Vascularised and Non-Vascularised Adipofascial Flap Applications in Tissue Trauma with Tendon Injury, Flap Viability and Tendon Healing a Hystological and Scintigraphical Rat Model Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
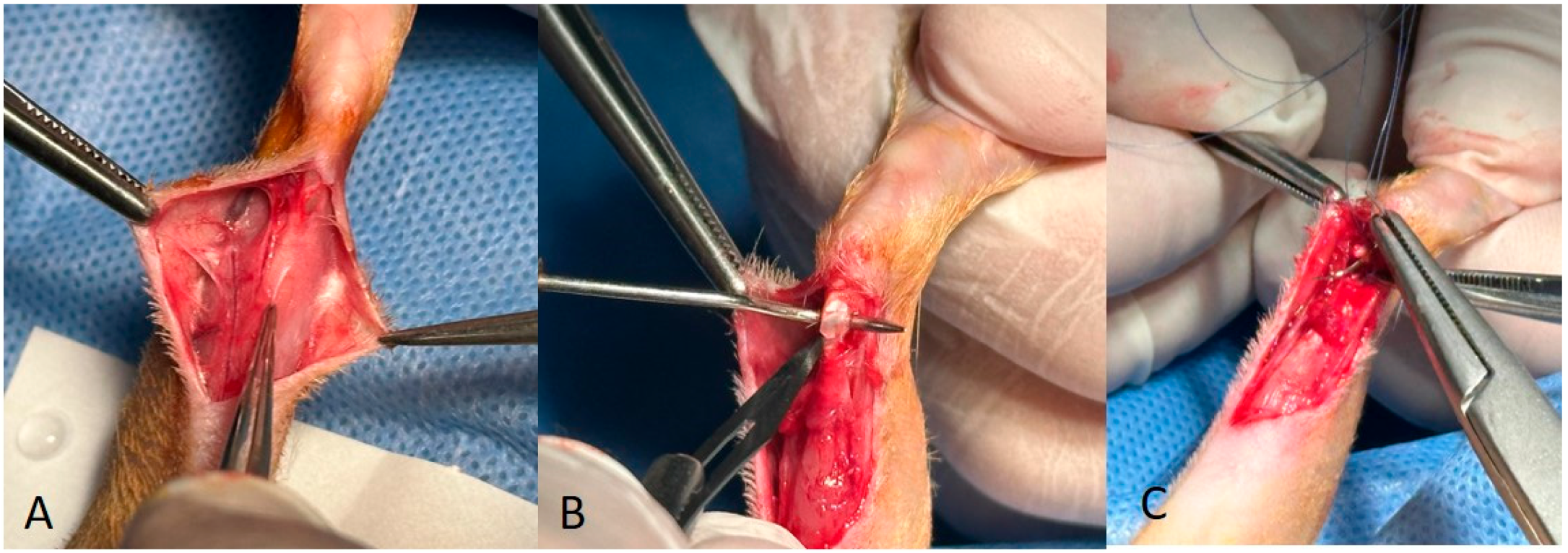
