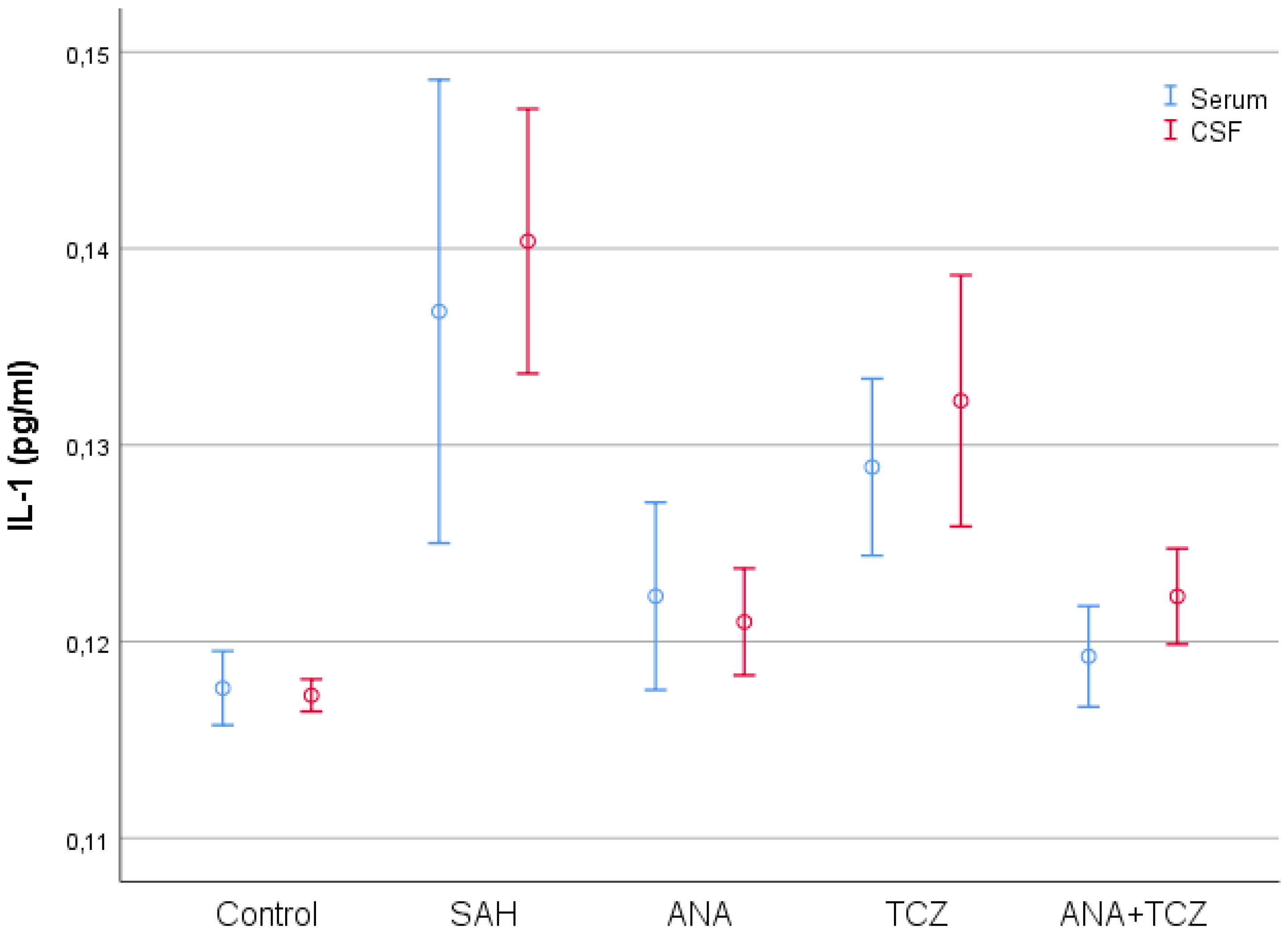
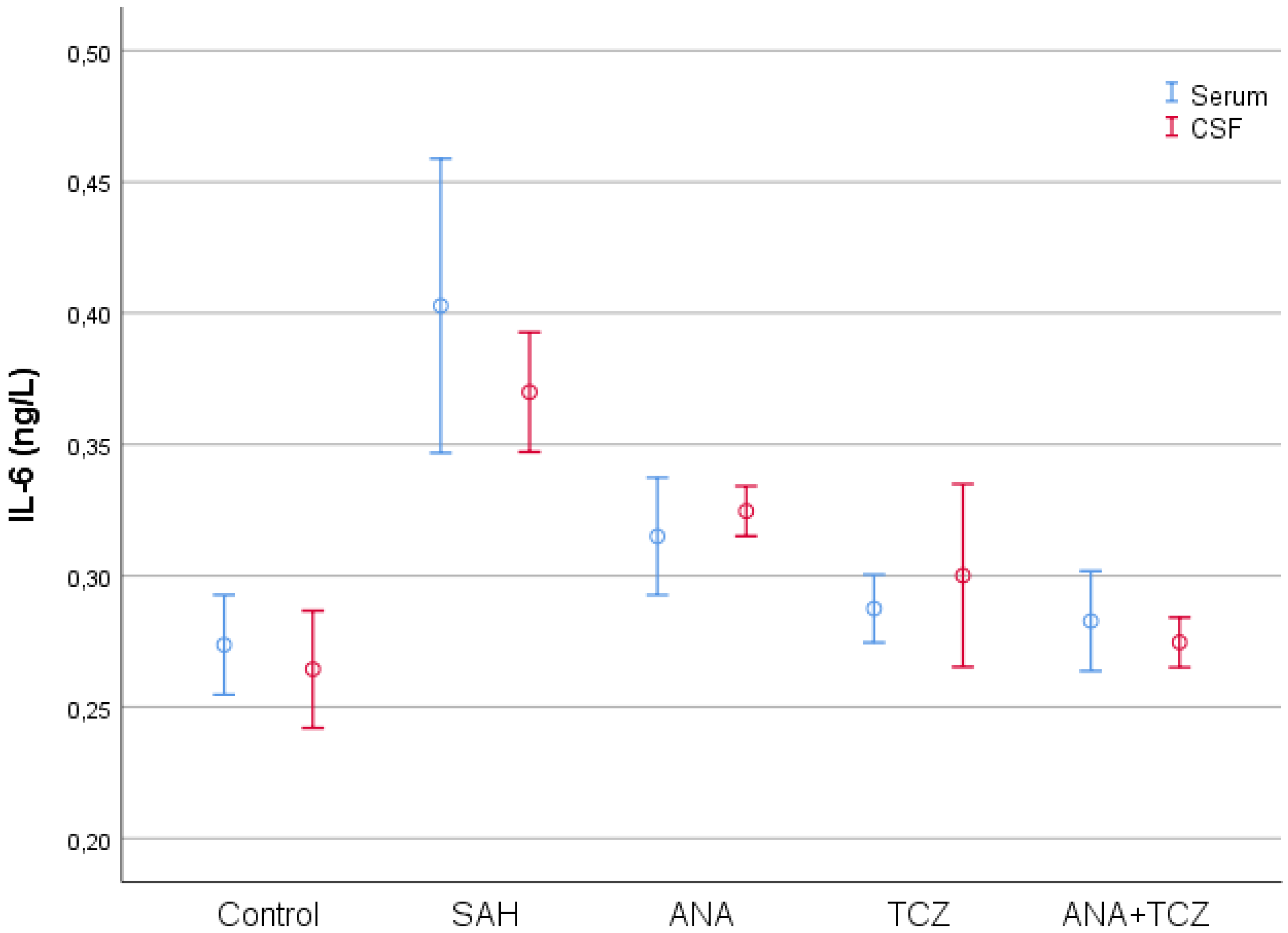
| Control (n = 8) | SAH (n = 8) | ANA (n = 8) | TCZ (n = 8) | ANA+TCZ (n = 8) | p | |
|---|---|---|---|---|---|---|
| Serum CRP (ng/mL) | 0.2487 ± 0.0144 a | 0.2786 ± 0.0106 b | 0.2525 ± 0.0143 a | 0.2569 ± 0.0226 ab | 0.2514 ± 0.0149 a | 0.004 |
| Serum TNF-α (ng/L) | 0.1789 ± 0.0007 a | 0.1821 ± 0.0025 b | 0.1802 ± 0.0014 ab | 0.1803 ± 0.0015 ab | 0.1798 ± 0.0014 ab | 0.008 |
| Serum IL-1 (pg/mL) | 0.1176 ± 0.0023 a | 0.1368 ± 0.0141 c | 0.1223 ± 0.0057 ab | 0.1289 ± 0.0054 bc | 0.1192 ± 0.0031 ab | <0.001 |
