A New Player in the Game: Can Exergame Be of Support in the Management of Atrial Fibrillation?
Abstract
1. Introduction
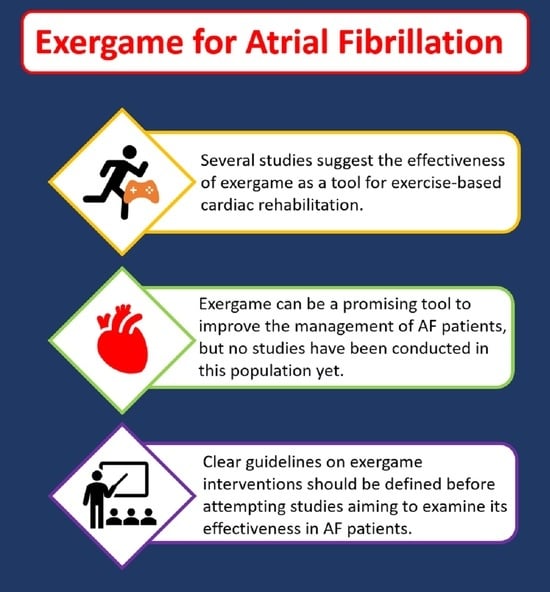
2. Select Player One: Exercise-Based Interventions in the Management of AF
3. Levelling-Up: Exergame as an Intervention for Cardiovascular Diseases
4. Let’s Start the Game: Potential Role of Exergame in the Management of AF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Lau, D.H.; Nattel, S.; Kalman, J.M.; Sanders, P. Modifiable risk factors and atrial fibrillation. Circulation 2017, 136, 583–596. [Google Scholar] [CrossRef]
- Larsson, S.C.; Drca, N.; Wolk, A. Alcohol consumption and risk of atrial fibrillation: A prospective study and dose-response meta-analysis. J. Am. Coll. Cardiol. 2014, 64, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Schlesinger, S.; Norat, T.; Riboli, E. Tobacco smoking and the risk of atrial fibrillation: A systematic review and meta-analysis of prospective studies. Eur. J. Prev. Cardiol. 2018, 25, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Anumonwo, J.M.B.; Kalifa, J. Risk Factors and Genetics of Atrial Fibrillation. Cardiol. Clin. 2014, 32, 485–494. [Google Scholar] [CrossRef]
- Lip, G.Y. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.F.; Joung, B.; Takahashi, Y.; Lim, T.W.; Choi, E.-K.; Chan, Y.-H.; Guo, Y.; Sriratanasathavorn, C.; Oh, S.; Okumura, K.; et al. 2021 Focused Update Consensus Guidelines of the Asia Pacific Heart Rhythm Society on Stroke Prevention in Atrial Fibrillation: Executive Summary. Arthritis Res. Ther. 2022, 122, 20–47. [Google Scholar] [CrossRef]
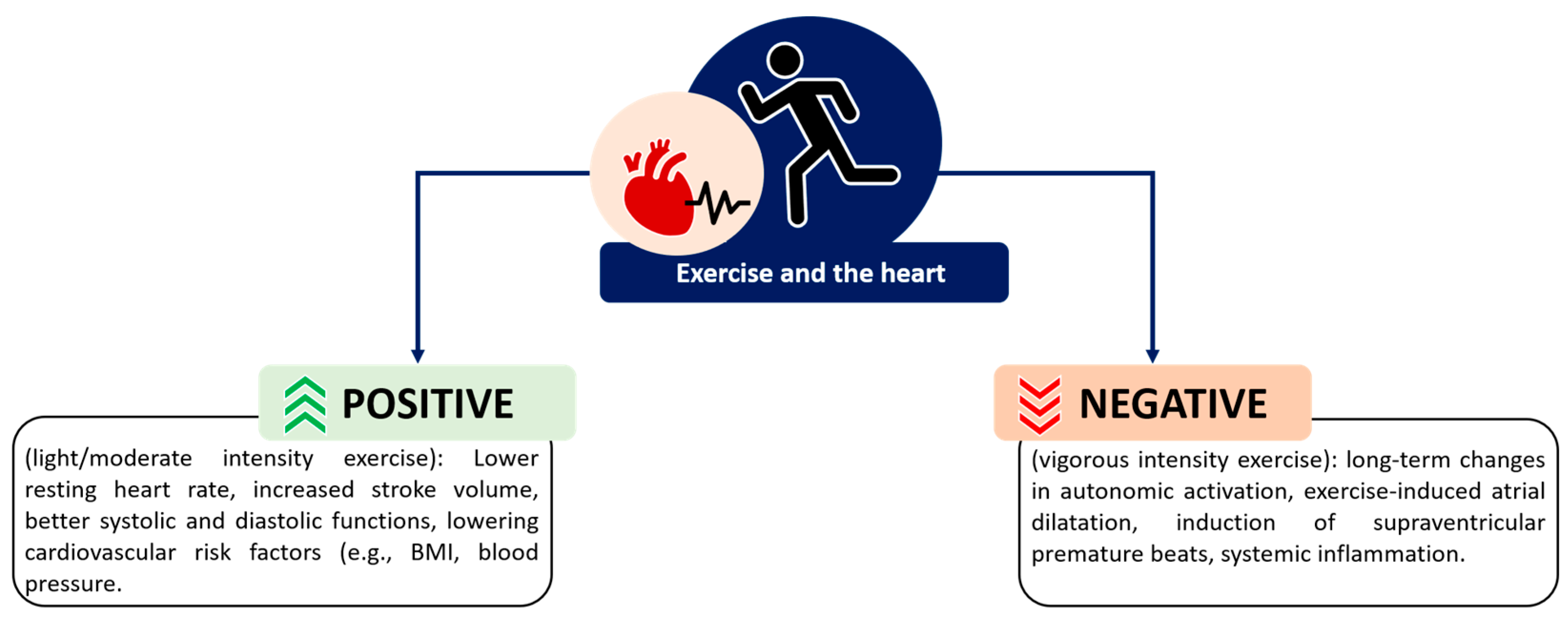
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Myrstad, M.; Malmo, V.; Ulimoen, S.R.; Tveit, A.; Loennechen, J.P. Exercise in individuals with atrial fibrillation. Clin. Res. Cardiol. 2019, 108, 347–354. [Google Scholar] [CrossRef]
- Giacomantonio, N.B.; Bredin, S.S.; Foulds, H.J.; Warburton, D.E. A systematic review of the health benefits of exercise rehabilitation in persons living with atrial fibrillation. Can. J. Cardiol. 2013, 29, 483–491. [Google Scholar] [CrossRef]
- Hamazaki, N.; Kamiya, K.; Fukaya, H.; Nozaki, K.; Ichikawa, T.; Matsuzawa, R.; Yamashita, M.; Uchida, S.; Maekawa, E.; Meguro, K.; et al. Effect of atrial fibrillation on response to exercise-based cardiac rehabilitation in older individuals with heart failure. Ann. Phys. Rehabil. Med. 2021, 64, 101466. [Google Scholar] [CrossRef] [PubMed]
- Nurkkala, V.-M.; Kalermo, J.; Jarvilehto, T. Development of exergaming simulator for gym training, exercise testing and rehabilitation. J. Commun. Comput. 2014, 11, 403–411. [Google Scholar]
- Bond, S.; Laddu, D.R.; Ozemek, C.; Lavie, C.J.; Arena, R. Exergaming and virtual reality for health: Implications for cardiac rehabilitation. Curr. Probl. Cardiol. 2019, 46, 100472. [Google Scholar] [CrossRef] [PubMed]
- Kappen, D.L.; Mirza-Babaei, P.; Nacke, L.E. Older adults’ physical activity and exergames: A systematic review. Int. J. Hum. Comput. Interact. 2019, 35, 140–167. [Google Scholar] [CrossRef]
