Abstract
Background: Although bleeding from gastric varices is less observed than esophageal variceal bleeding (VB) (25% vs. 64%), it is associated with an exceedingly high mortality rate of up to 45%. Current guidelines suggest that endoscopic cyanoacrylate injection therapy (ECI) is the first-line treatment for gastric variceal bleeding (GVB). A major concern, however, is the possibility of embolic incidents, which are clinically evident in approximately 1% of cases. There are no guidelines for secondary prophylaxis of GVB. Radiological treatments using a transjugular intrahepatic portosystemic shunt (TIPS) or balloon occlusive transvenous obliteration (BRTO) are considered viable. However, they are not universally inapplicable; for instance, in the setting of pulmonary hypertension (TIPS). EUS-guided combined injection therapy (EUS-CIT) (embolization coils + cyanoacrylate) is an emerging procedure with a perceived reduced risk of systemic embolization. Case presentation: A patient with alcoholic liver cirrhosis was subjected to EUS-CIT as a secondary prophylaxis for GVB. He had three VB episodes of prior presentation treated by endoscopic band ligation (EBL) and ECI. Due to recurrent episodes of bleeding, he was referred to TIPS, but was considered contraindicated due to severe pulmonary hypertension. EUS-CIT was conducted with two embolization coils inserted into the varix, followed by an injection of 1.5 mL of cyanoacrylate glue. A 19 Ga needle, 0.035″ 14/70 mm coils, non-diluted n-butyl-caynoacrylate, and a transgastric approach were utilized. There were no immediate complications. Complete obliteration of the GV was observed in a follow-up endoscopy on day 30. Subsequent endoscopies in months three and six showed no progression of gastric varices. Conclusions: Our initial experience with EUS-CIT suggests that it can be successfully used as secondary prophylaxis for recurrent GVB.
1. Introduction
Variceal bleeding (VB) is a common complication of liver cirrhosis with an annual incidence of 8–10% [1]. In long-term follow-ups, VB occurs in about 40% of compensated liver cirrhosis and up to 85% of decompensated liver cirrhosis patients [2]. While recently published guidelines and research indicate a gradual transition to non-invasive modalities for evaluating various gastrointestinal disorders, including gastro-oesophageal varices (GOV), endoscopy is still the cornerstone in managing VB [3,4,5,6].
Gastric varices (GV) represent a complex collection of vascular shunts between the porto-splenic venous system and the systemic veins of the abdomen and thorax. GV is uncommon (17–24% in patients with portal hypertension) compared to esophageal varices (EVs) (up to 85%). Sarin et al. established that bleeding from GV is rarer than EVs (25% vs. 64%, respectively) [7]. However, gastric variceal bleeding (GVB) is more severe and associated with higher mortality (45% vs. 20%, respectively) [7]. Sarin’s classification of gastric varices is the most commonly used system (Table 1).

Table 1.
Sarin’s classification of gastric varices.
Recent guidelines suggest that in GOV type 1, endoscopic band ligation (EBL) or endoscopic cyanoacrylate injection (ECI) are viable options [8,9,10]. In GOV type II and IGV type I, ECI is the preferred treatment modality [8,9,10].
The major drawback of ECI therapy is the potential for fatal adverse events (AEs). ECI complication rates range from 7% to as high as 20% [11]. The risk of glue detachment and subsequent embolism is a source of grave concern and manifests clinically in about 1% of cases, likely occurring in a significantly higher proportion of patients.
Endoscopic ultrasound (EUS) has become increasingly popular over the last two decades for treating various pancreatico-biliary disorders [12,13]. Until recently, its usage for vascular endotherapy had been overlooked. To reduce the incidence of embolic complications associated with glue injection therapy, Binmoeller et al. introduced an EUS-guided management of gastric varices combining coil and cyanoacrylate injections [14]. While still in its infancy and not universally adopted, initial studies have shown improved clinical outcomes compared to conventional ECI in terms of AEs (10% vs. 20%, systemic embolism being exceedingly rare), rebleeding rate (0% vs. 38%), and the need for reintervention (10% vs. 60%) [11].
