Age-Related Difference in Cognitive Performance under Severe Whole-Body Hyperthermia Parallels Cortisol and Physical Strain Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
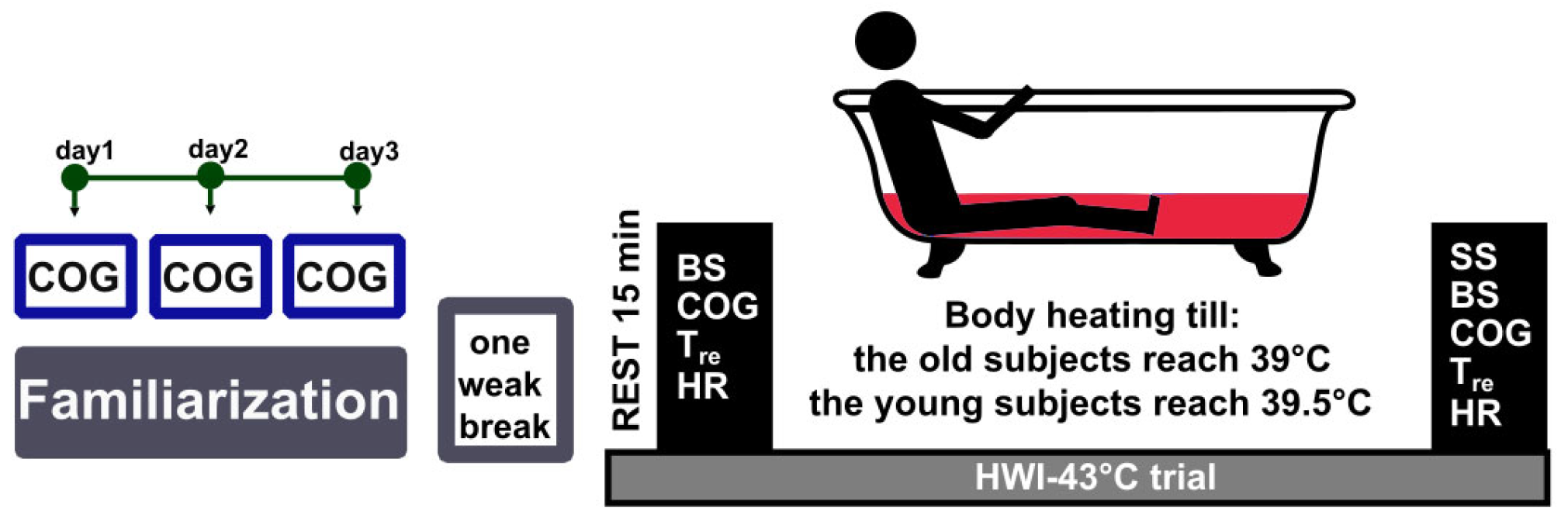
2.3. Study Design and Experimental Protocol
2.4. Measures
2.4.1. Measurement of Sweating Rate
2.4.2. Measurement of Rectal Temperature
2.4.3. Measurement of Heart Rate
2.4.4. Measurement of Physiological Strain Index
2.4.5. Measurement of Subjective Sensations
2.4.6. Measurement of Cognitive Functioning