| Serum IL-6 (ng/L) | 0.2737 ± 0.0227 a | 0.4028 ± 0.0671 b | 0.3150 ± 0.0268 a | 0.2875 ± 0.0155 a | 0.2828 ± 0.0228 a | <0.001 |
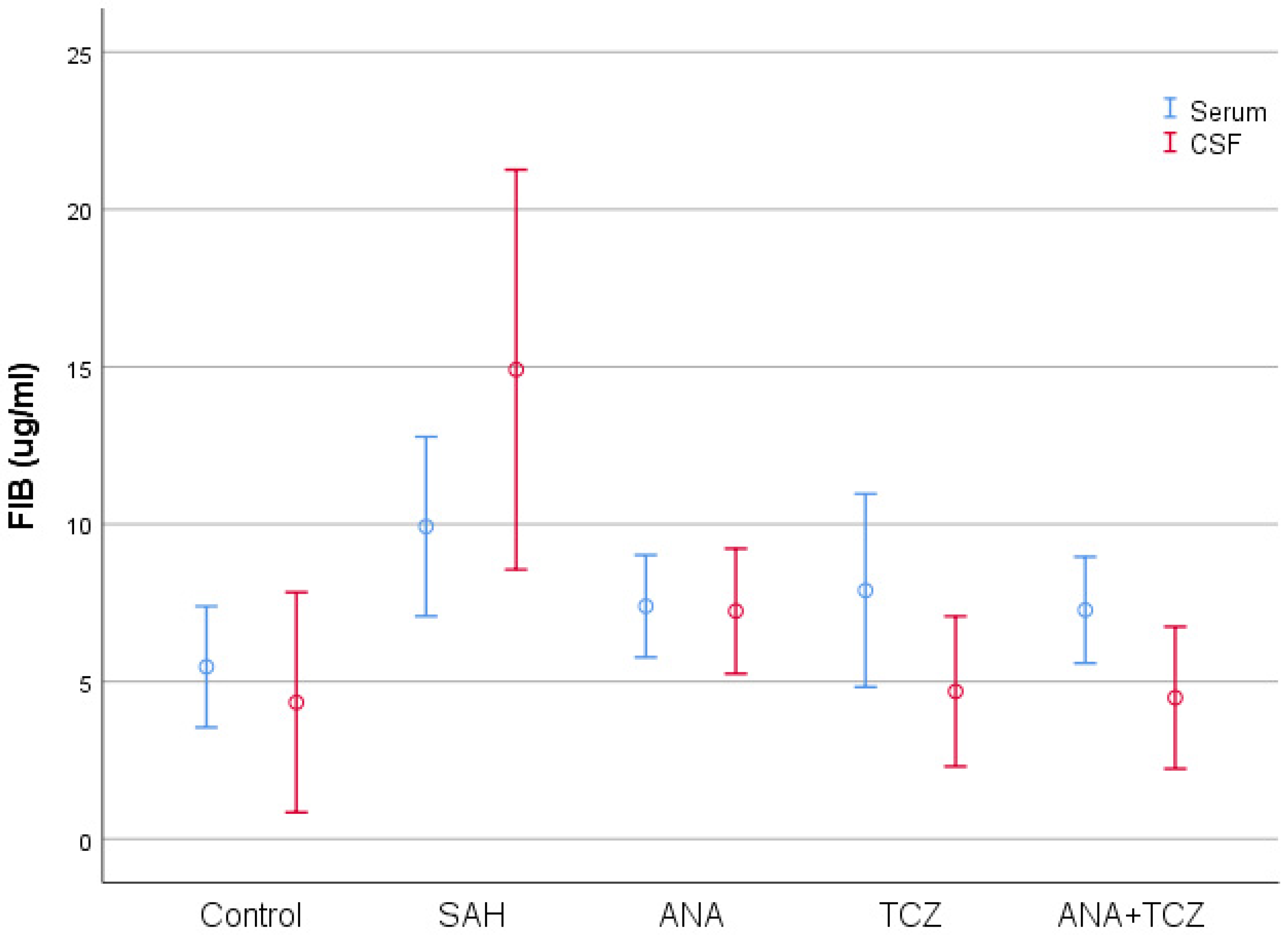
| Serum FIB (µg/mL) | 5.4680 ± 2.3009 a | 9.9280 ± 3.4066 b | 7.3954 ± 1.9423 ab | 7.8955 ± 3.6719 ab | 7.2732 ± 2.0222 ab | 0.048 |
| CSF CRP (ng/mL) | 0.2681 ± 0.0149 a | 0.2996 ± 0.0203 b | 0.2701 ± 0.0093 a | 0.2777 ± 0.0184 ab | 0.2720 ± 0.0162 a | 0.003 |