- Blasco-Peris, C.; Fuertes-Kenneally, L.; Vetrovsky, T.; Sarabia, J.M.; Climent-Paya, V.; Manresa-Rocamora, A. Effects of exergaming in patients with cardiovascular disease compared to conventional cardiac rehabilitation: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 3492. [Google Scholar] [CrossRef]
- Dawes, T.J.; Corden, B.; Cotter, S.; de Marvao, A.; Walsh, R.; Ware, J.S.; Cook, S.A.; O’regan, D.P. Moderate physical activity in healthy adults is associated with cardiac remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004712. [Google Scholar] [CrossRef] [PubMed]
- Morseth, B.; Løchen, M.L.; Ariansen, I.; Myrstad, M.; Thelle, D.S. The ambiguity of physical activity, exercise and atrial fibrillation. Eur. J. Prev. Cardiol. 2018, 25, 624–636. [Google Scholar]
- Everett, B.M.; Conen, D.; Buring, J.E.; Moorthy, M.; Lee, I.; Albert, C.M. Physical activity and the risk of incident atrial fibrillation in women. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 321–327. [Google Scholar] [CrossRef]
- Proietti, R.; Birnie, D.; Ziegler, P.D.; Wells, G.A.; Verma, A. Postablation Atrial Fibrillation Burden and Patient Activity Level: Insights from the DISCERN AF Study. J. Am. Heart Assoc. 2018, 7, e010256. [Google Scholar] [CrossRef]
- Bittman, J.; Thomson, C.J.; Lyall, L.A.; Alexis, S.L.; Lyall, E.T.; Cannatella, S.L.; Ebtia, M.; Fritz, A.; Freedman, B.K.; Alizadeh-Pasdar, N.; et al. Effect of an Exercise and Nutrition Program on Quality of Life in Patients with Atrial Fibrillation: The Atrial Fibrillation Lifestyle Project (ALP). CJC Open 2022, 4, 685–694. [Google Scholar] [CrossRef]
- Joensen, A.M.; Dinesen, P.; Svendsen, L.; Hoejbjerg, T.; Fjerbaek, A.; Andreasen, J.; Sottrup, M.; Lundbye-Christensen, S.; Vadmann, H.; Riahi, S. Effect of patient education and physical training on quality of life and physical exercise capacity in patients with paroxysmal or persistent atrial fibrillation: A randomized study. J. Rehabil. Med. 2019, 51, 442–450. [Google Scholar] [CrossRef]
- Osbak, P.; Mourier, M.; Henriksen, J.; Kofoed, K.; Jensen, G. Effect of physical exercise training on muscle strength and body composition, and their association with functional capacity and quality of life in patients with atrial fibrillation: A randomized controlled trial. J. Rehabil. Med. 2012, 44, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Hegbom, F.; Stavem, K.; Sire, S.; Heldal, M.; Orning, O.M.; Gjesdal, K. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int. J. Cardiol. 2007, 116, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.J.R.; Harrison, S.L.; Fazio-Eynullayeva, E.; Underhill, P.; Lane, D.A.; Thijssen, D.H.J.; Lip, G.Y.H. Exercise-Based Cardiac Rehabilitation and All-Cause Mortality among Patients with Atrial Fibrillation. J. Am. Heart Assoc. 2021, 10, e020804. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.J.; Lip, G.Y.; Thijssen, D.H. The counterintuitive role of exercise in the prevention and cause of atrial fibrillation. Am. J. Physiol. Circ. Physiol. 