Virtual Reality for Decreasing Procedural Pain during Botulinum Toxin Injection Related to Spasticity Treatment in Adults: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
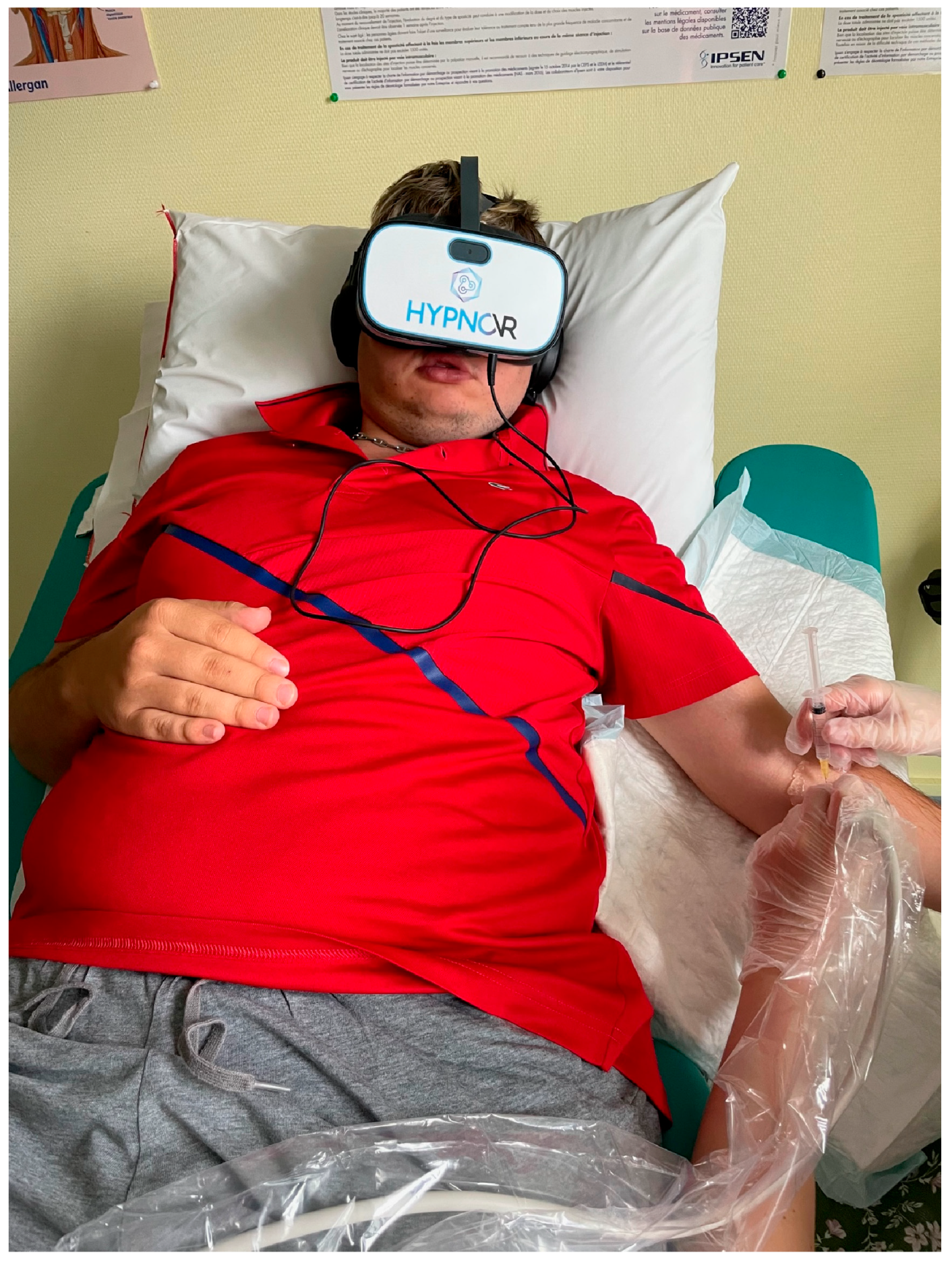
2.3. Procedure
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Primary and Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stump, E. WHO Report: Millions Have Neurological Disorders Worldwide. Neurol. Today 2007, 7, 25. [Google Scholar] [CrossRef]
- Chau, J.P.C.; Lo, S.H.S.; Choi, K.C.; Butt, L.; Zhao, J.; Thompson, D.R. Participation self-efficacy plays a mediation role in the association between mobility and social participation among stroke survivors. Heart Lung J. Crit. Care 2021, 50, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Lance, J. Pathophysiology of spasticity and clinical experience with baclofen. Spasticity Disord. Mot. Control 1980, 185–204. [Google Scholar]
- AFSSAPS. Recommandations de Bonne Pratique: Traitements Medicamenteux de la Spasticité; AGENCE FRANÇAISE DE SÉCURITÉ SANITAIRE DES PRODUITS DE SANTÉ: Saint Denis, France, 2009. [Google Scholar]
- Cameron, M.H.; Bethoux, F.; Davis, N.; Frederick, M. Botulinum Toxin for Symptomatic Therapy in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2014, 14, 463. [Google Scholar] [CrossRef] [PubMed]
- Chaléat-Valayer, E.; Parratte, B.; Colin, C.; Denis, A.; Oudin, S.; Bérard, C.; Bernard, J.; Bourg, V.; Deleplanque, B.; Dulieu, I.; et al. A French observational study of botulinum toxin use in the management of children with cerebral palsy: BOTULOSCOPE. Eur. J. Paediatr. Neurol. 2011, 15, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Brochard, S.; Blajan, V.; Lempereur, M.; Garlantezec, R.; Houx, L.; Le Moine, P.; Peudenier, S.; Lefranc, J.; Rémy-Néris, O. Determining the technical and clinical factors associated with pain for children undergoing botulinum toxin injections under nitrous oxide and anesthetic cream. Eur. J. Paediatr. Neurol. 2011, 15, 310–315. [Google Scholar] [CrossRef]
- Mathevon, L.; Davoine, P.; Tardy, M.; Bouchet, N.; Gornushkina, I.; Pérennou, D. Pain during botulinum toxin injections in spastic adults: Influence of patients’ clinical characteristics and of the procedure. Ann. Phys. Rehabil. Med. 2018, 61, e359. [Google Scholar] [CrossRef]
- Fisher, M.T.; Zigler, C.K.; Houtrow, A.J. Factors affecting procedural pain in children during and immediately after intramuscular botulinum toxin injections for spasticity. J. Pediatr. Rehabil. Med. 2018, 11, 193–197. [Google Scholar] [CrossRef]
- Bayon-Mottu, M.; Gambart, G.; Deries, X.; Tessiot, C.; Richard, I.; Dinomais, M. Pain during injections of botulinum toxin in children: Influence of the localization technique. Ann. Phys. Rehabil. Med. 2014, 57, 578–586. [Google Scholar] [CrossRef]
- Joindreau-Gaude, V.; Noël, N. Douleur et soins. In Proceedings of the Handicap Douleur Actes 24es Entret, Nanterre, France, 24–25 November 2011; Fondation Garches, Institut fédératif de recherche sur le handicap, Ed.; GM Santé: Paris, France, 2011; pp. 101–109. [Google Scholar]
