A Short-Duration Gonadotropin-Releasing Hormone Stimulation Test for the Diagnosis of Central Precocious Puberty
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Laboratory Tests
2.3. Clinical and Radiological Assessment
2.4. Ethical Statement
2.5. Statistical Analysis
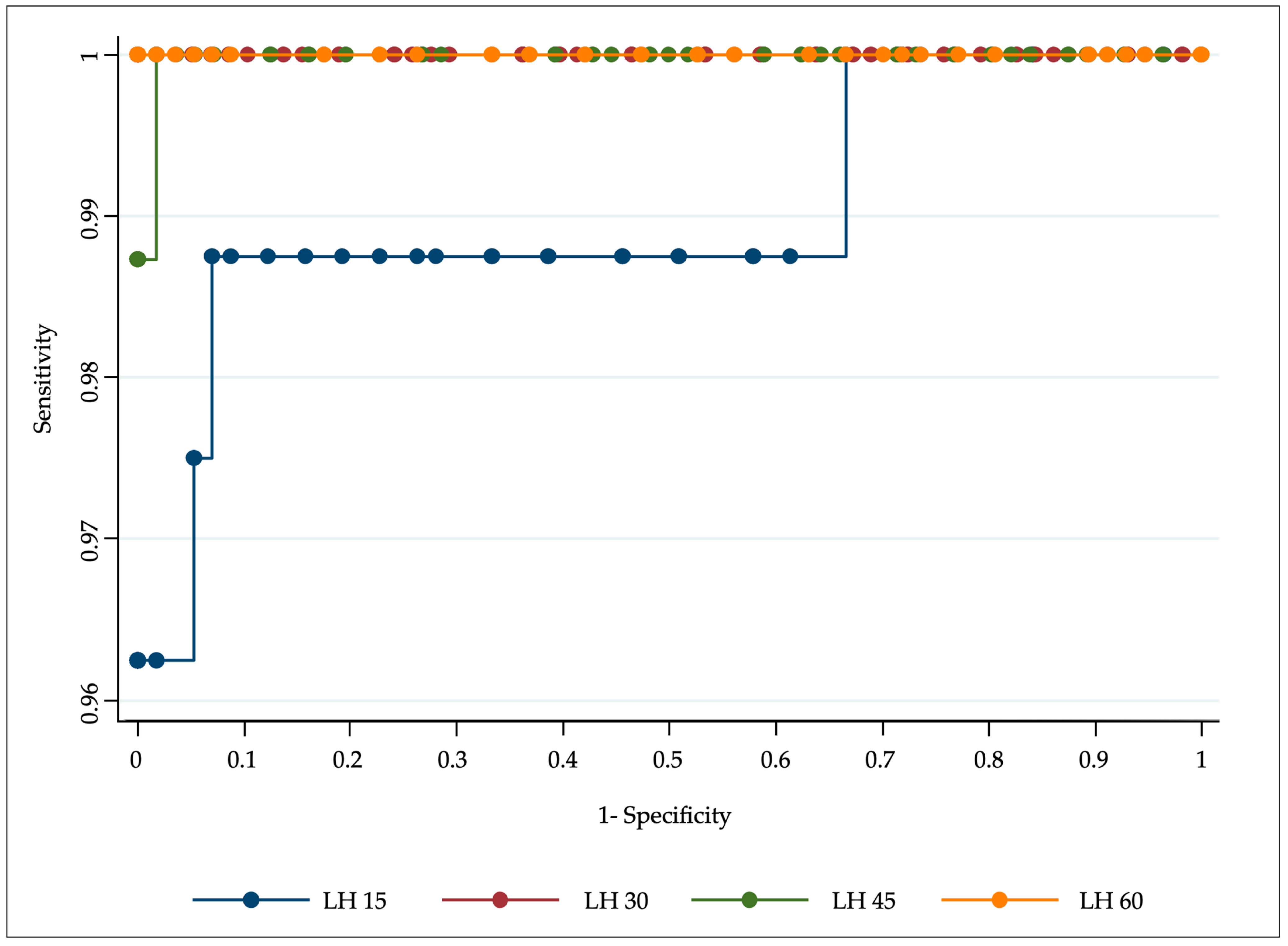
3. Results
3.1. GnRH Test in Females
3.2. GnRH Test in Males
3.3. Gender Differences
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carel, J.C.; Léger, J. Clinical practice. Precocious puberty. N. Engl. J. Med. 2008, 358, 2366–2377. [Google Scholar] [CrossRef]
- Maione, L.; Bouvattier, C.; Kaiser, U.B. Central precocious puberty: Recent advances in understanding the aetiology and in the clinical approach. Clin. Endocrinol. 2021, 4, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Bellotto, E.; Monasta, L.; Pellegrin, M.C.; Bossini, B.; Vidonis, G.; Conte, M.S.; Faleschini, E.; Barbi, E.; Tornese, G. Pattern and Features of Pediatric Endocrinology Referrals: A Retrospective Study in a Single Tertiary Center in Italy. Front. Pediatr. 2020, 8, 580588. [Google Scholar] [CrossRef] [PubMed]
- Poomthavorn, P.; Khlairit, P.; Mahachoklertwattana, P. Subcutaneous gonadotropin-releasing hormone agonist (triptorelin) test for diagnosing precocious puberty. Horm. Res. Paediatr. 2009, 72, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A. Laboratory monitoring of children with precocious puberty. Arch. Pediatr. Adolesc. Med. 1994, 148, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Potau, N.; Zampolli, M.; Virdis, R.; Gussinyé, M.; Carrascosa, A.; Saenger, P.; Vicens-Calvet, E. Use of leuprolide acetate response patterns in the early diagnosis of pubertal disorders: Comparison with the gonadotropin-releasing hormone test. J. Clin. Endocrinol. Metab. 1994, 78, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Houk, C.P.; Kunselman, A.R.; Lee, P.A. Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics 2009, 123, e1059–e1063. [Google Scholar] [CrossRef] [PubMed]
- Neely, E.K.; Wilson, D.M.; Lee, P.A.; Stene, M.; Hintz, R.L. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J. Pediatr. 1995, 127, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, O.H.; Hench, K.D.; Barnes, K.M.; Loriaux, D.L.; Cutler, G.B., Jr. Premature thelarche and central precocious puberty: The relationship between clinical presentation and the gonadotropin response to luteinizing hormone-releasing hormone. J. Clin. Endocrinol. Metab. 1988, 67, 474–479. [Google Scholar] [CrossRef]