2.2. Surgical Protocol
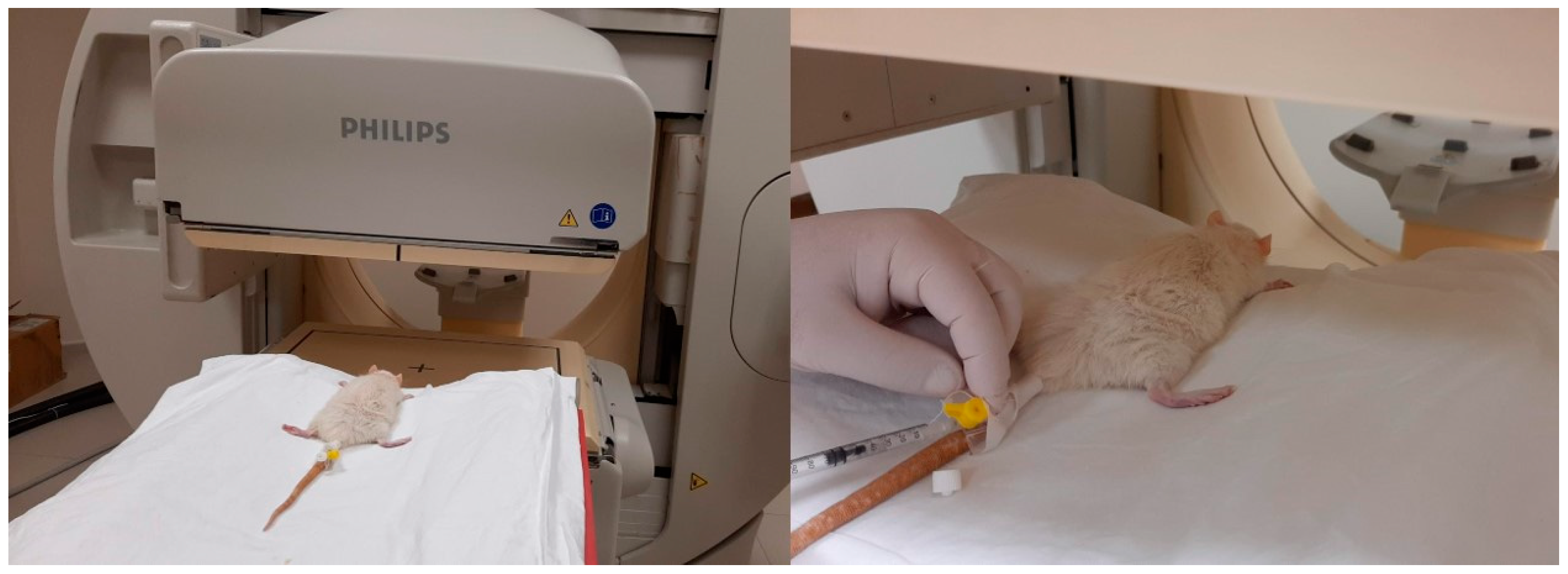
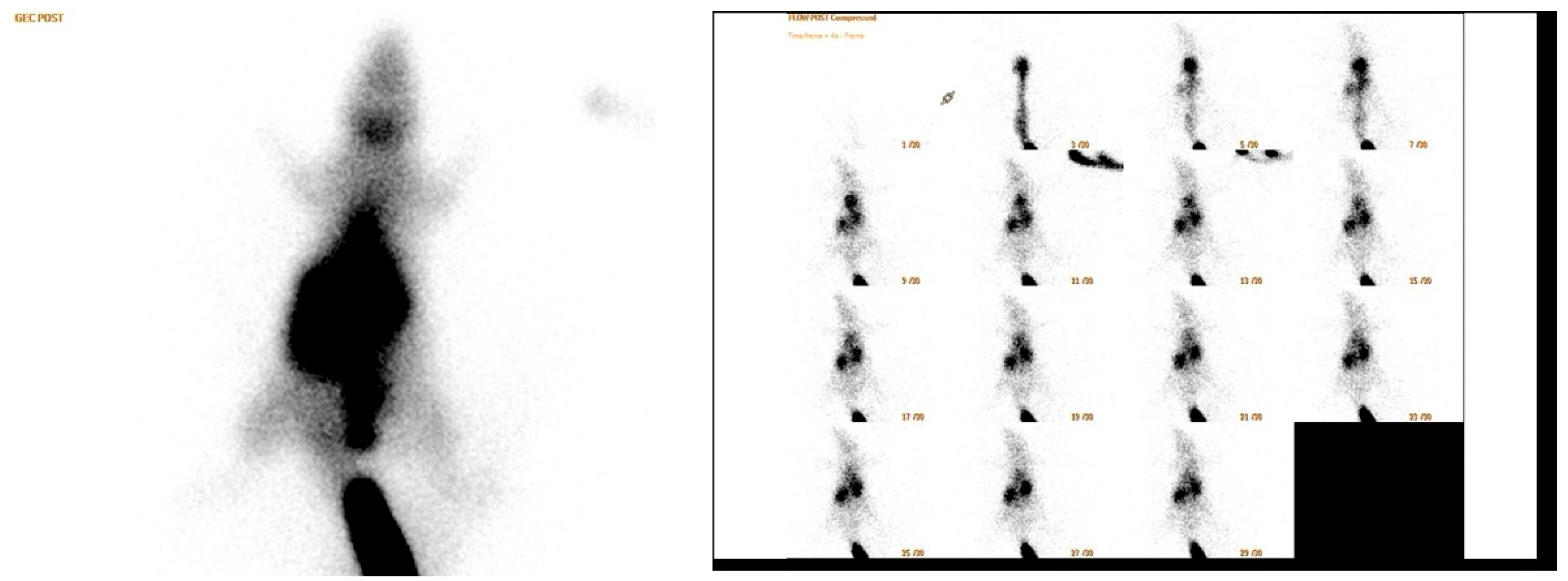
2.3. Scintigraphy Test
- Perfusion reserve = (early static count on right ROI − late static count on right ROI)/early static count on right ROI;
- R/L index = late ROI right/late ROI left.