2020, 319, H1051–H1058. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, H.; Cooper, R.; George, K.P.; Augustine, D.X.; Malhotra, A.; Paton, M.F.; Robinson, S.; Oxborough, D. The athlete’s heart: Insights from echocardiography. Echo Res. Pract. 2023, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Mascia, G.; Olivotto, I.; Brugada, J.; Arbelo, E.; Di Donna, P.; Della Bona, R.; Canepa, M.; Porto, I. Sport practice in hypertrophic cardiomyopathy: Running to stand still? Int. J. Cardiol. 2021, 345, 77–82. [Google Scholar] [CrossRef]
- Martinez, M.W.; Kim, J.H.; Shah, A.B.; Phelan, D.; Emery, M.S.; Wasfy, M.M.; Fernandez, A.B.; Bunch, T.J.; Dean, P.; Danielian, A.; et al. Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1453–1470. [Google Scholar] [CrossRef]
- Newman, W.; Parry-Williams, G.; Wiles, J.; Edwards, J.; Hulbert, S.; Kipourou, K.; Papadakis, M.; Sharma, R.; O’Driscoll, J. Risk of atrial fibrillation in athletes: A systematic review and meta-analysis. Br. J. Sports Med. 2021, 55, 1233–1238. [Google Scholar] [CrossRef]
- Elliott, A.D.; Linz, D.; Mishima, R.; Kadhim, K.; Gallagher, C.; E Middeldorp, M.; Verdicchio, C.V.; Hendriks, J.M.L.; Lau, D.H.; La Gerche, A.; et al. Association between physical activity and risk of incident arrhythmias in 402,406 individuals: Evidence from the UK Biobank cohort. Eur. Heart J. 2020, 41, 1479–1486. [Google Scholar] [CrossRef]
- Wilhelm, M.; Roten, L.; Tanner, H.; Wilhelm, I.; Schmid, J.-P.; Saner, H. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am. J. Cardiol. 2011, 108, 580–585. [Google Scholar] [CrossRef]
- Wilhelm, M.; Roten, L.; Tanner, H.; Schmid, J.-P.; Wilhelm, I.; Saner, H. Long-term cardiac remodeling and arrhythmias in nonelite marathon runners. Am. J. Cardiol. 2012, 110, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Nuoffer, J.-M.; Schmid, J.-P.; Wilhelm, I.; Saner, H. Comparison of pro-atrial natriuretic peptide and atrial remodeling in marathon versus non-marathon runners. Am. J. Cardiol. 2012, 109, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.R. Atrial fibrillation in athletes: Implicit literature-based connections suggest that overtraining and subsequent inflammation may be a contributory mechanism. Med. Hypotheses 2006, 66, 1085–1092. [Google Scholar] [CrossRef]
- Sanchis, L.; La Garza, M.S.-D.; Bijnens, B.; Giraldeau, G.; Grazioli, G.; Marin, J.; Gabrielli, L.; Montserrat, S.; Sitges, M. Gender influence on the adaptation of atrial performance to training. Eur. J. Sport Sci. 2017, 17, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Bayles, M.P. ACSM’s Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2023. [Google Scholar]
- Proietti, M.; Laroche, C.; Nieuwlaat, R.; Crijns, H.J.; Maggioni, A.P.; Lane, D.A.; Boriani, G.; Lip, G.Y. Increased burden of comorbidities and risk of cardiovascular death in atrial fibrillation patients in Europe over ten years: A comparison between EORP-AF pilot and EHS-AF registries. Eur. J. Intern. Med. 2018, 55, 28–34. [Google Scholar] [CrossRef]