- Cavallo, A.; Richards, G.; Busey, S.; Michaels, S. A simplified gonadotrophin-releasing hormone test for precocious puberty. Clin. Endocrinol. 1995, 42, 641–646. [Google Scholar] [CrossRef]
- Ab Rahim, S.N.; Omar, J.; Tuan Ismail, T.S. Gonadotropin-releasing hormone stimulation test and diagnostic cutoff in precocious puberty: A mini review. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Baens-Bailon, R.G.; Lee, P.A. Treatment of central precocious puberty: Comparison of urinary gonadotropin excretion and gonadotropin-releasing hormone (GnRH) stimulation tests in monitoring GnRH analog therapy. J. Clin. Endocrinol. Metab. 1996, 81, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Houk, C.P.; Kunselman, A.R.; Lee, P.A. The diagnostic value of a brief GnRH analogue stimulation test in girls with central precocious puberty: A single 30-minute post-stimulation LH sample is adequate. J. Pediatr. Endocrinol. Metab. 2008, 21, 1113–1118. [Google Scholar] [CrossRef]
- Kim, H.K.; Kee, S.J.; Seo, J.Y.; Yang, E.M.; Chae, H.J.; Kim, C.J. Gonadotropin-releasing hormone stimulation test for precocious puberty. Korean J. Lab. Med. 2011, 31, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Burlo, F.; Lorenzon, B.; Tamaro, G.; Fabretto, A.; Buonomo, F.; Peinkhofer, M.; Vidonis, V.; Vittori, G.; Faleschini, E.; Barbi, E.; et al. Prevalence and characteristics of thelarche variant. Front. Endocrinol. 2023, 14, 1303989. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.K.; Khadilkar, V.V.; Jahagirdar, R.; Khadilkar, A.V.; Lalwani, S.K. Evaluation of GnRH analogue testing in diagnosis and management of children with pubertal disorders. Indian J. Endocrinol. Metab. 2012, 16, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef]
- Greulich, W.W.; Pyle, S.I. Radiographic Atlas of Skeletal Development of the Hand and Wrist, 2nd ed.; Stanford University Press: Redwood City, CA, USA, 1959. [Google Scholar]
- The Italian Data Protection Authority. Authorisation No. 9/2014—General Authorisation to Process Personal Data for Scientific Research Purposes. Available online: https://www.garanteprivacy.it/web/guest/home/docweb/-/docweb-display/docweb/3786078 (accessed on 15 November 2023).