According to existing guidelines, there is a lack of high-quality data to suggest optimal therapeutic approaches for secondary prophylaxis of GVB. It is noted that transjugular intrahepatic portosystemic shunt (TIPS) and balloon occlusive retrograde transvenous obliteration (BRTO) probably perform similarly in cases of refractory bleeding [9].
In the current paper, we present a case of EUS-guided combined injection therapy (EUS-CIT) (coils + n-butyl-cyanoacrylate) for the secondary prophylaxis of gastric variceal bleeding in a patient with three previous bleeding episodes treated with EBL and ECI and was ineligible for TIPS due to severe pulmonary hypertension.
2. Case Description
The patient was a 65-year-old male with alcoholic cirrhosis. The diagnosis was established 4 years prior to the current presentation, and he abstained from alcohol consumption thereafter. Comorbidities included arterial hypertension, established 15 years prior to the initial diagnosis of liver cirrhosis for which he received regular therapy with angiotensin receptor blockers (Telmisartan 10 mg/day). In the past year, he experienced three episodes of variceal bleeding. Initially, he underwent an episode of hematemesis. Urgent upper endoscopy within six hours of symptoms onset revealed esophageal and variceal bleeding. EBL immediately controlled the bleeding. Two units of blood were transfused and the patient was discharged on post-procedure day four. No gastric varices were described at that point. An endoscopic follow-up was recommended within 20 days, but he refused. Non-selective beta-blockers (Propranolol 20 mg twice a day) were prescribed.
The patient was admitted again for hematemesis and melena 45 days later. Gastroscopy found no bleeding in the esophagus, but upon stomach examination, a type II GOV with a tortuous course and verices of about 2 cm was established. The bleeding spot was visualized. ECI was performed with 2 mL of N-butyl cyanocrylate injected under endoscopic guidance. The bleeding was immediately controlled. A transfusion of three units of blood was necessary to achieve a hemoglobin level of 93 g/L. He was discharged without any therapy modifications.
About two months later, the patient underwent another episode of upper gastrointestinal bleeding. Although I upper endoscopy revealed no signs of active bleeding, the presence of fresh blood in the stomach and a red spot on one of the fundal varices was observed. Endoscopic treatment was not commenced. Considering the refractory character of the bleeding, the patient’s eligibility for interventional treatment through TIPS was decided and he was referred to a tertiary center.
Pre-procedural echocardiography, however, showed severe pulmonary hypertension with a mean pulmonary artery pressure of >45 mmHg. Thus, he was considered contraindicated for TIPS. He was prescribed Tadalafil 10 mg twice daily and referred for reevaluation after 45 days. Follow-up echocardiography failed to establish any sonographic signs of improvement. At this point, he was referred to our center to discuss alternative therapeutic approaches.
2.1. Pre-Procedure Evaluation
At admission, the patient had not experienced any episodes of overt gastrointestinal bleeding in the last two months. Ultrasonography established typical cirrhotic transformation of the liver parenchyma (enlarged liver—craniocaudal diameter up to 200 mm, irregular contours, heterogenous structure, lobe disproportion) and an enlarged spleen (160/80 mm). Portal vein evaluation showed mild dilation up to 12 mm. Doppler ultrasound assessment was not performed due to a perceived low correlation with severe portal hypertension. There were only mild ascites established sonographically. Lab tests showed Hb—93 g/L, platelets—38 000 mm3, albumin—29 g/L, bilirubin—36.2 µmol/L, and an international normalized ratio (INR) of 1.36. There was no alteration in electrolytes or creatinine levels. Based on the clinical and lab findings, the patient was classified as class B according to the Child–Turcotte–Pugh classification (see Appendix A, Table A1) and had a model of end-stage liver disease (MELD-Na) score of 13.