- Francisco, G.E.; Balbert, A.; Bavikatte, G.; Bensmail, D.; Carda, S.; Deltombe, T.; Draulans, N.; Escaldi, S.; Gross, R.; Jacinto, J.; et al. A practical guide to optimizing the benefits of post-stroke spasticity interventions with botulinum toxin A: An international group consensus. J. Rehabil. Med. 2021, 53, jrm00134. [Google Scholar] [CrossRef]
- Nugud, A.; Alhoot, S.; Agabna, M.; Babiker, M.O.E.; El Bashir, H. Analgesia and sedation modalities used with botulinum toxin injections in children with cerebral palsy: A literature review. Sudan. J. Paediatr. 2021, 21, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.; Phadke, C.P.; Kam, A.; Ismail, F.; Boulias, C. Effect of topical anesthetics on needle insertion pain during botulinum toxin type A injections for limb spasticity. Arch. Phys. Med. Rehabil. 2012, 93, 1643–1647. [Google Scholar] [CrossRef]
- Gubbay, A.; Langdon, K. Effectiveness of sedation using nitrous oxide compared with enteral midazolam for botulinum toxin A injections in children. Dev. Med. Child Neurol. 2009, 51, 491–492. [Google Scholar] [CrossRef]
- Nilsson, S.; Brunsson, I.; Askljung, B.; Påhlman, M.; Himmelmann, K. A rectally administered combination of midazolam and ketamine was easy, effective and feasible for procedural pain in children with cerebral palsy. Acta Paediatr. 2017, 106, 458–462. [Google Scholar] [CrossRef]
- Weiss, R.A.; Lavin, P.T. Reduction of pain and anxiety prior to botulinum toxin injections with a new topical anesthetic method. Ophthalmic Plast. Reconstr. Surg. 2009, 25, 173–177. [Google Scholar] [CrossRef]
- Gambart, G.; Mette, F.; Pellot, A.-S.; Richard, I. Evaluation of analgesic protocol with nitrous oxide and EMLA cream during botulinum toxin injections in children. Ann. Readapt. Med. Phys. 2007, 50, 275–279. [Google Scholar] [CrossRef]
- Jones, T.; Moore, T.; Choo, J. The Impact of Virtual Reality on Chronic Pain. PLoS ONE 2016, 11, e0167523. [Google Scholar] [CrossRef]
- Trost, Z.; France, C.; Anam, M.; Shum, C. Virtual reality approaches to pain: Toward a state of the science. Pain 2021, 162, 325–331. [Google Scholar] [CrossRef]
- Goudman, L.; Jansen, J.; De Smedt, A.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality during Intrathecal Pump Refills in Children: A Case Series. J. Clin. Med. 2022, 11, 5877. [Google Scholar] [CrossRef]
- Luque-Moreno, C.; Kiper, P.; Solís-Marcos, I.; Agostini, M.; Polli, A.; Turolla, A.; Oliva-Pascual-Vaca, A. Virtual Reality and Physiotherapy in Post-Stroke Functional Re-Education of the Lower Extremity: A Controlled Clinical Trial on a New Approach. J. Pers. Med. 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Lambert, V.; Boylan, P.; Boran, L.; Hicks, P.; Kirubakaran, R.; Devane, D.; Matthews, A. Virtual reality distraction for acute pain in children. Cochrane Database Syst. Rev. 2020, 10, CD010686. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Houdayer, E.; Emedoli, D.; Locatelli, M.; Mortini, P.; Mandelli, C.; Raggi, A.; Iannaccone, S. Efficacy of virtual reality to reduce chronic low back pain: Proof-of-concept of a non-pharmacological approach on pain, quality of life, neuropsychological and functional outcome. PLoS ONE 2019, 14, e0216858. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Warty, R.R.; Sursas, J.A.; Payne, O.; Nair, A.; Krishnan, S.; Costa, F.d.S.; Wallace, E.M.; Vollenhoven, B. The Effectiveness of Virtual Reality in Managing Acute Pain and Anxiety for Medical Inpatients: Systematic Review. J. Med. Internet Res. 2020, 22, e17980. [Google Scholar] [CrossRef] [PubMed]
- Perrochon, A.; Borel, B.; Istrate, D.; Compagnat, M.; Daviet, J.-C. Exercise-based games interventions at home in individuals with a neurological disease: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.I.; Mahrer, N.E. Is Virtual Reality Ready for Prime Time in the Medical Space? A Randomized Control Trial of Pediatric Virtual Reality for Acute Procedural Pain Management. J. Pediatr. Psychol. 2018, 43, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Chau, B.; Chi, B.; Wilson, T. Decreasing pediatric pain and agitation during botulinum toxin injections for spasticity with virtual reality: Lessons learned from clinical use. J. Pediatr. Rehabil. Med. 2018, 11, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Sikka, N.; Shu, L.; Ritchie, B.; Amdur, R.L.; Pourmand, A. Virtual Reality-Assisted Pain, Anxiety, and Anger Management in the Emergency Department. Telemed. J. E-Health 2019, 25, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Almugait, M.; AbuMostafa, A. Author Correction: Comparison between the analgesic effectiveness and patients’ preference for virtual reality vs. topical anesthesia gel during the administration of local anesthesia in adult dental patients: A randomized clinical study. Sci. Rep. 2022, 12, 805. [Google Scholar] [CrossRef]
- Basak, T.; Demirtas, A.; Yorubulut, S.M. Virtual reality and distraction cards to reduce pain during intramuscular benzathine penicillin injection procedure in adults: A randomized controlled trial. J. Adv. Nurs. 2021, 77, 2511–2518. [Google Scholar] [CrossRef]
- Trompetto, C.; Marinelli, L.; Mori, L.; Puce, L.; Avanti, C.; Saretti, E.; Biasotti, G.; Amella, R.; Cotellessa, F.; Restivo, D.A.; et al. Effectiveness of Botulinum Toxin on Pain in Stroke Patients Suffering from Upper Limb Spastic Dystonia. Toxins 2022, 14, 39. [Google Scholar] [CrossRef]
- Baricich, A.; Battaglia, M.; Cuneo, D.; Cosenza, L.; Millevolte, M.; Cosma, M.; Filippetti, M.; Dalise, S.; Azzollini, V.; Chisari, C.; et al. Clinical efficacy of botulinum toxin type A in patients with traumatic brain injury, spinal cord injury, or multiple sclerosis: An observational longitudinal study. Front. Neurol. 2023, 14, 1133390. [Google Scholar] [CrossRef]