Forced-Choice Recognition Memory Test
Forward Digit-Span
Odd/Even Test
2.4.7. Measurement of Cortisol Concentration
2.5. Data Analyses
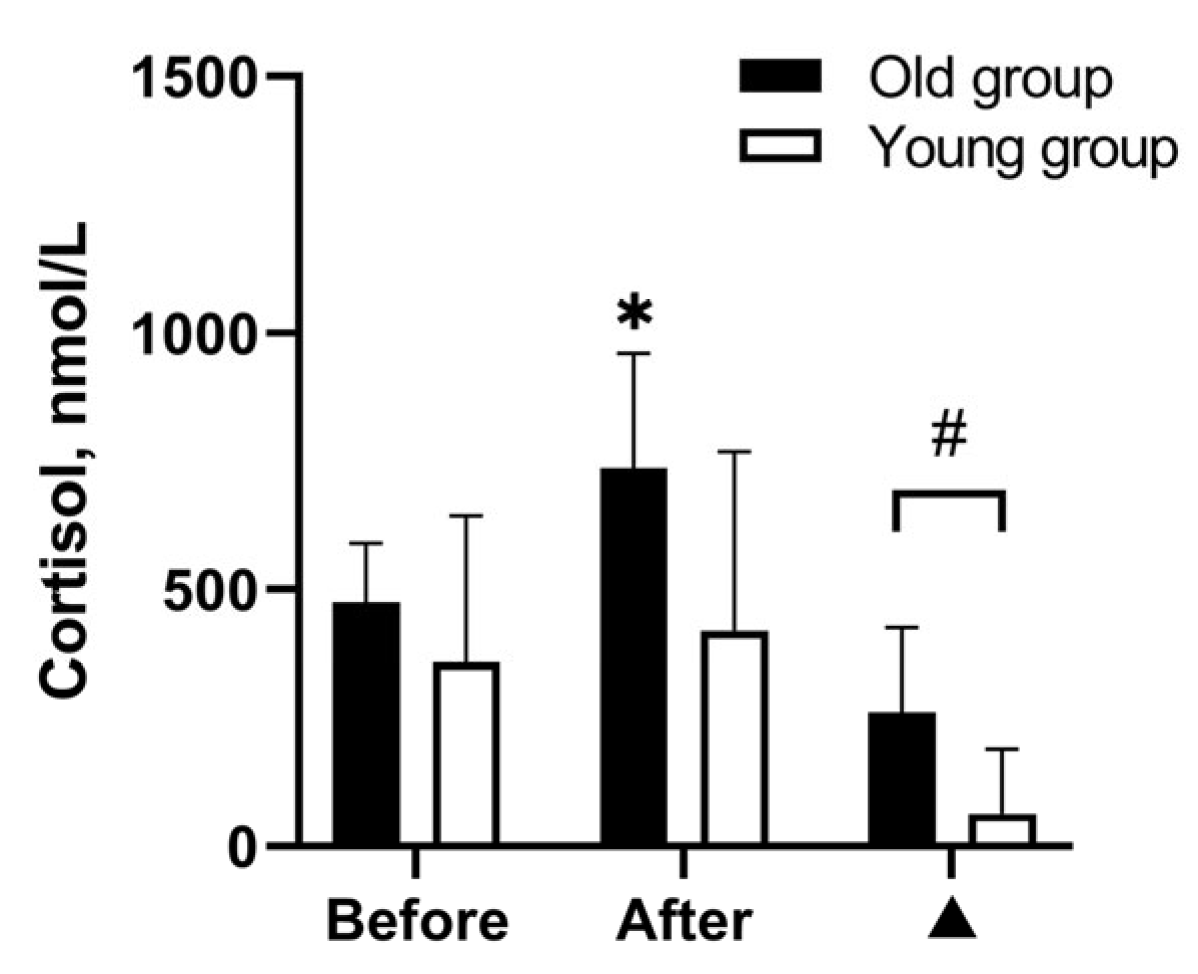
3. Results
3.1. Effects of HWI on Sweating Rate and Subjective Sensations
3.2. Physiological Response to Passive Heating
3.3. Effects of WBH on Cognitive Functioning
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat Population Structure and Ageing. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing (accessed on 28 June 2023).