- Kang, S.; Park, M.J.; Kim, J.M.; Yuk, J.S.; Kim, S.H. Ongoing increasing trends in central precocious puberty incidence among Korean boys and girls from 2008 to 2020. PLoS ONE 2023, 18, e0283510. [Google Scholar] [CrossRef]
- Fava, D.; Pepino, C.; Tosto, V.; Gastaldi, R.; Pepe, A.; Paoloni, D.; Strati, M.F.; Angelelli, A.; Calandrino, A.; Tedesco, C.; et al. Precocious Puberty Diagnoses Spike, COVID-19 Pandemic, and Body Mass Index: Findings From a 4-year Study. J. Endocr. Soc. 2023, 7, bvad094. [Google Scholar] [CrossRef]
- Peinkhofer, M.; Bossini, B.; Penco, A.; Giangreco, M.; Pellegrin, M.C.; Vidonis, V.; Vittori, G.; Grassi, N.; Faleschini, E.; Barbi, E.; et al. Reduction in pediatric growth hormone deficiency and increase in central precocious puberty diagnoses during COVID 19 pandemics. Ital. J. Pediatr. 2022, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, P.; Lin, Y.; Raman, V.; Haymond, M. A single sample GnRHa stimulation test in the diagnosis of precocious puberty. Int. J. Pediatr. Endocrinol. 2012, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, R.; Milenkovic, T.; Soldatovic, I.; Pekic, S.; Mitrovic, K.; Todorovic, S. Triptorelin stimulated luteinizing hormone concentrations for diagnosing central precocious puberty: Study of diagnostic accuracy. Endocrine 2022, 75, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Liu, J.; Fu, P.; Zhou, Y.; Li, Z.; Liu, P. The Diagnostic Utility of the Basal Luteinizing Hormone Level and Single 60-Minute Post GnRH Agonist Stimulation Test for Idiopathic Central Precocious Puberty in Girls. Front. Endocrinol. 2021, 12, 713880. [Google Scholar] [CrossRef] [PubMed]
- Cantas-Orsdemir, S.; Eugster, E.A. Update on central precocious puberty: From etiologies to outcomes. Expert Rev. Endocrinol. Metab. 2019, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R. Interpretation of reproductive hormones before, during and after the pubertal transition-Identifying health and disordered puberty. Clin. Endocrinol. 2021, 95, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Lee, Y.S.; Yu, J. Basal serum luteinizing hormone value as the screening biomarker in female central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Chung, I.H. Morning basal luteinizing hormone, a good screening tool for diagnosing central precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 27–33. [Google Scholar] [CrossRef]
- Kandemir, N.; Demirbilek, H.; Özön, Z.A.; Gönç, N.; Alikaşifoglu, A. GnRH stimulation test in precocious puberty: Single sample is adequate for diagnosis and dose adjustment. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 12–17. [Google Scholar] [CrossRef]