- Anwar, K.; Barnes, M.P. A pilot study of a comparison between a patient scored numeric rating scale and clinician scored measures of spasticity in multiple sclerosis. NeuroRehabilitation 2009, 24, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.; de Araújo Val, S.; Magalhães, F.; Marinho, V.; Ayres, C.; Teixeira, S.; Bastos, V.H. Virtual reality exposure therapy for neuro-psychomotor recovery in adults: A systematic review. Disabil. Rehabil. Assist. Technol. 2021, 16, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Mallari, B.; Spaeth, E.K.; Goh, H.; Boyd, B.S. Virtual reality as an analgesic for acute and chronic pain in adults: A systematic review and meta-analysis. J. Pain Res. 2019, 12, 2053–2085. [Google Scholar] [CrossRef] [PubMed]
- Eijlers, R.; Utens, E.M.W.J.; Staals, L.M.; de Nijs, P.F.A.; Berghmans, J.M.; Wijnen, R.M.H.; Hillegers, M.H.J.; Dierckx, B.; Legerstee, J.S. Systematic Review and Meta-analysis of Virtual Reality in Pediatrics: Effects on Pain and Anxiety. Anesth. Analg. 2019, 129, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H. How does distraction work in the management of pain? Curr. Pain Headache Rep. 2005, 9, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Indovina, P.; Barone, D.; Gallo, L.; Chirico, A.; De Pietro, G.; Giordano, A. Virtual Reality as a Distraction Intervention to Relieve Pain and Distress During Medical Procedures: A Comprehensive Literature Review. Clin. J. Pain 2018, 34, 858–877. [Google Scholar] [CrossRef]
- Abiodun, A.O.; Adesina, M.A. Virtual reality: A breakthrough in pain management? World News Nat. Sci. 2019, 23, 24–33. [Google Scholar]
- Wismeijer, A.A.J.; Vingerhoets, A.J.J.M. The use of virtual reality and audiovisual eyeglass systems as adjunct analgesic techniques: A review of the literature. Ann. Behav. Med. 2005, 30, 268–278. [Google Scholar] [CrossRef]
- Rutter, C.E.; Dahlquist, L.M.; Weiss, K.E. Sustained efficacy of virtual reality distraction. J. Pain 2009, 10, 391–397. [Google Scholar] [CrossRef]
- Birnie, K.A.; Noel, M.; Parker, J.A.; Chambers, C.T.; Uman, L.S.; Kisely, S.R.; McGrath, P.J. Systematic review and meta-analysis of distraction and hypnosis for needle-related pain and distress in children and adolescents. J. Pediatr. Psychol. 2014, 39, 783–808. [Google Scholar] [CrossRef] [PubMed]
- Koller, D.; Goldman, R.D. Distraction techniques for children undergoing procedures: A critical review of pediatric research. J. Pediatr. Nurs. 2012, 27, 652–681. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.; Brivio, E.; Riva, G.; Baños, R.M. Immersive Versus Non-immersive Experience: Exploring the Feasibility of Memory Assessment Through 360° Technology. Front. Psychol. 2019, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Goudman, L.; Jansen, J.; Billot, M.; Vets, N.; De Smedt, A.; Roulaud, M.; Rigoard, P.; Moens, M. Virtual Reality Applications in Chronic Pain Management: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e34402. [Google Scholar] [CrossRef]
- Patterson, D.R.; Hoffman, H.G.; Chambers, G.; Bennetts, D.; Hunner, H.H.; Wiechman, S.A.; Garcia-Palacios, A.; Jensen, M.P. Hypnotic Enhancement of Virtual Reality Distraction Analgesia during Thermal Pain: A Randomized Trial. Int. J. Clin. Exp. Hypn. 2021, 69, 225–245. [Google Scholar] [CrossRef]
