Cystic Angiomyofibroblastoma of the Uterus Mimicking Ovarian Cancer
Abstract
1. Introduction
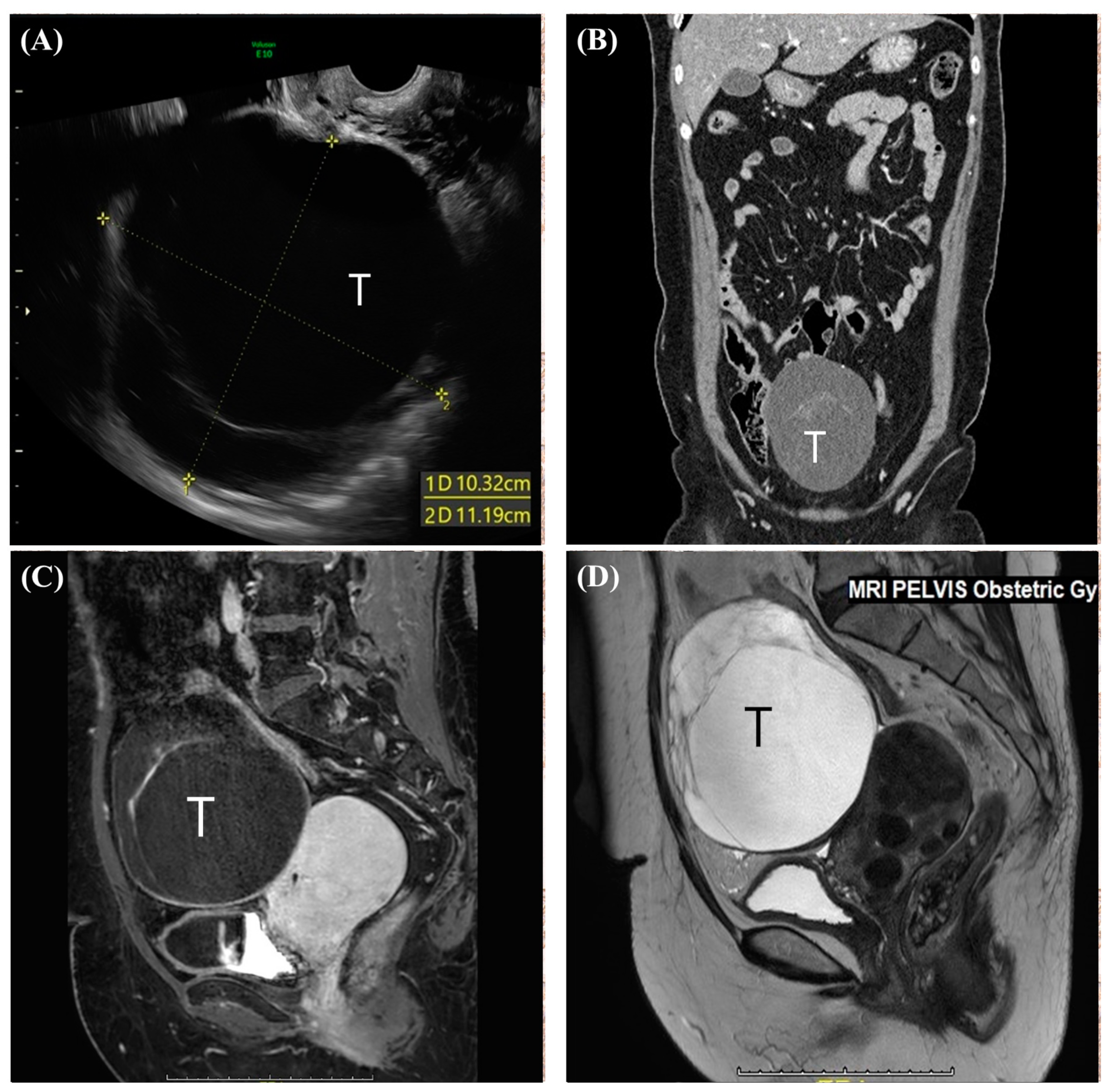
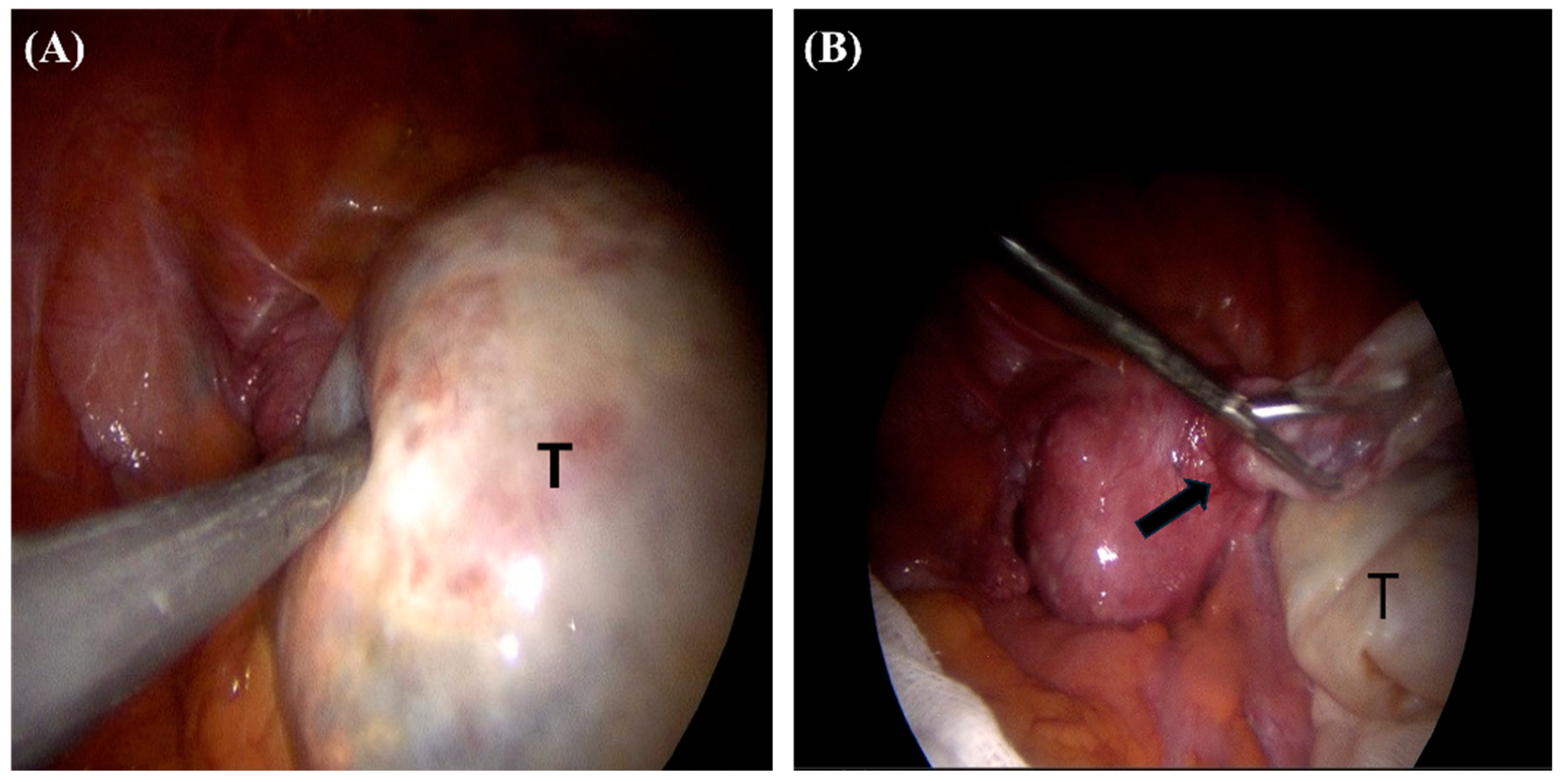
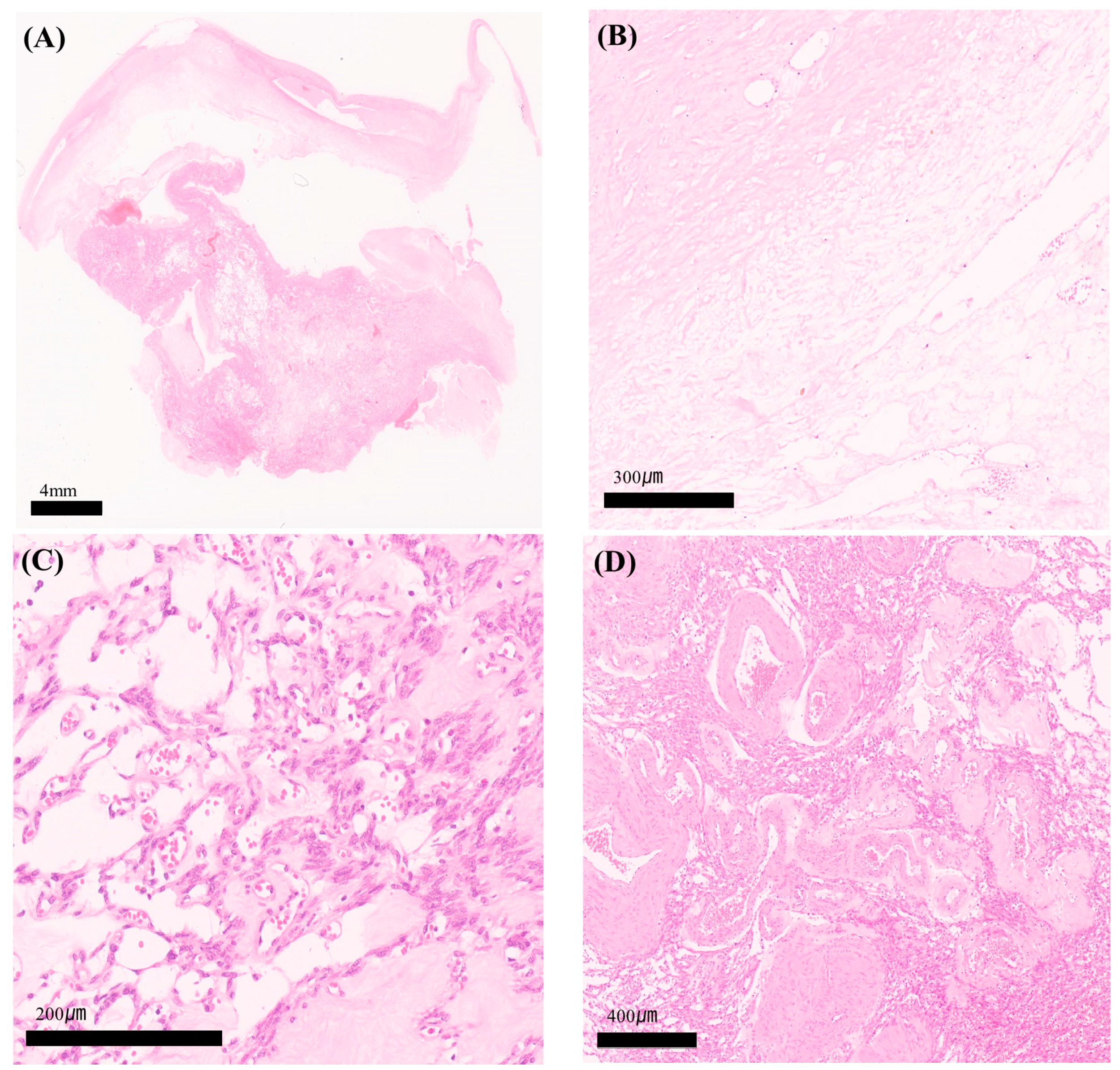
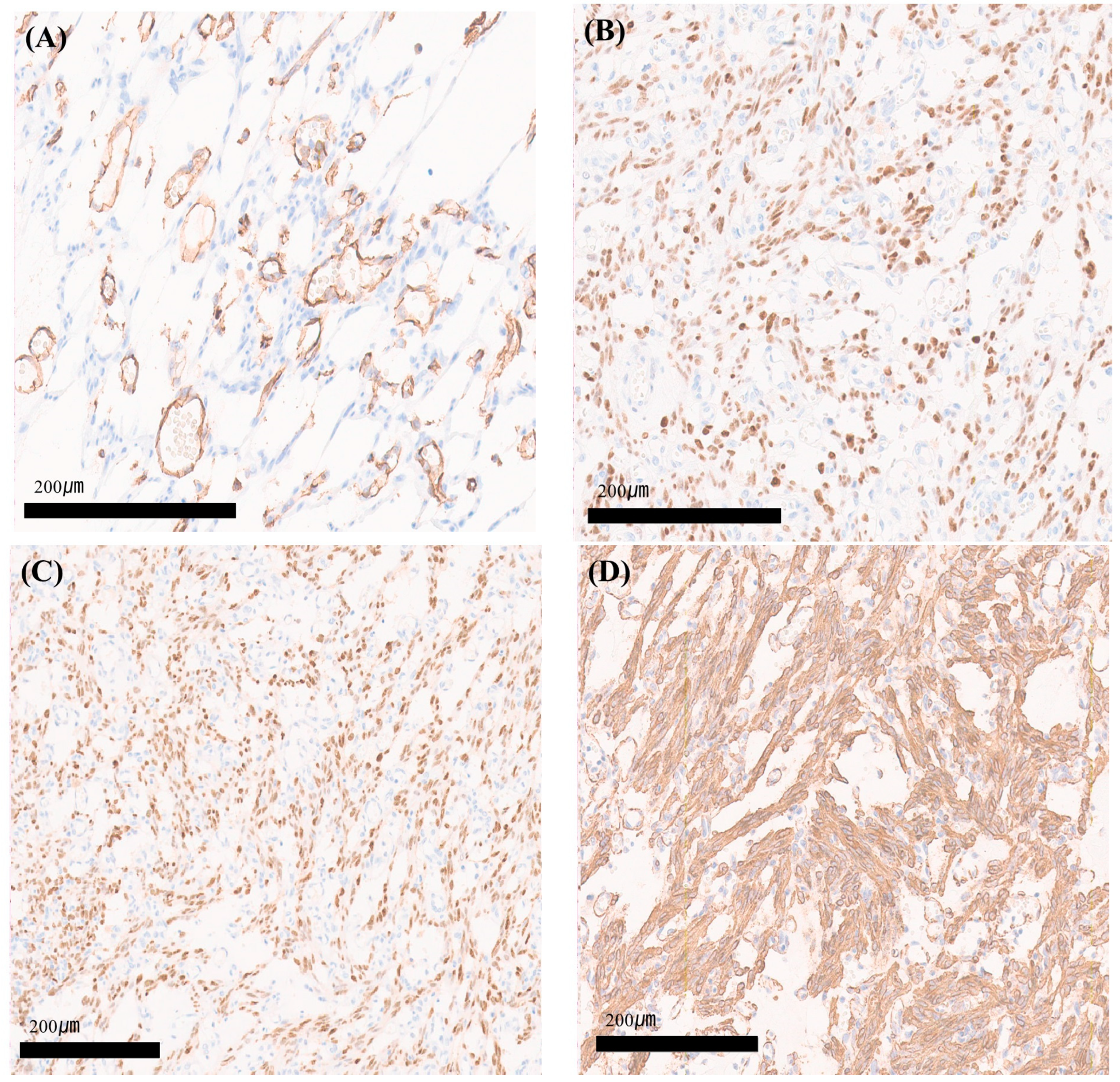
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fletcher, C.D.; Tsang, W.Y.; Fisher, C.; Lee, K.C.; Chan, J.K. Angiomyofibroblastoma of the vulva. A benign neoplasm distinct from aggressive angiomyxoma. Am. J. Surg. Pathol. 1992, 16, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Poljak, N.K.; Kljajić, Z.; Petricević, J.; Forempoher, G.; Simunić, M.M.; Colović, Z.; Kontić, M. Polypoid angiomyofibroblastoma tumor of nasal cavity: Case report. Coll. Antropol. 2013, 37, 301–304. [Google Scholar] [PubMed]
- Zhu, J.; Su, S.; Li, H. Angiomyofibroblastoma of the mediastinum: A case report and literature review. Medicine 2016, 95, e5484. [Google Scholar] [CrossRef]
- Kobayashi, T.; Suzuki, K.; Arai, T.; Sugimura, H. Angiomyofibroblastoma arising from the fallopian tube. Obstet. Gynecol. 1999, 94 Pt 2, 833–834. [Google Scholar] [PubMed]