| CSF TNF-α (ng/L) | 0.1794 ± 0.0003 a | 0.1825 ± 0.0018 b | 0.1803 ± 0.0009 a | 0.1801 ± 0.0010 a | 0.1800 ± 0.0010 a | <0.001 |
| CSF IL-1 (pg/mL) | 0.1173 ± 0.0010 a | 0.1404 ± 0.0081 c | 0.1210 ± 0.0033 a | 0.1323 ± 0.0076 b | 0.1223 ± 0.0029 a | <0.001 |
| CSF IL-6 (ng/L) | 0.2644 ± 0.0267 a | 0.3700 ± 0.0273 c | 0.3246 ± 0.0114 b | 0.3001 ± 0.0417 ab | 0.2746 ± 0.0114 a | <0.001 |
| CSF FIB (µg/mL) | 4.3375 ± 4.1829 a | 14.910 ± 7.5979 b | 7.2388 ± 2.3763 a | 4.6888 ± 2.8536 a | 4.4856 ± 2.6973 a | <0.001 |
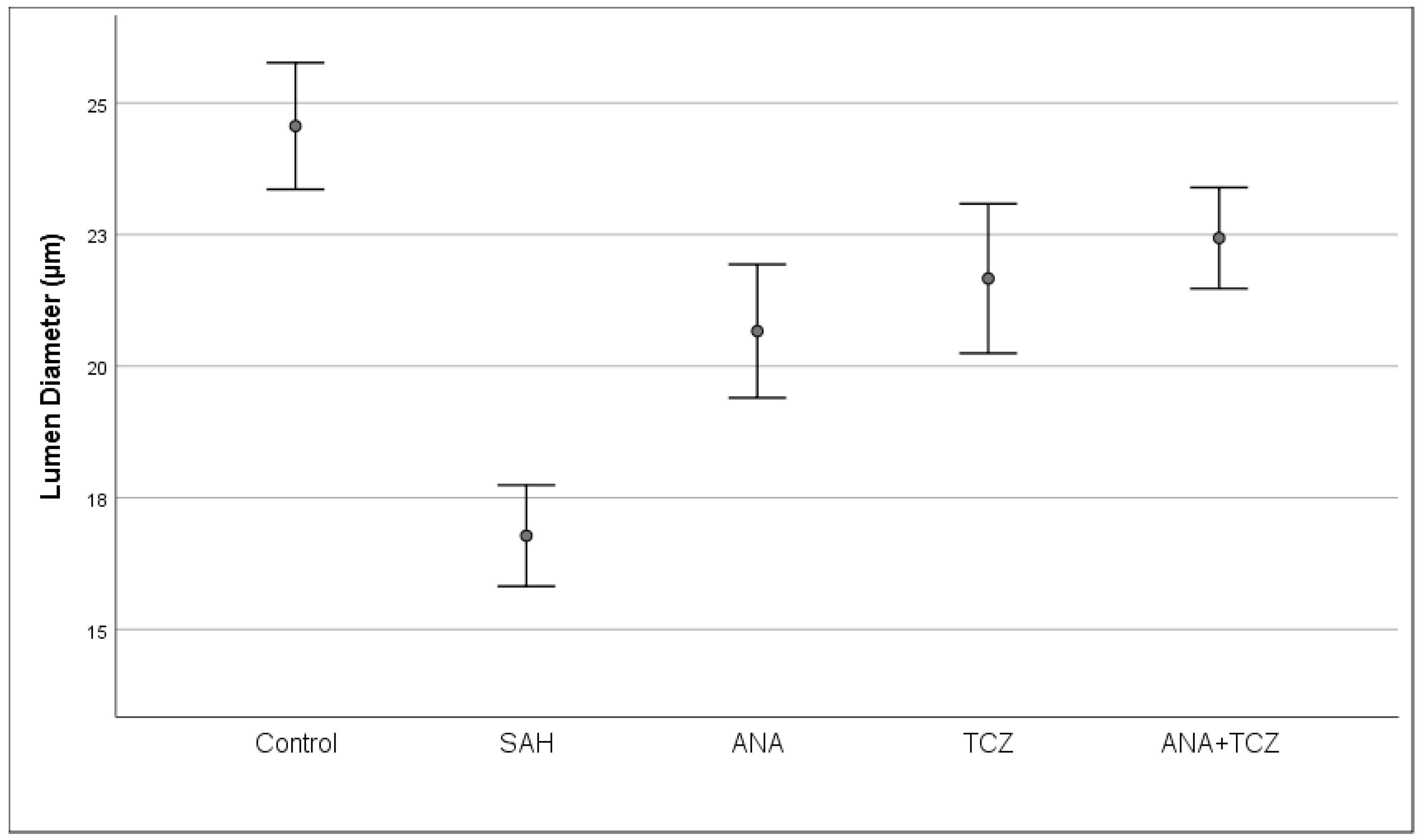