- Frost, L.; Vestergaard, P.; Mosekilde, L. Hyperthyroidism and risk of atrial fibrillation or flutter: A population-based study. Arch. Intern. Med. 2004, 164, 1675–1678. [Google Scholar] [CrossRef]
- Vlachos, K.; Mascia, G.; Martin, C.A.; Bazoukis, G.; Frontera, A.; Cheniti, G.; Letsas, K.P.; Efremidis, M.; Georgopoulos, S.; Gkalapis, C.; et al. Atrial fibrillation in Brugada syndrome: Current perspectives. J. Cardiovasc. Electrophysiol. 2020, 31, 975–984. [Google Scholar] [CrossRef]
- Platonov, P.G.; McNitt, S.; Polonsky, B.; Rosero, S.Z.; Zareba, W. Atrial fibrillation in long QT syndrome by genotype. Circ. Arrhythmia Electrophysiol. 2019, 12, e007213. [Google Scholar] [CrossRef]
- Leo, D.G.; Ozdemir, H.; Lane, D.A.; Lip, G.Y.H.; Keller, S.S.; Proietti, R. At the heart of the matter: How mental stress and negative emotions affect atrial fibrillation. Front. Cardiovasc. Med. 2023, 10, 1171647. [Google Scholar] [CrossRef]
- Severino, P.; Mariani, M.V.; Maraone, A.; Piro, A.; Ceccacci, A.; Tarsitani, L.; Maestrini, V.; Mancone, M.; Lavalle, C.; Pasquini, M.; et al. Triggers for atrial fibrillation: The role of anxiety. Cardiol. Res. Pract. 2019, 2019, 1208505. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, A.; Ising, M.; Unschuld, P.G.; Kern, N.; Lucae, S.; Pütz, B.; Uhr, M.; Binder, E.B.; Holsboer, F.; E Keck, M. Regulation of the hypothalamic–pituitary–adrenocortical system in patients with panic disorder. Neuropsychopharmacology 2006, 31, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.W.; Fisher, A.J.; Newman, M.G.; Granger, D.A. Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder. Psychophysiology 2016, 53, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Middeldorp, M.E.; Ariyaratnam, J.; Lau, D.; Sanders, P. Lifestyle modifications for treatment of atrial fibrillation. Heart 2020, 106, 325–332. [Google Scholar] [CrossRef]
- Chung, M.K.; Eckhardt, L.L.; Chen, L.Y.; Ahmed, H.M.; Gopinathannair, R.; Joglar, J.A.; Noseworthy, P.A.; Pack, Q.R.; Sanders, P.; Trulock, K.M.; et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: A scientific statement from the American Heart Association. Circulation 2020, 141, E750–E772. [Google Scholar] [CrossRef]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Koziel, M.; Yang, P.-S.; Guo, Y.; et al. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: Impact on clinical outcomes—A systematic review and meta-analysis of 285,000 patients. Thromb. Haemost. 2022, 122, 406–414. [Google Scholar] [CrossRef]
- Nelson, G.A.; McNaught-Mitchell, M.P.; Roopchand-Martin, S.D.; Gordon, C.M. Wii Fit plus exercise training for persons with cardiac disease. Cardiopulm. Phys. Ther. J. 2015, 26, 73–77. [Google Scholar] [CrossRef]
- Klompstra, L.; Jaarsma, T.; Strömberg, A. Exergaming to increase the exercise capacity and daily physical activity in heart failure patients: A pilot study. BMC Geriatr. 2014, 14, 119. [Google Scholar]