An upper endoscopy was performed, which established grade II esophageal varices (Baveno classification, see Appendix A, Table A2) and large, type I GOV, tortuous varices measuring about 1.5 cm with red spots. Based on these endoscopic findings, the patient was considered at high risk of recurrent bleeding. Considering concomitant pulmonary hypertension, an increased risk of potentially fatal embolic AEs was perceived. It was then decided that EUS-CIT (coil + cyanoacrylate) might be safer than conventional ECI for avoiding thromboembolism.
A planned intervention was thoroughly explained to the patient, with all possible outcomes, AEs, and viable alternatives diligently described. Written and oral informed consent was obtained before the procedure.
The impaired coagulation was concerning so an adequate correction was planned. A total of 500 mL of fresh frozen plasma was transfused the night before the procedure. A total of 12 units of thrombocyte concentrate were also transfused immediately before and during the intervention. Prophylactic antibiotic treatment with 2 g of Ceftriaxone/d intravenously was initiated. A proton pump inhibitor was not prescribed. The patient was advised to continue his routine therapy (Telmisartan, Tadalafil, Propranolol).
2.2. Technique Description
This procedure was executed under general anesthesia with tracheal intubation using a combination of fentanyl, midazolam, sevoflurane, suxamethonium (Lysthenon), atracurium besylate (Tracrium), and propofol.
The patient was placed in a left lateral position. A curvilinear echoendoscope (Olympus GF-UCT180, Olympus, Hamburg, Germany), combined with a Hitachi-Aloka ProSound Alpha 7 (Hitachi, Tokyo, Japan) ultrasound device, was introduced and positioned at the level of the cardia. Insufflation with CO2 was used instead of ambient air (Olympus UCR, Hamburg, Germany). A total of 200 mL of 0.9% sodium chloride was installed in the stomach to improve acoustic coupling. Once at the level of the cardia, the procedure was performed under sonographic guidance. Gastric varices were visualized sonographically, and a Doppler ultrasound was performed to confirm the presence of blood flow. The widest diameter of the varix was 10 mm. We aimed to embolize the vessels located under the mucosa and not the perforated vessel (despite being easily identified) due to the perceived risk of embolization. Although a transcrural approach was intended, the position of the endoscope was inadequate, so a transgastric puncture was attempted.
Once an adequate position was achieved sonographically, the varix was punctured with a 19 Ga fine needle aspiration (FNA) needle (ExpectTM needle; Boston Scientific, Marlborough, MA, USA). As anticipated, a transgastric approach was associated with a more angulated position of the tip of the endoscope, leading to a more difficult puncture of the target vessel and insertion of the coils. Blood was aspirated to verify an adequate position, followed by rinsing the needle with 4 mL of 5% glucose solution. Next, two 0.035 inch 14 mm/7 cm embolization coils (Nester Embolization coil; Cook Medical, Bloomington, IN, USA) were introduced into the varix. The technique consisted of inserting the coils into the FNA needle, and then pushing them out into the varix using a needle stylet. The procedure was executed under combined ultrasound and fluoroscopic guidance using Philips BV Pulsera C-arm (Philips, Best, The Netherlands). Although primarily used as a scaffold for subsequent glue injection, an immediate reduction of blood flow in the varix was observed upon coil deployment.
Following coil insertion, another aspiration of blood and rinsing with glucose solution were executed to reconfirm the position prior to glue injection. Eventually, 1.5 mL of pure (not mixed with Lipiodol) N-2-butyl cyanoacrylate was injected into the varix. To compensate for the volume retained in the needle, the cyanoacrylate syringe was filled before application with 1 mL more cyanoacrylate than intended for injection. The needle was withdrawn from the vessel but not from the endoscope to minimize the risk of endoscope damage. The controlled Doppler examination showed that blood flow was almost absent from the target vessels.