- Shimizu, M.; Kobayashi, T.; Chiba, H.; Senoo, I.; Ito, H.; Matsukura, K.; Saito, S. Adult Spinal Deformity and Its Relationship with Height Loss: A 34-Year Longitudinal Cohort Study. BMC Musculoskelet. Disord. 2020, 21, 422. [Google Scholar] [CrossRef] [PubMed]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing Trends in Regional Heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef] [PubMed]
- Meade, R.D.; Akerman, A.P.; Notley, S.R.; McGinn, R.; Poirier, P.; Gosselin, P.; Kenny, G.P. Physiological Factors Characterizing Heat-Vulnerable Older Adults: A Narrative Review. Environ. Int. 2020, 144, 105909. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Hu, S.; Yu, C.W. Cognitive Performance in a Warming Planet. Indoor Buil. Environ. 2022, 31, 2195–2198. [Google Scholar] [CrossRef]
- Raz, N.; Lindenberger, U.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Williamson, A.; Dahle, C.; Gerstorf, D.; Acker, J.D. Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cereb. Cortex. 2005, 15, 1676–1689. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Park, D.C. Human Neuroscience and the Aging Mind: A New Look at Old Problems. J. Gerontol. B Psychol. Sci. Soc. Sci. 2010, 65B, 405–415. [Google Scholar] [CrossRef]
- Bherer, L. Cognitive Plasticity in Older Adults: Effects of Cognitive Training and Physical Exercise. Ann. N. Y. Acad. Sci. 2015, 1337, 1–6. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Snyder, A.Z.; Vincent, J.L.; Lustig, C.; Head, D.; Raichle, M.E.; Buckner, R.L. Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron 2007, 56, 924–935. [Google Scholar] [CrossRef]
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The Effects of Stress and Stress Hormones on Human Cognition: Implications for the Field of Brain and Cognition. Brain. Cogn. 2007, 65, 209–237. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress Signalling Pathways That Impair Prefrontal Cortex Structure and Function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Guergova, S.; Dufour, A. Thermal Sensitivity in the Elderly: A Review. Ageing. Res. Rev. 2011, 10, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, M.; Nishimura, Y.; Mikami, Y.; Kouda, K.; Sakurai, Y.; Yoshioka, I.; Kinoshita, T.; Kojima, D.; Tajima, F. Attenuation of Core Temperature Elevation and Interleukin-6 Excretion during Head-out Hot Water Immersion in Elderly People. J. Phys. Ther. Sci. 2020, 32, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Blatteis, C.M. Age-Dependent Changes in Temperature Regulation—A Mini Review. Gerontology 2012, 58, 289–295. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Barie, P.S.; Bartlett, J.G.; Bleck, T.; Carroll, K.; Kalil, A.C.; Linden, P.; Maki, D.G.; Nierman, D.; Pasculle, W.; et al. Guidelines for Evaluation of New Fever in Critically Ill Adult Patients: 2008 Update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit. Care. Med. 2008, 36, 1330–1349. [Google Scholar] [CrossRef]
- Liu, K.; Sun, G.; Li, B.; Jiang, Q.; Yang, X.; Li, M.; Li, L.; Qian, S.; Zhao, L.; Zhou, Z.; et al. The Impact of Passive Hyperthermia on Human Attention Networks: An fMRI Study. Behav. Brain. Res. 2013, 243, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Qian, S.; Jiang, Q.; Liu, K.; Li, B.; Li, M.; Zhao, L.; Zhou, Z.; von Deneen, K.M.; Liu, Y. Hyperthermia-Induced Disruption of Functional Connectivity in the Human Brain Network. PLoS ONE 2013, 8, e61157. [Google Scholar] [CrossRef] [PubMed]