- García-Bravo, S.; Cano-De-La-Cuerda, R.; Domínguez-Paniagua, J.; Campuzano-Ruiz, R.; Barreñada-Copete, E.; López-Navas, M.J.; Araujo-Narváez, A.; García-Bravo, C.; Florez-Garcia, M.; Botas-Rodríguez, J.; et al. Effects of Virtual Reality on Cardiac Rehabilitation Programs for Ischemic Heart Disease: A Randomized Pilot Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 8472. [Google Scholar] [CrossRef]
- Vieira, Á.; Melo, C.; Machado, J.; Gabriel, J. Virtual reality exercise on a home-based phase III cardiac rehabilitation program, effect on executive function, quality of life and depression, anxiety and stress: A randomized controlled trial. Disabil. Rehabil. Assist. Technol. 2018, 13, 112–123. [Google Scholar] [CrossRef]
- Jaarsma, T.; Klompstra, L.; Ben Gal, T.; Ben Avraham, B.; Boyne, J.; Bäck, M.; Chialà, O.; Dickstein, K.; Evangelista, L.; Hagenow, A.; et al. Effects of exergaming on exercise capacity in patients with heart failure: Results of an international multicentre randomized controlled trial. Eur. J. Heart Fail. 2021, 23, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Fuschillo, S.; Papa, A.; Di Minno, M.N.D.; Maniscalco, M. Exergaming as a supportive tool for home-based rehabilitation in the COVID-19 pandemic era. Games Health J. 2020, 9, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Shields, G.E.; Wells, A.; Doherty, P.; Heagerty, A.; Buck, D.; Davies, L.M. Cost-effectiveness of cardiac rehabilitation: A systematic review. Heart 2018, 104, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Chindhy, S.; Taub, P.R.; Lavie, C.J.; Shen, J. Current challenges in cardiac rehabilitation: Strategies to overcome social factors and attendance barriers. Expert Rev. Cardiovasc. Ther. 2020, 18, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, M.; Batalik, L.; Antoniou, V.; Pepera, G. Safety of home-based cardiac rehabilitation: A systematic review. Heart Lung 2022, 55, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Yang, S. Defining exergames & exergaming. Proc. Meaningful Play. 2010, 2010, 21–23. [Google Scholar]
- Beach, C.; Montoye, A.H.; Steeves, J.A. Differences in physical activity during walking and two Pokémon Go playing Styles. Games Health J. 2021, 10, 130–138. [Google Scholar]
- Shameli, A.; Althoff, T.; Saberi, A.; Leskovec, J. How gamification affects physical activity: Large-scale analysis of walking challenges in a mobile application. In Proceedings of the 26th International Conference on World Wide Web Companion, Perth, Australia, 3–7 April 2017. [Google Scholar]
- Agmon, M.; Perry, C.K.; Phelan, E.; Demiris, G.; Nguyen, H.Q. A pilot study of Wii Fit exergames to improve balance in older adults. J. Geriatr. Phys. Ther. 2011, 34, 161–167. [Google Scholar] [CrossRef]
- Höchsmann, C.; Walz, S.P.; Schäfer, J.; Holopainen, J.; Hanssen, H.; Schmidt-Trucksäss, A. Mobile Exergaming for Health—Effects of a serious game application for smartphones on physical activity and exercise adherence in type 2 diabetes mellitus—study protocol for a randomized controlled trial. Trials 2017, 18, 103. [Google Scholar] [CrossRef]