- Early count percentage = early static count on ROI right/early static count on ROI whole body
- Late count percentage = late static count at right ROI/late static count at total body ROI
2.4. Histopathological Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Chang, S.M. Distally based sural adipofascial turnover flap for coverage of complicated wound in the foot and ankle region. Ann. Plast. Surg. 2020, 84, 580–587. [Google Scholar] [CrossRef] [PubMed]
- de Santos, A.L.; da Silva, C.G. Biomechanical Evaluation of Tendon Regeneration with Adipose-derived Stem Cell. J. Orthop. Res. 2019, 37, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.A.; Evans, C.H. The role of the paratenon in Achilles tendon healing: A study in rats. Am. J. Sports Med. 2018, 46, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Battiston, B. Efficiency of Hyaloglide® in the prevention of the recurrence of adhesions after tenolysis of flexor tendons in zone II: A randomized, controlled, multicentre clinical trial. J. Hand Surg. (Eur. Vol.) 2010, 35, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lucchina, S.; Maggiulli, F. Can an adipofascial flap be used to prevent adhesions after plating of the proximal phalanx? A case report. Chir. Main 2015, 34, 86–90. [Google Scholar] [CrossRef]
- Hirase, Y.; Kojima, T. Double-layered free temporal fascia flap as a two-layered tendon-gliding surface. Plast. Reconstr. Surg. 1991, 88, 707–712. [Google Scholar] [CrossRef]
- del Piñal, F.; Moraleda, E. Outcomes of free adipofascial flaps combined with tenolysis in scarred beds. J. Hand Surg. 2014, 39, 269–279. [Google Scholar] [CrossRef]
- Roulet, S.; De Luca, L. The medial adipofascial flap for infected tibia fractures reconstruction: 10 years of experience with 59 cases. Ann. Chir. Plast. Esthétique 2021, 66, 234–241. [Google Scholar] [CrossRef]
- Chung, K.C.; Cederna, P.S. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J. Hand Surg. 2002, 27, 525–533. [Google Scholar] [CrossRef]
- Acartürk, T.O.; Tunc, S. Versatility of the perforator-based adipose, adipofascial, and fasciocutaneous flaps in reconstruction of distal leg and foot defects. J. Foot Ankle Surg. 2016, 55, 362–367. [Google Scholar] [CrossRef]
- Adams, S.B., Jr.; Thorpe, M.A. Stem cell-bearing suture improves Achilles tendon healing in a rat model. Foot Ankle Int. 2014, 35, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, S.; Adanır, O. Effect of platelet-rich plasma for treatment of Achilles tendons in free-moving rats after surgical incision and treatment. Acta Orthop. Traumatol. Turc. 2015, 49, 544–551. [Google Scholar]