At this point, the endoscopic reevaluation showed moderate amounts of blood in the fundus of the stomach, but no active bleeding. Gentle probing with a cannula was performed, with the varices established to have a hard consistency.
Figure 1, Figure 2 and Figure 3 present post-procedural endoscopic, endosonographic, and fluoroscopic findings.
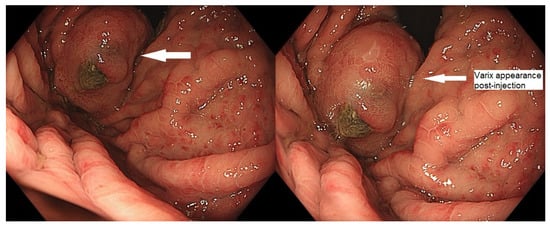
Figure 1.
Endoscopic appearance of gastric varix post-embolization.
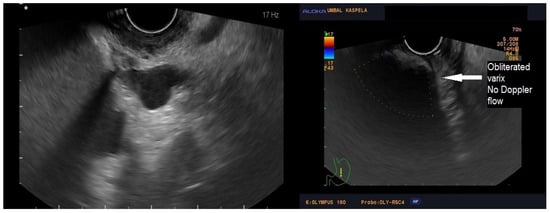
Figure 2.
Ultrasonography showing absent Doppler flow in the treated vessel.
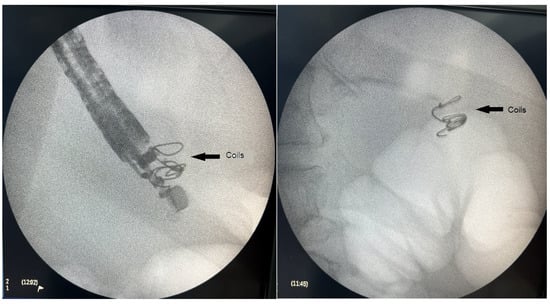
Figure 3.
Fluoroscopic image of the introduced embolization coils.
2.3. Post-Procedure
The post-procedure period was uneventful. The patient restored their fluid intake the same day, a semi-solid diet the day after, and was discharged on post-procedure day two. Lab tests showed only a minor decrease in hemoglobin to 86 g/L. No vasoactive medications or proton pump inhibitors were prescribed. An antibiotic prophylaxis was administered until discharge.
A follow-up endoscopy was performed 30 days after the intervention. The Doppler EUS showed no blood flow in the gastric varices. According to the existing guidelines, EBL (Super7; Boston Scientific, Marlborough, MA, USA) of EVs was electively performed to achieve eradication. Upper endoscopies were performed at 3 and 6 months without progression signs of GOV.
The patient has not experienced any bleeding episodes at nine months post-procedure.
A summary of the disease course is provided in Table 2.

Table 2.
Overview of liver disease course.
3. Discussion
The current case delineates some of the risk factors for GVB. Our patient experienced two prior episodes of GVB. The presence of red spots, decompensated liver cirrhosis, and the location and size (>10 mm) of the varices (GOV type II) confirmed the patient’s exceedingly high risk of recurrent hemorrhage. An interesting observation was the lack of gastric varices at the initial presentation. This finding correlates with Sarin et al.’s study in which GV develops in about 9% of patients following obliteration of EVs [7].
The current standard of care for GVB is ECI. Existing data suggest that ECI is superior to alternative methods of hemostasis (ethanol-based sclerotherapy or EBL) for the immediate control of bleeding (93.9% vs. 79.5%, p = 0.03) [15].
Glue embolism is associated with unfavorable clinical outcomes despite being rare. The largest study on the subject reported clinically manifested pulmonary embolism in 0.7% of cases [16]. The real incidence of glue emboli may be significantly higher, but the lack of prominent symptoms often hinders diagnosis.