| Lumen diameter (µm) | 24.5610 ± 1.4389 a | 16.7790 ± 1.1489 c | 20.6656 ± 1.5151 b | 21.6643 ± 1.6980 b | 22.4331 ± 1.1484 b | <0.001 |
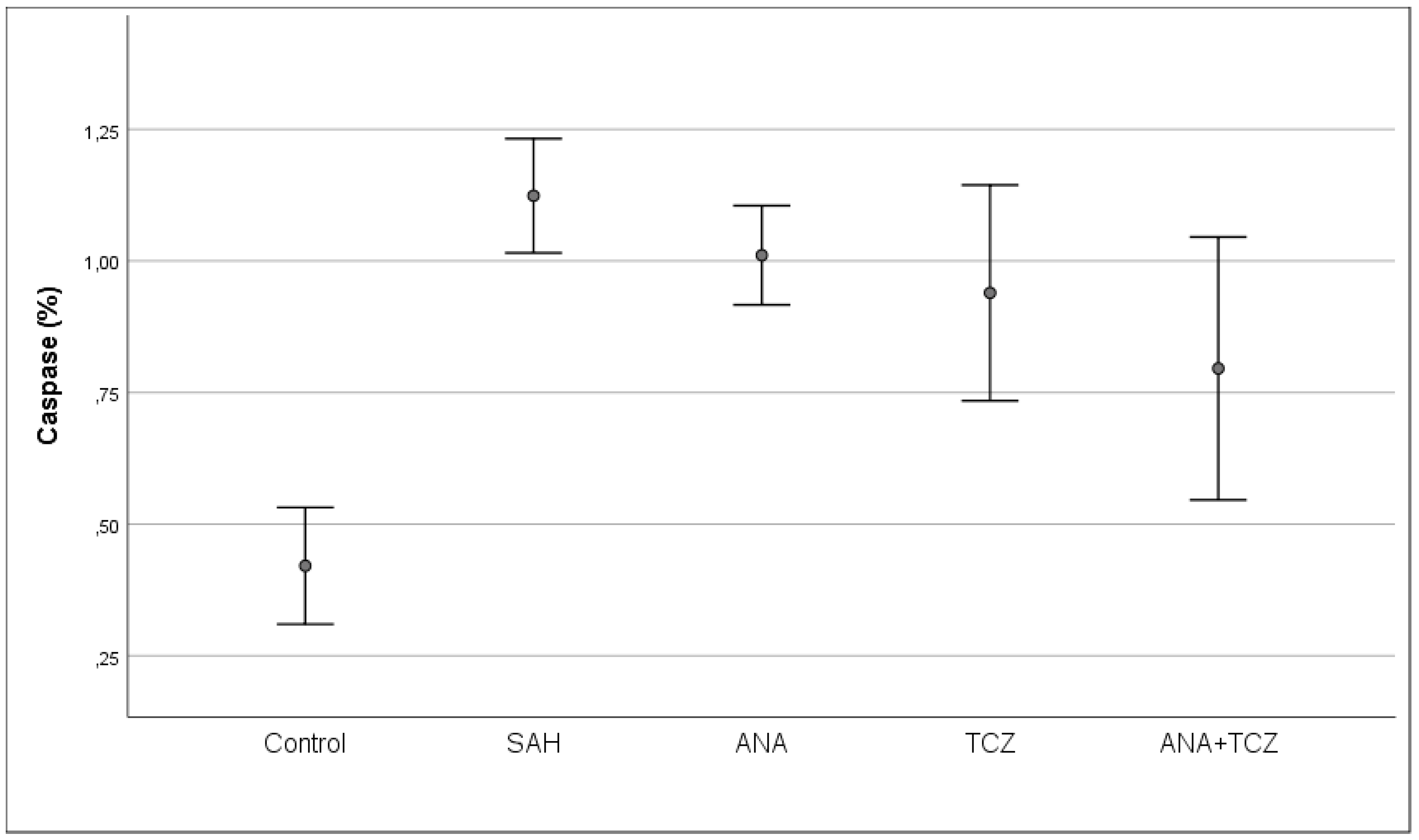
| Caspase (%) | 0.4209 ± 0.1326 a | 1.1238 ± 0.1174 c | 1.0108 ± 0.1126 bc | 0.9393 ± 0.2451 bc | 0.7958 ± 0.2986 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kılıç, G.; Engin, B.E.; Halabi, A.; Tuncer, C.; Sungur, M.A.; Alpay, M.; Kurtuluş, A.; Soylu, H.; Gök, A. The Comparative Effects of Anakinra and Tocilizumab on Inflammation and Cerebral Vasospasm in an Experimental Subarachnoid Hemorrhage Model. Medicina 2024, 60, 2025. https://doi.org/10.3390/medicina60122025