- Rousseaux, F.; Bicego, A.; Ledoux, D.; Massion, P.; Nyssen, A.-S.; Faymonville, M.-E.; Laureys, S.; Vanhaudenhuyse, A. Hypnosis Associated with 3D Immersive Virtual Reality Technology in the Management of Pain: A Review of the Literature. J. Pain Res. 2020, 13, 1129–1138. [Google Scholar] [CrossRef]
- Rousseaux, F.; Dardenne, N.; Massion, P.B.; Ledoux, D.; Bicego, A.; Donneau, A.-F.; Faymonville, M.-E.; Nyssen, A.-S.; Vanhaudenhuyse, A. Virtual reality and hypnosis for anxiety and pain management in intensive care units: A prospective randomised trial among cardiac surgery patients. Eur. J. Anaesthesiol. 2022, 39, 58–66. [Google Scholar] [CrossRef]
- Rousseaux, F.; Panda, R.; Toussaint, C.; Bicego, A.; Niimi, M.; Faymonville, M.-E.; Nyssen, A.; Laureys, S.; Gosseries, O.; Vanhaudenhuyse, A. Virtual reality hypnosis in the management of pain: Self-reported and neurophysiological measures in healthy subjects. Eur. J. Pain 2023, 27, 148–162. [Google Scholar] [CrossRef]
- Langlois, P.; Perrochon, A.; David, R.; Rainville, P.; Wood, C.; Vanhaudenhuyse, A.; Pageaux, B.; Ounajim, A.; Lavallière, M.; Debarnot, U.; et al. Hypnosis to manage musculoskeletal and neuropathic chronic pain: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 135, 104591. [Google Scholar] [CrossRef]
- Bani Mohammad, E.; Ahmad, M. Virtual reality as a distraction technique for pain and anxiety among patients with breast cancer: A randomized control trial. Palliat. Support. Care 2019, 17, 29–34. [Google Scholar] [CrossRef]


| Variables | Mean ± SD/n (%) |
|---|---|
| Age (years) | 49.9 ± 10.6 |
| Sex Male Female | |
| 8 (47.1%) | |
| 9 (52.9%) | |
| Disease diagnosis Stroke Cerebral palsy Multiple sclerosis Cervical myelopathy Hereditary spastic paraplegia Cerebrospinal meningitis | |
| 9 (52.9%) | |
| 3 (17.6%) | |
| 2 (11.8%) | |
| 1 (5.9%) | |
| 1 (5.9%) | |
| 1 (5.9%) | |
| Use of analgesic drugs No Yes | |
| 14 (82.4%) | |
| 3 (17.6%) | |
| Baseline pain intensity NRS (0–10) median (min–max) | 0 (0–4) |
| Trapezius | Levator Scapulae | Pectoralis Major | Pectoralis Minor | Teres Major | Deltoid | Biceps Brachii | Brachialis | Brachioradialis | Triceps Brachii | Pronator Teres | Flexor Carpi Ulnaris | Flexor Carpi Radialis | Flexor Digitorum Superficialis | Flexor Digitorum Profundus | Flexor Pollicis Longus | Flexor Pollicis Brevis | Adductor Pollicis | Abductor Digiti Minimi of Hand | Interossei Dorsales | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | ||||||||||||||||||||
| P2 | 1 | 1 | 1 | 1 | ||||||||||||||||
| P3 | ||||||||||||||||||||
| P4 | 1 | 1 | 1 | |||||||||||||||||
| P5 | ||||||||||||||||||||
| P6 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||
| P7 | ||||||||||||||||||||
| P8 | ||||||||||||||||||||
| P9 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||
| P10 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||
| P11 | 1 | 1 | ||||||||||||||||||
| P12 | ||||||||||||||||||||