In the presented case, ECI was the preferred first-line treatment according to existing guidelines. It outlines certain pitfalls in applying the procedure, particularly the inability to verify the presence of complete variceal occlusion. The anticipated risk of glue embolism generally leads to an insufficient volume of glue injection, resulting in incomplete variceal obliteration.
Currently, there are no clear recommendations on the optimal secondary prophylaxis for GVB. In general, TIPS and BRTO are considered viable options with comparable clinical outcomes [8]. TIPS is an effective modality for managing EVs. However, GV’s tendency to bleed at lower portal pressures may lead to a rebleeding rate as high as 50% after TIPS [17].
In the current paper, the patient had absolute contraindications for TIPS [18]. BRTO was not discussed due to a lack of expertise. Such a decision would probably be inappropriate considering the patient’s prior history of EV bleeding. We established that BRTO leads to EV progression in 30–35% of cases [19]. Alternative modalities for secondary prophylaxis were evaluated after the patient was considered ineligible for radiological interventions.
Applying EUS-CIT as a secondary prophylaxis for GVB was extensively investigated in a recent meta-analysis by Chandan et al. [20]. They established that EUS-CIT is highly effective for both primary and secondary prophylaxis of GVB, achieving 95.4% and 88.7% treatment efficiency, respectively. The pooled rate of GV obliteration was 87%, with an 11.9% complication rate. In only 7.7% of patients, salvage therapy (mainly TIPS) was needed. Comparing EIT and EUS-CIT, EUS-CIT had an improved rebleeding rate when compared to EIT.
The EUS-CIT technique includes the initial puncture of the gastric varices under endosonographic guidance with an FNA needle. In our report, we used a 19 Ga needle, which was used in most cases and described in the literature [11,14,21,22,23,24]. The rationale is that 19 Ga needles can accommodate 0.035″ embolization coils, which are the most common choice.
There are two modalities for variceal puncture reported in the literature. Binmoeller et al. used the so-called “transcrural” access to describe the technique. This approach includes puncturing the varix through the distal esophageal and crus of the diaphragm. Afterward, the “transgastric” approach was described, which consists of direct puncturing of the varix through an overlying stomach mucosa. Although the transgastric approach is associated with a higher risk of bleeding and technical difficulty, it may be preferable when there is a large EV present. Comparative studies failed to achieve significant differences between the clinical outcomes of the two techniques. In the current case, we used a transgastric approach to visualize the gastric vessels through the esophagus, which was inadequate.
Another aspect under debate is the optimal choice of target vessel. Romero-Castro et al. speculated that selecting perforated vessels would induce better GV occlusion while reducing the total glue volume needed and, hence, the risk of systemic embolization [25]. On the other hand, selecting a perforating vessel carries a risk of inadvertent injection into an efferent vessel or peri-gastric branch of the splenic vein, which has potentially disastrous consequences. To avoid these potential pitfalls, we decided to inject directly into the varix, an agreed-upon approach in more recent studies [14,23].
The standard approach to coil introduction involves positioning the puncture needle at a distance from the opposite wall of the vessel to reduce perforation risk [26]. The diameter of the coils should be 20–30% larger than the diameter of the vessel to preclude migration [26]. We followed those recommendations with the largest diameter of the varix being 10 mm. We also used two 14 mm/7 cm 0.035″ embolization coils.
Regarding the glue injection technique, there are certain discrepancies between different authors. Recent AGA guidelines warn against using plant-based oils in combination with cyanoacrylate due to increased embolization risk [8]. Binmoeller et al. used 2-octyl-cyanocrylate, which has a significantly longer polymerization time [14]. Based on existing guidelines and our own experience, we opted to inject “pure” N-butyl cyanoacrylate. Rinsing the needle properly is essential to avoid premature polymerization. We used glucose solution for rinsing based on our experience with ECI, suggesting that 5% glucose solution slightly delays the polymerization of cyanoacrylate.