Kılıç G, Engin BE, Halabi A, Tuncer C, Sungur MA, Alpay M, Kurtuluş A, Soylu H, Gök A. The Comparative Effects of Anakinra and Tocilizumab on Inflammation and Cerebral Vasospasm in an Experimental Subarachnoid Hemorrhage Model. Medicina. 2024; 60(12):2025. https://doi.org/10.3390/medicina60122025
Chicago/Turabian StyleKılıç, Güven, Berk Enes Engin, Amir Halabi, Cengiz Tuncer, Mehmet Ali Sungur, Merve Alpay, Adem Kurtuluş, Hakan Soylu, and Ali Gök. 2024. "The Comparative Effects of Anakinra and Tocilizumab on Inflammation and Cerebral Vasospasm in an Experimental Subarachnoid Hemorrhage Model" Medicina 60, no. 12: 2025. https://doi.org/10.3390/medicina60122025
APA StyleKılıç, G., Engin, B. E., Halabi, A., Tuncer, C., Sungur, M. A., Alpay, M., Kurtuluş, A., Soylu, H., & Gök, A. (2024). The Comparative Effects of Anakinra and Tocilizumab on Inflammation and Cerebral Vasospasm in an Experimental Subarachnoid Hemorrhage Model. Medicina, 60(12), 2025. https://doi.org/10.3390/medicina60122025