- Donath, L.; Rössler, R.; Faude, O. Effects of virtual reality training (exergaming) compared to alternative exercise training and passive control on standing balance and functional mobility in healthy community-dwelling seniors: A meta-analytical review. Sports Med. 2016, 46, 1293–1309. [Google Scholar] [CrossRef]
- Sato, K.; Kuroki, K.; Saiki, S.; Nagatomi, R. Improving walking, muscle strength, and balance in the elderly with an exergame using Kinect: A randomized controlled trial. Games Health J. 2015, 4, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez i Badia, S.; Avelino, J.; Bernardino, A.; Cameirao, M.S.; Munoz, J.E.; Cardoso, H.; Gonccalves, A.; Paulino, T.; Ribeiro, R.; Simao, H.; et al. Development and Validation of a Mixed Reality Exergaming Platform for Fitness Training of Older Adults, in Everyday Virtual and Augmented Reality; Springer: Berlin/Heidelberg, Germany, 2023; pp. 119–145. [Google Scholar]
- Taylor, J.L.; Bonikowske, A.R.; Olson, T.P. Optimizing outcomes in cardiac rehabilitation: The importance of exercise intensity. Front. Cardiovasc. Med. 2021, 8, 734278. [Google Scholar] [PubMed]
- Vanhees, L.; Hansen, D. Modalities of Exercise Training in Cardiac Rehabilitation. In Textbook of Sports and Exercise Cardiology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 881–896. [Google Scholar]
- Cacciata, M.C.; Stromberg, A.; Klompstra, L.; Jaarsma, T.; Kuriakose, M.; Lee, J.-A.; Lombardo, D.; Evangelista, L.S. Facilitators and challenges to exergaming: Perspectives of patients with heart failure. J. Cardiovasc. Nurs. 2022, 37, 281. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable sensors for remote health monitoring. Sensors 2017, 17, 130. [Google Scholar]
- Kelly, J.T.; Campbell, K.L.; Gong, E.; Scuffham, P. The Internet of Things: Impact and implications for health care delivery. J. Med. Internet Res. 2020, 22, e20135. [Google Scholar] [CrossRef]
- Epstein, E.; Patel, N.; Maysent, K.; Taub, P.R. Cardiac rehab in the COVID era and beyond: mHealth and other novel opportunities. Curr. Cardiol. Rep. 2021, 23, 42. [Google Scholar] [CrossRef]
| Study ID, Year, Country | Design, Study Population | Intervention | Outcome(s) | Results | Conclusion |
|---|---|---|---|---|---|
| [20] Bittman, 2022, Canada | RCT; Non-permanent, nonvalvular AF; Intervention n = 34 (mean age 63.7 ± 8.6 years, female n = 11); Control n = 38 (mean age 61.0 ± 9.7 years, female n = 17). | Nutritional plan and exercise program with 200 min/week (month 1 to 6) plus 2 weekly sessions of supervised cardiac rehabilitation (month 4 to 6) | QoL (SF-36) | QoL: [Intervention (I), Control (C), mean ± SD—vitality I: 13.2 ± 20.4; C: 1.0 ± 14.9, p < 0.001; social functioning I: 14.7 ± 24.1; C: 2.4 ± 21.2, p = 0.018; emotional well-being I: 5.5 ± 14.1; C: −1.0 ± 13.3, p = 0.017; and general health perceptions I: 8.1 ± 12.3; C: 2.7 ± 13.3, p = 0.009]. | (+) exercise training improved QoL in AF patients. |