- Kilkenny, C.; Browne, W.J. The ARRIVE Guidelines Checklist AnimalResearch:Reporting In Vivo Experiments. Br. J. Pharmacol. 2010, 8, 8–9. [Google Scholar]
- Koc, M.; Yucens, M. Do adipofascial flaps affect the mechanical properties of a repaired tendon? A biomechanical rat model study. Hand Surg. Rehabil. 2019, 38, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Oztermeli, A.; Okyay, M.F. An immunohistochemical and biomechanical evaluation of the effect of fat graft on tendon healing. J. Orthop. Surg. 2023, 31, 10225536231220839. [Google Scholar] [CrossRef]
- Gir, P.; Cheng, A. Pedicled-perforator (propeller) flaps in lower extremity defects: A systematic review. J. Reconstr. Microsurg. 2012, 28, 595–602. [Google Scholar] [CrossRef]
- Goil, P.; Sharma, A.K. Comparison of the outcomes of adipofascial and two–staged fasciocutaneous reverse sural flap in patients with lower leg trauma. J. Clin. Orthop. Trauma 2021, 14, 113–120. [Google Scholar] [CrossRef]
- Schmidt, K.; Jakubietz, M. The distally based adipofascial sural artery flap: Faster, safer, and easier? A long-term comparison of the fasciocutaneous and adipofascial method in a multimorbid patient population. Plast. Reconstr. Surg. 2012, 130, 360–368. [Google Scholar] [CrossRef]
- Losco, L.; Lo Torto, F. Modified single pedicle reverse adipofascial flap for fingertip reconstruction. Microsurgery 2019, 39, 221–227. [Google Scholar] [CrossRef]
- Laoulakos, D.H.; Tsetsonis, C.H. The dorsal reverse adipofascial flap for fingertip reconstruction. Plast. Reconstr. Surg. 2003, 112, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Idone, F.; Sisti, A. Fenestrated adipofascial reverse flap: A modified technique for the reconstruction of fingertip amputations. J. Investig. Surg. 2017, 30, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Facchin, F.; Sonda, R. Multi-dorsal metacarpal artery perforator adipofascial turnover flap for index to little finger reconstruction: Anatomical study and clinical application. Hand Surg. Rehabil. 2021, 40, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Beanlands, R.S.; Dawood, F. Are the kinetics of technetium-99m methoxyisobutyl isonitrile affected by cell metabolism and viability? Circulation 1990, 82, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Kato, T. Measurement of technetium-99m sestamibi signals in rats administered a mitochondrial uncoupler and in a rat model of heart failure. PLoS ONE 2015, 10, e0117091. [Google Scholar] [CrossRef] [PubMed]
- Crane, P.; Laliberté, R. Effect of mitochondrial viability and metabolism on technetium-99m-sestamibi myocardial retention. Eur. J. Nucl. Med. 1993, 20, 20–25. [Google Scholar] [CrossRef]