After the procedure was completed, the needle was withdrawn from the varix, but not from the endoscope. The latter was still in the patient. Glue-induced damage from endoscopes might be irreversible, so every effort should be made to avoid it.
EBL was conducted during a follow-up endoscopy at week 4. These results align with existing guidelines for pursuing the complete eradication of EVs [9].
4. Conclusions
Our initial experience with EUS-CIT suggests that it is a viable alternative to ECI therapy after achieving excellent variceal occlusion and minimizing the risk of systemic embolization. It can be an effective secondary prophylaxis for recurrent GVB. EUS-CIT is a valid alternative for patients with contraindications for radiological interventions (TIPS and/or BRTO) and may be considered in the presence of adequate expertise.
Author Contributions
Conceptualization: K.A. and R.D.; methodology: K.A.; investigation: K.A.; resources: R.D.; data curation: M.K.; writing—original draft preparation: Y.B.-K.; writing—review and editing: M.K. and K.A.; visualization: Y.B.-K.; supervision: R.D.; project administration: Y.B.-K.; funding acquisition: M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University hospital “Kaspela” (protocol No. 49/23112023 from 23 November 2023) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study. Written informed consent was obtained from the patient to publish this paper.
Data Availability Statement
Data are available upon request due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| VB | variceal bleeding |
| GOV | gastro-esophageal varices |
| GV | gastric varices |
| EVs | esophageal varices |
| GVB | gastric variceal bleeding |
| IGV | isolated gastric varices |
| EBL | endoscopic band ligation |
| ECI | endoscopic cyanoacrylate injection |
| AEs | adverse events |
| EUS | endoscopic ultrasound |
| TIPS | transjugular intrahepatic portosystemic shunt |
| BRTO | balloon occlusive retrograde transvenous obliteration |
| EUS-CIT | endoscopic ultrasound combined with injection therapy |
| INR | international normalized ratio |
| FNA | fine needle aspiration |
Appendix A

Table A1.
The Child–Turcotte–Pugh (CTP) classification of liver cirrhosis [27].
Table A1.
The Child–Turcotte–Pugh (CTP) classification of liver cirrhosis [27].
| Parameter | Points Assigned | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Ascites | Absent | Mild to moderate (diuretic responsiveness) | Severe (diuretic refractory) |
| Hepatic encephalopaty | None | Grades 1–2 | Grades 3–4 |
| Bilirubin (µmol/L) | <34.2 | 34.2–51.3 | >51.3 |
| INR | <1.7 | 1.7–2.3 | >2.3 |
| Albumin (g/L) | >35 | 28–35 | <28 |
CPT classification; Child A—5–6 points—well-compensated; Child B—7–9 points—significant functional compromise; Child C—10–15 points—decompensated.

Table A2.
Baveno classification of esophageal varices [3].
Table A2.
Baveno classification of esophageal varices [3].