| [23] Hegbom, 2007, Norway | RCT; Chronic AF; Intervention n = 13 (mean age 62 ± 7, female n = 13); Control n = 15 (mean age 65 ± 7, female n = 13). | 24 training sessions (1.25 h × 3 days/week) of aerobic exercise and muscular strengthening | QoL (SF-36); exercise capacity (Borg Scale 17) | QoL: [mean score ± SD, physical functioning pre 82 ± 14 vs. 86 ± 10 post, p = 0.01; bodily pain pre 82 ± 17 vs. post 92 ± 14, p = 0.01; vitality pre 61 ± 14 vs. 68 ± 13 post, p = 0.01; and role-emotional pre 85 ± 28 vs. post 94 ± 20, p = 0.01]. Exercise capacity: increased by 41% (±36%) | (+) exercise training improved QoL and exercise capacity in AF patients. |
| [21] Joensen, 2019, Denmark | RCT; Paroxysmal or persistent AF; Intervention n = 28 (mean age 62.2 ± 10.0, female n = 11); Control n = 24 (mean age 60.2 ± 8.9, female n = 7). | 6-month exercise intervention consisting of two weekly sessions of supervised cardiac rehabilitation (with at least 30 min of aerobic exercise at ≥70% of maximum exercise capacity). | QoL (AF-QoL-18; exercise capacity (ergometer cycle test) | QoL: [Intervention (I), Comparator (C), mean ± SD—I: baseline 48.4 ± 22.8 to 6 months 68.0 ± 15.2, vs. C: baseline 51.6 ± 22.3 to 6 months 59.2 ± 27.3, p = 0.031]. Exercise capacity: [Intervention: mean ± SD, 176 ± 48 pre vs. 190 ± 55 at 6 months, p = 0.026] | (+) exercise training improved QoL and exercise capacity in AF patients. |
| [22] Osbak, 2012, Denmark | RCT; Permanent AF; Intervention n = 24 (mean age 69.5 7.3, female n = 6); Control n = 23 (mean age 70.9± 8.3, female n = 6). | 12-week exercise intervention (1 h/3 times per week of supervised training) | QoL (SF-36; MLHF-Q); exercise capacity (6MWT) | QoL: [mean score ± SD, SF-36: physical functioning pre 82 ± 14 vs. 86 ± 10 post, p = 0.01; bodily pain pre 82 ± 17 vs. post 92 ± 14, p = 0.01; vitality pre 61 ± 14 vs. 68 ± 13 post, p = 0.01; role-emotional pre 85 ± 28 vs. post 94 ± 20, p = 0.01; and MLHF-Q: p = 0.03]. Exercise capacity: [mean score(meters), SD, Intervention (504.4 ± 85.1 pre vs. 569.9 ± 92.6 post) vs. control (453.1 ± 100.1 pre vs. 454.1 ± 95.7 post), p = 0.001]. intervention decreased patients’ resting pulse [mean ± SD: 94.8 ± 22.4 to 86.3 ± 22.5 beats/min, p = 0.049]. | (+) exercise training improved QoL and exercise capacity and decreased resting heart rate in AF patients. |
| [24] Buckley, 2021, UK | Retrospective study on international database | N/A | Mortality | Mortality: 68% lower odds of all-cause mortality [odds ratio: 0.32; 95% CI: 0.29–0.35]. | (+) exercise training reduced the mortality rate in AF patients. |
| Study ID, Year, Country | Design, Study Population | Intervention | Outcome(s) | Results | Conclusion |
|---|---|---|---|---|---|
| [50] Garcia-Bravo, 2020, Spain | RCT; Ischemic heart disease; Intervention n = 10 (mean age 48.7 ± 6.66, gender not reported); Control n = 10 (mean age 53.7 ± 10.3, gender not reported). | 8 weeks of exergame consisting of 2 × 60 min/week aerobic sessions using the Microsoft XBOX with the Kinect sensor | Exercise capacity (6MWT); QoL (SF-36); depression level (Beck-II depression inventory) | Exercise capacity: [mean ± SD, distance: 457.80 ± 132.00 pre vs. 513.00 ± 117.00 post, p = 0.005. Quality of life and level of depression: SF-36 general health: p = 0.049, SF-36 social function: p = 0.010, Beck-II depression inventory: p = 0.012]. | (+) exergame improved exercise capacity and quality of life and reduced the level of depression. |