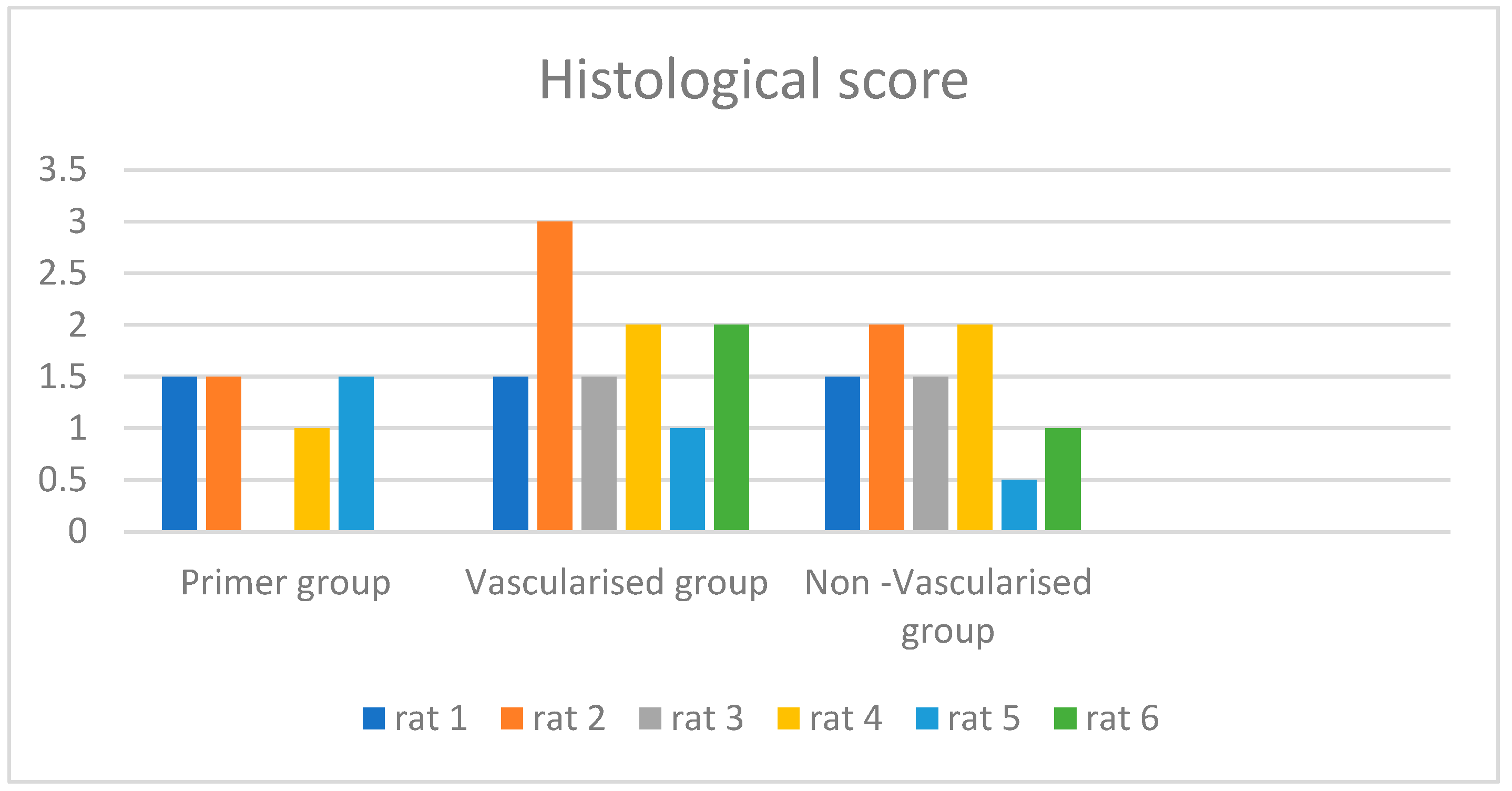
| Rat | Histological Score | Late R/L İndex | Perfusion Reserve | Static Late Discussion |
|---|---|---|---|---|
| Primer group rat 1 | 1.5 | 1.77 | 0.3 | 4 |
| Primer group rat 2 | 1.5 | 1.43 | 0.25 | 2 |
| Primer group rat 3 | ||||
| Primer group rat 4 | 1 | 1.02 | 0.01 | 1 |
| Primer group rat 5 | 1.5 | 1.23 | 0.12 | 3 |
| Primer group rat 6 | ||||
| Vascularised group rat 1 | 1.5 | 1.57 | 0.34 | 3 |
| Vascularised group rat 2 | 3 | 1.45 | 0.19 | 1 |
| Vascularised group rat 3 | 1.5 | 1.74 | 0.1 | 2 |
| Vascularised group rat 4 | 2 | 1.88 | 0.29 | 3 |
| Vascularised group rat 5 | 1 | 1.16 | 0.31 | 1 |
| Vascularised group rat 6 | 2 | 1.28 | 0.08 | 2 |
| Non-Vascularised group rat 1 | 1.5 | 1.52 | 0.19 | 4 |
| Non-Vascularised group rat 2 | 2 | 1.62 | 0.15 | 1 |
| Non-Vascularised group rat 3 | 1.5 | 1.76 | 0.45 | 4 |
| Non-Vascularised group rat 4 | 2 | 2.66 | 0.03 | 4 |
| Non-Vascularised group rat 5 | 0.5 | 1.15 | 0.24 | 3 |
| Non-Vascularised group rat 6 | 1 | 1.91 | 0.06 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yucens, M.; Aydemir, A.N.; Sengoz, T.; Mete, G.A.; Ök, N.; Koç, M.R.; Demirkan, A.F. Vascularised and Non-Vascularised Adipofascial Flap Applications in Tissue Trauma with Tendon Injury, Flap Viability and Tendon Healing a Hystological and Scintigraphical Rat Model Study. Medicina 2024, 60, 1689. https://doi.org/10.3390/medicina60101689
Yucens M, Aydemir AN, Sengoz T, Mete GA, Ök N, Koç MR, Demirkan AF. Vascularised and Non-Vascularised Adipofascial Flap Applications in Tissue Trauma with Tendon Injury, Flap Viability and Tendon Healing a Hystological and Scintigraphical Rat Model Study. Medicina. 2024; 60(10):1689. https://doi.org/10.3390/medicina60101689
Chicago/Turabian StyleYucens, Mehmet, Ahmet Nadir Aydemir, Tarık Sengoz, Gulcin Abban Mete, Nusret Ök, Mehmet Rauf Koç, and Ahmet Fahir Demirkan. 2024. "Vascularised and Non-Vascularised Adipofascial Flap Applications in Tissue Trauma with Tendon Injury, Flap Viability and Tendon Healing a Hystological and Scintigraphical Rat Model Study" Medicina 60, no. 10: 1689. https://doi.org/10.3390/medicina60101689
APA StyleYucens, M., Aydemir, A. N., Sengoz, T., Mete, G. A., Ök, N., Koç, M. R., & Demirkan, A. F. (2024). Vascularised and Non-Vascularised Adipofascial Flap Applications in Tissue Trauma with Tendon Injury, Flap Viability and Tendon Healing a Hystological and Scintigraphical Rat Model Study. Medicina, 60(10), 1689. https://doi.org/10.3390/medicina60101689