- Chin, V.L.; Cai, Z.; Lam, L.; Shah, B.; Zhou, P. Evaluation of puberty by verifying spontaneous and stimulated gonadotropin values in girls. J. Pediatr. Endocrinol. Metab. 2015, 28, 387–392. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Yu, Y.; Yang, P.; Li, H.; Shen, Y.; Huang, X.; Liu, S. Evaluation of basal sex hormone levels for activation of the hypothalamic-pituitary-gonadal axis. J. Pediatr. Endocrinol. Metab. 2018, 31, 323–329. [Google Scholar] [CrossRef]
- Penco, A.; Bossini, B.; Giangreco, M.; Vidonis, V.; Vittori, G.; Grassi, N.; Pellegrin, M.C.; Faleschini, E.; Barbi, E.; Tornese, G. Should Pediatric Endocrinologists Consider More Carefully When to Perform a Stimulation Test? Front. Endocrinol. 2021, 12, 660692. [Google Scholar] [CrossRef]
- Varimo, T.; Huttunen, H.; Miettinen, P.J.; Kariola, L.; Hietamäki, J.; Tarkkanen, A.; Hero, M.; Raivio, T. Precocious Puberty or Premature Thelarche: Analysis of a Large Patient Series in a Single Tertiary Center with Special Emphasis on 6- to 8-Year-Old Girls. Front. Endocrinol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Brito, V.N.; Latronico, A.C.; Arnhold, I.J.; Mendonça, B.B. Update on the etiology, diagnosis and therapeutic management of sexual precocity. Arq. Bras. Endocrinol. Metabol. 2008, 52, 18–31. [Google Scholar] [CrossRef]



| All Patients | Females | Females with Pubertal Response (N = 111) | Females with Prepubertal Response (N = 58) | p Value (Pubertal vs. Prepubertal Females) | Males with Pubertal Response | p Value (Pubertal Females vs. Males) | p Value (Females vs. Males) | |
|---|---|---|---|---|---|---|---|---|
| Number | 188 | 169 | 111 | 58 | / | 19 | / | |
| Age (years) | 8.1 [7.6–8.6] | 8.0 [7.5–8.5] | 8.3 [7.8–8.7] | 7.7 [6.7–8.0] | <0.0001 | 9.0 [8.3–9.4] | <0.0001 | <0.0001 |
| Age at puberty (years) | 7.0 [7.0–7.9] | 7.5 [6.9–7.8] | 7.5 [7.0–7.9] | 7.0 [6.3–7.5] | 0.011 | 8.5 [7.9–8.9] | <0.0001 | <0.0001 |
| Tanner stage (%) (2/3/4) | 69/30/1 | 69/30/1 | 53/46/1 | 93/7/0 | / | 69/25/6 | / | / |
| Bone age (years) | 10.0 [8.8–11.0] | 10.0 [8.8–11.0] | 10.0 [8.8–11.0] | 8.8 [7.8–8.8] | <0.001 | 10.0 [9.0–12.0] | 0.496 | 0.026 |
| Age and bone age difference (years) | 1.6 [0.8–2.6] | 1.6 [0.8–2.6] | 2.1 [1.3–2.8] | 0.8 [0.1–1.5] | <0.001 | 1.6 [0.7–2.9] | 0.259 | 0.889 |
| Height velocity (SDS) | 1.9 [0.8–4.4] | 1.9 [0.8–4.5] | 2.4 [0.8–4.6] | 1.8 [0.7–3.3] | 0.456 | 1.9 [−0.2–3.7] | 0.634 | 0.732 |
| Basal LH (mU/mL) | 0.5 [0.2–1.2] | 0.3 [0.2–1.0] | 0.8 [0.3–2.0] | 0.2 [0.1–0.2] | <0.001 | 1.1 [0.5–1.2] | 0.656 | 0.139 |
| Basal FSH (mU/mL) | 3.0 [1.8–4.8] | 2.9 [1.6–4.6] | 4.1 [2.8–5.4] | 1.8 [1.3–2.7] | <0.001 | 3.0 [1.0–3.7] | <0.0001 | 0.018 |