- Law, K.-S.; Pan, S.-T.; Wu, M.-P. Effective treatment of tubal angiomyofibroblastoma via laparoscopic complete resection. Heliyon 2020, 6, e04123. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chen, Y.-R.; Tsai, H.-D.; Cheng, Y.-M.; Hsiao, Y.-H. Angiomyofibroblastoma of the Broad Ligament: A Case Report. Int. J. Gynecol. Pathol. 2017, 36, 471–475. [Google Scholar] [CrossRef]
- Nielsen, G.P.; Young, R.H.; Dickersin, G.R.; Rosenberg, A.E. Angiomyofibroblastoma of the Vulva with Sarcomatous Transformation (“Angiomyofibrosarcoma”). Am. J. Surg. Pathol. 1997, 21, 1104–1108. [Google Scholar] [CrossRef]
- Haroon, S.; Irshad, L.; Zia, S.; Ali, A.H.; Dowlah, T.U.; Rashid, K.; Malik, U.A.; Khan, A.N.; Irfan, M.; A Hashmi, A. Aggressive Angiomyxoma, Angiomyofibroblastoma, and Cellular Angiofibroma of the Lower Female Genital Tract: Related Entities with Different Outcomes. Cureus 2022, 14, e29250. [Google Scholar] [CrossRef]
- Laskin, W.B.; Fetsch, J.F.; A Tavassoli, F. Angiomyofibroblastoma of the female genital tract: Analysis of 17 cases including a lipomatous variant. Hum. Pathol. 1997, 28, 1046–1055. [Google Scholar] [CrossRef]
- Babala, P.; Biro, C.; Klacko, M.; Miklos, P.; Ondrus, D. Angiomyofibroblastoma of the cervix uteri: A case report. Klin. Onkol. Cas. Ceske Slov. Onkol. Spol. 2011, 24, 133–136. [Google Scholar]
- Kim, M.J.; Ni Kuk, J.; Sung, L.J.H.; Hyun, C.L.; Shim, S.S.; Park, C.M.; Kim, S.Y. Angiomyofibroblastoma of the uterine cervix in a breast cancer patient: A case report. Korean J. Obstet. Gynecol. 2011, 54, 330–333. [Google Scholar] [CrossRef][Green Version]