- Brazaitis, M.; Eimantas, N.; Daniuseviciute, L.; Vitkauskiene, A.; Paulauskas, H.; Skurvydas, A. Two Strategies for the Acute Response to Cold Exposure but One Strategy for the Response to Heat Stress. Int. J. Hyperth. 2015, 31, 325–335. [Google Scholar] [CrossRef]
- Bayley, P.J.; Wixted, J.T.; Hopkins, R.O.; Squire, L.R. Yes/No Recognition, Forced-Choice Recognition, and the Human Hippocampus. J. Cogn. Neurosci. 2008, 20, 505–512. [Google Scholar] [CrossRef][Green Version]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Gagnon, D.; Adams, A.; Rivas, E.; Cullum, C.M.; Crandall, C.G. Cognitive and Perceptual Responses during Passive Heat Stress in Younger and Older Adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R847–R854. [Google Scholar] [CrossRef]
- Baranauskiene, N.; Wang, J.; Eimantas, N.; Solianik, R.; Brazaitis, M. Age-related Differences in the Neuromuscular Performance of Fatigue-provoking Exercise under Severe Whole-body Hyperthermia Conditions. Scand. J. Med. Sci. Sports 2023, 33, 1621–1637. [Google Scholar] [CrossRef]
- Leliavski, A.; Dumbell, R.; Ott, V.; Oster, H. Adrenal Clocks and the Role of Adrenal Hormones in the Regulation of Circadian Physiology. J. Biol. Rhythm. 2015, 30, 20–34. [Google Scholar] [CrossRef]
- Li, S.; Lu, A.; Li, B.; Wang, Y. Circadian Rhythms on HyJpothalamic-Pituitary-Adrenal Axis Hormones and Cytokines of Collagen Induced Arthritis in Rats. J. Autoimmun. 2004, 22, 277–285. [Google Scholar] [CrossRef]
- Moran, D.S.; Shitzer, A.; Pandolf, K.B. A Physiological Strain Index to Evaluate Heat Stress. Am. J. Physiol. 1998, 275, R129–R134. [Google Scholar] [CrossRef] [PubMed]
- Solianik, R.; Skurvydas, A.; Mickevičienė, D.; Brazaitis, M. Intermittent Whole-Body Cold Immersion Induces Similar Thermal Stress but Different Motor and Cognitive Responses between Males and Females. Cryobiology 2014, 69, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Solianik, R.; Skurvydas, A.; Urboniene, D.; Eimantas, N.; Daniuseviciute, L.; Brazaitis, M. Similar Cold Stress Induces Sex-Specific Neuroendocrine and Working Memory Responses. Cryo Lett. 2015, 36, 120–127. [Google Scholar]
- Solianik, R.; Brazaitis, M.; Skurvydas, A. Sex-Related Differences in Attention and Memory. Medicina 2016, 52, 372–377. [Google Scholar] [CrossRef]
- Finkel, D.; Reynolds, C.A.; McArdle, J.J.; Gatz, M.; Pedersen, N.L. Latent Growth Curve Analyses of Accelerating Decline in Cognitive Abilities in Late Adulthood. Dev. Psychol. 2003, 39, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Hedden, T.; Gabrieli, J.D.E. Insights into the Ageing Mind: A View from Cognitive Neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef]
- Nyberg, L.; Lövdén, M.; Riklund, K.; Lindenberger, U.; Bäckman, L. Memory Aging and Brain Maintenance. Trends Cogn. Sci. 2012, 16, 292–305. [Google Scholar] [CrossRef]
- Salthouse, T. Consequences of Age-Related Cognitive Declines. Annu. Rev. Psychol. 2012, 63, 201–226. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Talarico, J.N.; Marin, M.-F.; Sindi, S.; Lupien, S.J. Effects of Stress Hormones on the Brain and Cognition: Evidence from Normal to Pathological Aging. Dement. Neuropsychol. 2011, 5, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Vedhara, K.; Hyde, J.; Gilchrist, I.D.; Tytherleigh, M.; Plummer, S. Acute Stress, Memory, Attention and Cortisol. Psychoneuroendocrinology 2000, 25, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Brazaitis, M.; Paulauskas, H.; Eimantas, N.; Daniuseviciute, L.; Volungevicius, G.; Skurvydas, A. Motor Performance Is Preserved in Healthy Aged Adults Following Severe Whole-Body Hyperthermia. Int. J. Hyperth. 2019, 36, 65–74. [Google Scholar] [CrossRef]