| Size | Description |
|---|---|
| Small | Minimally elevated varices above the esophageal mucosa surface |
| Medium | Tortuous varices occupying less than one third of the esophageal surface |
| Large | Varices occupying more than one-third of the esophageal surface |
References
- Alqahtani, S.A.; Jang, S. Pathophysiology and Management of Variceal Bleeding. Drugs 2021, 81, 647–667. [Google Scholar] [CrossRef]
- Baiges, A.; Hernández-Gea, V. Management of Liver Decompensation in Advanced Chronic Liver Disease: Ascites, Hyponatremia, and Gastroesophageal Variceal Bleeding. Clin. Drug Investig. 2022, 42, 25–31. [Google Scholar] [CrossRef]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2021, 76, 959–974. [Google Scholar] [CrossRef]
- Hristov, B.; Andonov, V.; Doykov, D.; Doykova, K.; Valova, S.; Nacheva-Georgieva, E.; Uchikov, P.; Kostov, G.; Doykov, M.; Tilkian, E. Evaluation of Liver Stiffness Measurement by Means of 2D-SWE for the Diagnosis of Esophageal Varices. Diagnostics 2023, 13, 356. [Google Scholar] [CrossRef]
- Nacheva-Georgieva, E.L.; Doykov, D.I.; Hristov, B.K.; Doykova, K.A.; Doykov, M.I. Comparison of Point Shear Wave Elastography and 2-Dimensional Shear Wave Elastography Values of Liver Metastases from Colorectal Cancer. Gastroenterol. Insights 2023, 14, 271–281. [Google Scholar] [CrossRef]
- Hristov, B.; Andonov, V.; Doykov, D.; Tsvetkova, S.; Doykova, K.; Doykov, M. Evaluation of Ultrasound-Based Point Shear Wave Elastography for Differential Diagnosis of Pancreatic Diseases. Diagnostics 2022, 12, 841. [Google Scholar] [CrossRef]
- Sarin, S.K.; Lahoti, D.; Saxena, S.P.; Murthy, N.S.; Makwana, U.K. Prevalence, classification and natural history of gastric varices: A long-term follow-up study in 568 portal hypertension patients. Hepatology 1992, 16, 1343–1349. [Google Scholar] [CrossRef]
- Henry, Z.; Patel, K.; Patton, H.; Saad, W. AGA Clinical Practice Update on Management of Bleeding Gastric Varices: Expert Review. Clin. Gastroenterol. Hepatol. 2021, 19, 1098–1107.e1. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Duboc, M.C.; Garcia-Pagan, J.C.; Fuccio, L.; Karstensen, J.G.; Hucl, T.; Jovanovic, I.; Awadie, H.; Hernandez-Gea, V.; Tantau, M.; et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 1094–1120. [Google Scholar] [CrossRef]
- Tripathi, D.; Stanley, A.J.; Hayes, P.C.; Patch, D.; Millson, C.; Mehrzad, H.; Austin, A.; Ferguson, J.W.; Olliff, S.P.; Hudson, M.; et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015, 64, 1680–1704. [Google Scholar] [CrossRef]
- Bazarbashi, A.N.; Wang, T.J.; Jirapinyo, P.; Thompson, C.C.; Ryou, M. Endoscopic Ultrasound-Guided Coil Embolization With Absorbable Gelatin Sponge Appears Superior to Traditional Cyanoacrylate Injection for the Treatment of Gastric Varices. Clin. Transl. Gastroenterol. 2020, 11, e00175. [Google Scholar] [CrossRef]
- van der Merwe, S.W.; van Wanrooij, R.L.J.; Bronswijk, M.; Everett, S.; Lakhtakia, S.; Rimbas, M.; Hucl, T.; Kunda, R.; Badaoui, A.; Law, R.; et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 185–205. [Google Scholar] [CrossRef]
- Hristov, B.; Radev, D.; Uchikov, P.; Kostov, G.; Doykov, M.; Valova, S.; Tilkiyan, E. Clinical Outcomes of EUS-Guided Choledochoduodenostomy for Biliary Drainage in Unresectable Pancreatic Cancer: A Case Series. Medicina 2023, 59, 351. [Google Scholar] [CrossRef]
- Binmoeller, K.F.; Weilert, F.; Shah, J.N.; Kim, J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest. Endosc. 2011, 74, 1019–1025. [Google Scholar] [CrossRef]