- Lee, C.L.; Ng, B.K.; Nurismah, M.I.; Chew, K.T.; Aruku, N.; Lim, P.S. Concurrent utero-vaginal prolapse with cervical angiomyofibroblastoma: A rare disease with distinct entity. J. Surg. Acad. 2015, 5, 58–61. [Google Scholar]
- Wong, Y.P.; Tan, G.C.; Ng, P.F. Cervical angiomyofibroblastoma: A case report and review of literature. J. Obstet. Gynaecol. 2017, 37, 681–682. [Google Scholar] [CrossRef]
- Roncati, L.; Pusiol, T.; Piscioli, F.; Barbolini, G.; Maiorana, A. Undetermined cervical smear due to angiomyofibroblastoma of the cervix uteri. J. Obstet. Gynaecol. 2017, 37, 829–830. [Google Scholar] [CrossRef]
- Büyüktalanci, D.; Yiğit, S.; Altindağ, S.D.; Aydoğmuş, H.; Gençdal, S. Angiomyofibroblastoma of the uterine cervix in a patient with triple negative breast cancer: A case report. Anatol. Curr. Med. J. 2021, 3, 181–184. [Google Scholar] [CrossRef]
- Güvendi, G.F.; Suntur, M.; Ayazoğlu, M.S.; Okcu, O.; Bedir, R. Angiomyofibroblastoma of the lower genital tract of women: Report of two cases. J. Clin. Obstet. Gynecol. 2021, 31, 31–34. [Google Scholar] [CrossRef]