| [52] Jaarsma, 2021, Sweden, Italy, Israel, the Netherlands, Germany and the USA | International Multicentre RCT; Heart failure; Intervention n = 305 (mean age 66 ± 12, female n = 85); Control n = 300 (mean age 67 ± 11, female n = 90). | 12-month, 5 × 30 min weekly sessions with the Nintendo Wii Sports software | Exercise capacity (6MWT); self-reported PA level; patients outcome measures | No statistically significant differences between groups [p > 0.05]. | (=) exergame did not show statistically significant effects compared to traditional exercise. |
| [49] Klompstra, 2014, Sweden | Pilot study; Heart Failure; n = 32 (mean age 63 ± 14, female n = 10); | 12-week, 20 min × day session using the Nintendo Wii Sports | Exercise capacity (6MWT) | Exercise capacity: [mean ± SD: 501 ± 95 m pre vs. 521 ± 101 m post, p < 0.05]. | (+) exergame improved exercise capacity. |
| [48] Nelson, 2014, Jamaica | Single group pre-post test; Cardiac disease; n = 28 (mean age 62.1 ± 11.4, female n = 15). | 6 weeks consisting of 3 × 40 min/week training sessions with the Nintendo Wii Fit Plus software | Exercise capacity (6MWT) | Exercise capacity: [mean ± SD, from 461.93 m (SD 5 105.87) pre to 498.22 m (SD 5 132.95) post, p < 0.001]. | (+) exergame improved exercise capacity. |
| [51] Vieira, 2018, Portugal | RCT; Patients who completed phase II cardiac rehab; Exergame n = 11 (mean age 55 9.0, gender not reported); Booklet group n = 11 (mean age 59 11.3, gender not reported); Control group n = 11 (mean age 59 5.8, gender not reported). | 6-month, 3 × 60–90 min weekly session using the Microsoft XBOX Kinect | QoL (MacNew questionnaire); depression, anxiety, and stress (Depression, Anxiety, and Stress Scale 21) | No statistically significant differences between groups [p > 0.05] | (=) exergame did not show statistically significant effects compared to control (traditional exercise; usual care). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leo, D.G.; Proietti, R. A New Player in the Game: Can Exergame Be of Support in the Management of Atrial Fibrillation? Medicina 2024, 60, 172. https://doi.org/10.3390/medicina60010172
Leo DG, Proietti R. A New Player in the Game: Can Exergame Be of Support in the Management of Atrial Fibrillation? Medicina. 2024; 60(1):172. https://doi.org/10.3390/medicina60010172
Chicago/Turabian StyleLeo, Donato Giuseppe, and Riccardo Proietti. 2024. "A New Player in the Game: Can Exergame Be of Support in the Management of Atrial Fibrillation?" Medicina 60, no. 1: 172. https://doi.org/10.3390/medicina60010172
APA StyleLeo, D. G., & Proietti, R. (2024). A New Player in the Game: Can Exergame Be of Support in the Management of Atrial Fibrillation? Medicina, 60(1), 172. https://doi.org/10.3390/medicina60010172