- Qiao, W.; Ren, Y.; Bai, Y.; Liu, S.; Zhang, Q.; Zhi, F. Cyanoacrylate injection versus band ligation in the endoscopic management of acute gastric variceal bleeding: Meta-analysis of randomized, controlled studies based on the PRISMA statement. Medicine 2015, 94, e1725. [Google Scholar] [CrossRef]
- Cheng, L.F.; Wang, Z.Q.; Li, C.Z.; Lin, W.; Yeo, A.E.; Jin, B. Low incidence of complications from endoscopic gastric variceal obturation with butylcyanoacrylate. Clin. Gastroenterol. Hepatol. 2010, 8, 760–766. [Google Scholar] [CrossRef]
- Butler, J.R.; Eckert, G.J.; Zyromski, N.J.; Leonardi, M.J.; Lillemoe, K.D.; Howard, T.J. Natural history of pancreatitis-induced splenic vein thrombosis: A systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB 2011, 13, 839–845. [Google Scholar] [CrossRef]
- Manguso, G.; Vignone, A.; Merli, M.; Miotti, C.; Caputo, A.; Vizza, C.D.; Badagliacca, R. Hemodynamic Evaluation of the Right Heart-Pulmonary Circulation Unit in Patients Candidate to Transjugular Intrahepatic Portosystemic Shunt. J. Clin. Med. 2022, 11, 461. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, J.H.; Sinn, D.H.; Song, Y.B.; Gwak, G.-Y.; Choi, M.S.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Effect of balloon-occluded retrograde transvenous obliteration on the natural history of coexisting esophageal varices. J. Clin. Gastroenterol. 2008, 42, 974–979. [Google Scholar] [CrossRef]
- Chandan, S.; Nguyen, A.K.; Mohan, B.P.; Deliwala, S.; Ramai, D.; Kassab, L.L.; Muthusamy, A.; Facciorusso, A.; Kamal, F.; Bilal, M.; et al. EUS-guided therapies for primary and secondary prophylaxis in gastric varices-An updated systematic review and meta-analysis. Endosc. Ultrasound 2023, 12, 351–361. [Google Scholar] [CrossRef]
- Castellanos, E.R.; Seron, P.; Gisbert, J.P.; Cosp, X.B. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst. Rev. 2015, 5, CD010180. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, S.; Pawlak, K.; Błaszczyk, Ł.; Jagielski, M.; Wiechowska-Kozłowska, A. Endoscopic Ultrasound-Guided Treatment of Gastric Varices Using Coils and Cyanoacrylate Glue Injections: Results after 1 Year of Experience. J. Clin. Med. 2019, 8, 1786. [Google Scholar] [CrossRef]
- Kouanda, A.; Binmoeller, K.; Hamerski, C.; Nett, A.; Bernabe, J.; Shah, J.; Bhat, Y.; Watson, R. Safety and efficacy of EUS-guided coil and glue injection for the primary prophylaxis of gastric variceal hemorrhage. Gastrointest. Endosc. 2021, 94, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.M.; Weilert, F.; Fredrick, R.T.; Kane, S.D.; Shah, J.N.; Hamerski, C.M.; Binmoeller, K.F. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: A large U.S. experience over 6 years (with video). Gastrointest. Endosc. 2016, 83, 1164–1172. [Google Scholar] [CrossRef]
- Romero-Castro, R.; Pellicer-Bautista, F.J.; Jimenez-Saenz, M.; Marcos-Sanchez, F.; Caunedo-Alvarez, A.; Ortiz-Moyano, C.; Gomez-Parra, M.; Herrerias-Gutierrez, J.M. EUS-guided injection of cyanoacrylate in perforating feeding veins in gastric varices: Results in 5 cases. Gastrointest. Endosc. 2007, 66, 402–407. [Google Scholar] [CrossRef]
- Robles-Medranda, C.; Oleas, R.; Valero, M.; Puga-Tejada, M.; Baquerizo-Burgos, J.; Ospina, J.; Pitanga-Lukashok, H. Endoscopic ultrasonography-guided deployment of embolization coils and cyanoacrylate injection in gastric varices versus coiling alone: A randomized trial. Endoscopy 2020, 52, 268–275. [Google Scholar] [CrossRef]
- Tsoris, A.; Marlar, C.A. Use of the Child Pugh Score in Liver Disease. [Updated 2023 Mar 13]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542308/ (accessed on 8 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).