- Figueiredo, G.; O’Shea, A.; Neville, G.M.; Lee, S.I. Rare Mesenchymal Tumors of the Pelvis: Imaging and Pathologic Correlation. RadioGraphics 2022, 42, 143–158. [Google Scholar] [CrossRef]
- Kwack, J.Y.; Kim, S.; Lee, J.S.; Kwon, Y.-S. Case Report of Vaginal Angiomyofibroblastoma and Differential Diagnosis of Other Benign Mesenchymal Tumors in the Female Genitalia. J. Clin. Obstet. Gynecol. 2024, 34, 36–40. [Google Scholar] [CrossRef]
- Cree, I.A.; White, V.A.; Indave, B.I.; Lokuhetty, D. Revising the WHO classification: Female genital tract tumours. Histopathology 2020, 76, 151–156. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Qian, H.-L.; Jin, H.-M. Local recurrent vaginal aggressive angiomyxoma misdiagnosed as cellular angiomyofibroblastoma: A case report. Exp. Ther. Med. 2016, 11, 1893–1895. [Google Scholar] [CrossRef][Green Version]
- Varras, M.; Akrivis, C.; Demou, A.; Kitsiou, E.; Antoniou, N. Angiomyofibroblastoma of the vagina in a postmenopausal breast cancer patient treated with tamoxifen: Clinicopathologic analysis of a case and review of the literature. Int. J. Gynecol. Cancer 2006, 16, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Wang, Z.; Li, Y.; Cui, G. Giant pelvic angiomyofibroblastoma: Case report and literature review. Diagn. Pathol. 