| P13 | 1 | 1 | 1 | 1 | ||||||||||||||||
| P14 | 1 | 1 | 1 | 1 | ||||||||||||||||
| P15 | 1 | 1 | 1 | 1 | ||||||||||||||||
| P16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||
| P17 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||
| % | 1.1% | 1.1% | 3.4% | 1.1% | 1.1% | 1.1% | 4.5% | 10.1% | 3.4% | 1.1% | 1.1% | 3.4% | 3.4% | 7.9% | 6.7% | 2.2% | 1.1% | 3.4% | 2.2% | 3.4% |
| Rectus Femoris | Adductor Longus | Tibialis Posterior | Gastrocnemius Medial Head | Gastrocnemius Lateral Head | Soleus | Flexor Digitorum Longus | Flexor Hallucis Longus | |
|---|---|---|---|---|---|---|---|---|
| P1 | 1 | 1 | 1 | 1 | ||||
| P2 | 1 | 1 | 1 | |||||
| P3 | 1 | |||||||
| P4 | ||||||||
| P5 | 1 | 1 | 1 | 1 | ||||
| P6 | ||||||||
| P7 | 1 | 1 | 1 | |||||
| P8 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| P9 | ||||||||
| P10 | ||||||||
| P11 | 1 | 1 | ||||||
| P12 | 1 | 1 | ||||||
| P13 | ||||||||
| P14 | 1 | 1 | 1 | |||||
| P15 | 1 | 1 | 1 | 1 | 1 | |||
| P16 | ||||||||
| P17 | ||||||||
| % | 3.4% | 2.2% | 5.6% | 7.9% | 7.9% | 7.9% | 1.1% | 1.1% |
| Variables | Without VR | With VR | p-Value |
|---|---|---|---|
| Anxiety during procedure | 2.1 ± 3.0 | 1.3 ± 2.1 | 0.054 |
| Perceived percentage of improvement Pain intensity Level of anxiety | - | ||
| 39.7% ± 30.9% | |||
| 21.5% ± 25.0% | |||
| Patient satisfaction (0–10) | - | 7.9 ± 1.6 | |
| Agreed to reuse VR for next injection Yes No | - | ||
| 15 (88.2%) | |||
| 2 (11.8%) | |||
| Does the patient recommend VR for other patients? Yes No | - | ||
| 17 (100%) | |||
| 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, R.; Dumas, A.; Ojardias, E.; Duval, S.; Ounajim, A.; Perrochon, A.; Luque-Moreno, C.; Moens, M.; Goudman, L.; Rigoard, P.; et al. Virtual Reality for Decreasing Procedural Pain during Botulinum Toxin Injection Related to Spasticity Treatment in Adults: A Pilot Study. Medicina 2024, 60, 23. https://doi.org/10.3390/medicina60010023
David R, Dumas A, Ojardias E, Duval S, Ounajim A, Perrochon A, Luque-Moreno C, Moens M, Goudman L, Rigoard P, et al. Virtual Reality for Decreasing Procedural Pain during Botulinum Toxin Injection Related to Spasticity Treatment in Adults: A Pilot Study. Medicina. 2024; 60(1):23. https://doi.org/10.3390/medicina60010023
Chicago/Turabian StyleDavid, Romain, Alexis Dumas, Etienne Ojardias, Solène Duval, Amine Ounajim, Anaïck Perrochon, Carlos Luque-Moreno, Maarten Moens, Lisa Goudman, Philippe Rigoard, and et al. 2024. "Virtual Reality for Decreasing Procedural Pain during Botulinum Toxin Injection Related to Spasticity Treatment in Adults: A Pilot Study" Medicina 60, no. 1: 23. https://doi.org/10.3390/medicina60010023
APA StyleDavid, R., Dumas, A., Ojardias, E., Duval, S., Ounajim, A., Perrochon, A., Luque-Moreno, C., Moens, M., Goudman, L., Rigoard, P., & Billot, M. (2024). Virtual Reality for Decreasing Procedural Pain during Botulinum Toxin Injection Related to Spasticity Treatment in Adults: A Pilot Study. Medicina, 60(1), 23. https://doi.org/10.3390/medicina60010023