| Basal LH/FSH ratio | 0.16 [0.08–0.34] | 0.15 [0.07–0.32] | 0.21 [0.10–0.44] | 0.10 [0.06–0.16] | <0.001 | 0.37 [0.13–0.66] | 0.03 | 0.001 |
| Peak LH (mU/mL) | 10.2 [4.5–21.5] | 5.8 [2.8–7.5] | 17.4 [10.2–28.6] | 2.7 [1.7–3.3] | <0.001 | 11.6 [7.1–22.8] | 0.058 | 0.241 |
| Peak FSH (mU/mL) | 10.8 [8.3–14.3] | 11.3 [8.9–15.1] | 10.9 [9.0–13.4] | 12.3 [8.1–18.7] | 0.236 | 6.3 [3.7–8.5] | <0.0001 | <0.0001 |
| Peak LH/FSH ratio | 0.41 [0.25–1.24] | 0.39 [0.25–1.17] | 0.61 [0.29–1.57] | 0.27 [021–0.38] | <0.001 | 0.64 [0.31–1.40] | 0.705 | 0.320 |
| Estradiol (pg/mL) | / | 18.5 [10.4–34.1] | 16.8 [10.9–34.6] | 21.0 [5.0–33.8] | 0.497 | / | / | / |
| Testosterone (ng/mL) | / | / | / | / | / | 0.19 [0.09–1.01] | / | / |
| Basal | 15 m | 30 m | 45 m | 60 m | 90 m | 120 m | |
|---|---|---|---|---|---|---|---|
| Cumulative frequency LH > 5 mU/mL | 6 | 72 | 106 | 110 | 110 | 110 | 111 |
| % | 5.4 | 64.9 | 95.5 | 99.1 | 99.1 | 99.1 | 100 |
| Cumulative frequency LH > 3.3 mU/mL | 16 | 81 | 107 | 110 | 110 | 110 | 111 |
| % | 14.4 | 73 | 96.4 | 99.1 | 99.1 | 99.1 | 100 |
| Basal | 15 m | 30 m | 45 m | 60 m | 90 m | 120 m | |
|---|---|---|---|---|---|---|---|
| Cumulative frequency LH > 5 mU/mL | 0 | 12 | 16 | 19 | 19 | 19 | 19 |
| % | 0 | 63.2 | 84.2 | 100 | 100 | 100 | 100 |
| Cumulative frequency LH > 3.3 mU/mL | 1 | 14 | 16 | 19 | 19 | 19 | 19 |
| % | 5.3 | 73.7 | 84.2 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrin, M.C.; Marzin, C.; Monasta, L.; Tamaro, G.; Vidonis, V.; Vittori, G.; Faleschini, E.; Barbi, E.; Tornese, G. A Short-Duration Gonadotropin-Releasing Hormone Stimulation Test for the Diagnosis of Central Precocious Puberty. Medicina 2024, 60, 24. https://doi.org/10.3390/medicina60010024
Pellegrin MC, Marzin C, Monasta L, Tamaro G, Vidonis V, Vittori G, Faleschini E, Barbi E, Tornese G. A Short-Duration Gonadotropin-Releasing Hormone Stimulation Test for the Diagnosis of Central Precocious Puberty. Medicina. 2024; 60(1):24. https://doi.org/10.3390/medicina60010024
Chicago/Turabian StylePellegrin, Maria Chiara, Chiara Marzin, Lorenzo Monasta, Gianluca Tamaro, Viviana Vidonis, Giada Vittori, Elena Faleschini, Egidio Barbi, and Gianluca Tornese. 2024. "A Short-Duration Gonadotropin-Releasing Hormone Stimulation Test for the Diagnosis of Central Precocious Puberty" Medicina 60, no. 1: 24. https://doi.org/10.3390/medicina60010024
APA StylePellegrin, M. C., Marzin, C., Monasta, L., Tamaro, G., Vidonis, V., Vittori, G., Faleschini, E., Barbi, E., & Tornese, G. (2024). A Short-Duration Gonadotropin-Releasing Hormone Stimulation Test for the Diagnosis of Central Precocious Puberty. Medicina, 60(1), 24. https://doi.org/10.3390/medicina60010024