2014, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Matsui-Horiguchi, M.; Fujiwara, M.; Kaketa, M.; Kawano, M.; Ohtsubo-Shimoyamada, R.; Ohse, H. Angiomyofibroblastoma of the vulva: Report of a case with immunohistochemical and molecular analysis. Int. J. Gynecol. Pathol. 2003, 22, 277–284. [Google Scholar] [CrossRef]
- Kurose, K.; Mine, N.; Iida, A.; Nagai, H.; Harada, H.; Araki, T.; Emi, M. Three aberrant splicing variants of the HMGIC gene transcribed in uterine leiomyomas. Genes Chromosomes Cancer 2001, 30, 212–217. [Google Scholar] [CrossRef] [PubMed]
| Case | Age | Size (cm) | Presentation | Para | Treatment | Outcome | IHC | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 44 | 2 | Polypoid mass | ND | ND | ND | desmin/vimentin (+/+), E/P (ND/ND) αSMA/CD34 (ND/ND), S100 (ND) | Babala, P., et al. [10] |
| 2 * | 43 | 3 × 3 × 2.5 | No Sx. | 3 | Local excision | ND | desmin/vimentin (+/+), E/P (+/+) αSMA/CD34 (−/−), S100 (ND) | Kim, M.J., et al. [11] |
| 3 | 53 | 4 × 3 | Vaginal mass | 3 | LAVH & BSO | 2 years | desmin/vimentin (+/ND), E/P (ND/ND) αSMA/CD34 (+/+), S100 (ND) | Lee, C., et al. [12] |
| 4 | 32 | 1.2 | Vaginal spotting | ND | Local excision | ND | desmin/vimentin (−/ND), E/P (+/+)αSMA/CD34 (ND/−), S100 (-) | Wong, Y.P., et al. [13] |
| 5 | 48 | 1 | Vaginal spotting | ND | Local excision(conization) | ND | desmin/vimentin (+/ND), E/P (+/+) αSMA/CD34 (+/+), S100 (ND) | Roncati, L., et al. [14] |
| 6 * | 40 | 6 × 5 | Vaginal bleeding | ND | TAH & BSO | ND | desmin/vimentin (+/+), E/P (+/+) αSMA/CD34 (−/−), S100 (ND) | Büyüktalancı, D.Ö., et al. [15] |
| 7 | 45 | 2.5 × 2 × 1 | AUB | ND | Local excision | ND | desmin/vimentin (+/+), E/P (+/+) αSMA/CD34 (ND/+), S100 (ND) | Güvendi, G.F., et al. [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.Y.; An, H.J.; Jo, I.A.; Shin, J.K.; Choi, W.J.; Baek, J.C. Cystic Angiomyofibroblastoma of the Uterus Mimicking Ovarian Cancer. Medicina 2024, 60, 1645. https://doi.org/10.3390/medicina60101645
Jo JY, An HJ, Jo IA, Shin JK, Choi WJ, Baek JC. Cystic Angiomyofibroblastoma of the Uterus Mimicking Ovarian Cancer. Medicina. 2024; 60(10):1645. https://doi.org/10.3390/medicina60101645
Chicago/Turabian StyleJo, Jae Yoon, Hyo Jung An, In Ae Jo, Jeong Kyu Shin, Won Jun Choi, and Jong Chul Baek. 2024. "Cystic Angiomyofibroblastoma of the Uterus Mimicking Ovarian Cancer" Medicina 60, no. 10: 1645. https://doi.org/10.3390/medicina60101645
APA StyleJo, J. Y., An, H. J., Jo, I. A., Shin, J. K., Choi, W. J., & Baek, J. C. (2024). Cystic Angiomyofibroblastoma of the Uterus Mimicking Ovarian Cancer. Medicina, 60(10), 1645. https://doi.org/10.3390/medicina60101645