- Palve, S.S.; Palve, S.B. Impact of Aging on Nerve Conduction Velocities and Late Responses in Healthy Individuals. J. Neurosci. Rural. Pract. 2018, 09, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Mather, M. The Emotion Paradox in the Aging Brain. Ann. N. Y. Acad. Sci. 2012, 1251, 33–49. [Google Scholar] [CrossRef]
- Brazaitis, M.; Paulauskas, H.; Eimantas, N.; Obelieniene, D.; Baranauskiene, N.; Skurvydas, A. Heat Transfer and Loss by Whole-Body Hyperthermia during Severe Lower-Body Heating Are Impaired in Healthy Older Men. Exp. Gerontol. 2017, 96, 12–18. [Google Scholar] [CrossRef]
- Teichner, W.H. Reaction Time in the Cold. J. Appl. Psychol. 1958, 42, 54–59. [Google Scholar] [CrossRef]
- Kenny, G.P.; Yardley, J.; Brown, C.; Sigal, R.J.; Jay, O. Heat Stress in Older Individuals and Patients with Common Chronic Diseases. CMAJ 2010, 182, 1053–1060. [Google Scholar] [CrossRef]
- Eimonte, M.; Paulauskas, H.; Daniuseviciute, L.; Eimantas, N.; Vitkauskiene, A.; Dauksaite, G.; Solianik, R.; Brazaitis, M. Residual Effects of Short-Term Whole-Body Cold-Water Immersion on the Cytokine Profile, White Blood Cell Count, and Blood Markers of Stress. Int. J. Hyperther. 2021, 38, 696–707. [Google Scholar] [CrossRef]
- Baker, F.C.; Siboza, F.; Fuller, A. Temperature Regulation in Women: Effects of the Menstrual Cycle. Temperature 2020, 7, 226–262. [Google Scholar] [CrossRef] [PubMed]
- Neff, L.M.; Hoffmann, M.E.; Zeiss, D.M.; Lowry, K.; Edwards, M.; Rodriguez, S.M.; Wachsberg, K.N.; Kushner, R.; Landsberg, L. Core Body Temperature Is Lower in Postmenopausal Women than Premenopausal Women: Potential Implications for Energy Metabolism and Midlife Weight Gain. Cardiovasc. Endocrinol. 2016, 5, 151–154. [Google Scholar] [CrossRef] [PubMed]
| Measure | Young Men (n = 10) M (SD) | Old Men (n = 9) M (SD) | p |
|---|---|---|---|
| Age, years | 20.8 (0.6) | 68.9 (5.7) | <0.001 |
| Height, cm | 180.8 (6.5) | 176.6 (6.4) | 0.182 |
| Weight, kg | 78.3 (11.1) | 83.3 (15.7) | 0.494 |
| Body mass index, kg/m2 | 23.9 (3.0) | 26.5 (3.3) | 0.149 |
| Body fat, % | 15.6 (6.13) | 24.9 (6.7) | 0.003 |
| Body surface area, m2 | 1.98 (0.15) | 2.00 (0.21) | 0.849 |
| Rating | Thermal Sensation | Shivering/Sweating Sensation | Comfort Sensation |
|---|---|---|---|
| 1 | Very cold | Vigorously shivering | Comfortable |
| 2 | Cold | Moderately shivering | Uncomfortable |
| 3 | Cool | Slightly shivering | Little comfortable |
| 4 | Slightly cool | Not at all | Comfortable |
| 5 | Neutral | Slightly sweating | Extremely uncomfortable |
| 6 | Slightly warm | Moderately sweating | |
| 7 | Heavily sweating | Sweating running off in many places | |
| 8 | Hot | ||
| 9 | Very hot |
| Measure | Old Group (n = 9) M (SD) | Young Group (n = 10) M (SD) |
|---|---|---|
| Weight loss, kg | 1.36 (0.34) | 1.43 (0.38) |
| Comfort sensation, points | 1.56 (0.73) | 2.10 (0.74) |
| Sweating sensation, points | 5.67 (0.50) | 5.85 (0.47) |
| Thermal sensation, points | 7.00 (0.50) | 7.50 (0.53) |
| Measure | Group (n) | Before HWI–43 °C M (SD) | After HWI–43 °C M (SD) |
|---|---|---|---|
| HR, bpm | Old (9) | 58.22 (10.07) | 114.67 (12.32) * |
| Young (10) | 66.7 (12.34) | 149.3 (24.71) *,# | |
| HR during HWI–43 °C, bpm | Old (9) | 96.8 (12.0) | |
| Young (10) | 134.1 (21.1) # | ||
| PSI during HWI–43 °C | Old (9) | 7.32 (0.41) | |
| Young (10) | 8.68 (0.97) # | ||
| Time to target Tre, min | Old (9) | 88.2 (16.2) | |
| Young (10) | 62.7 (21.3) # | ||
| Measure | Group (n) | Before HWI–43 °C M (SD) | After HWI–43 °C M (SD) | ▲ M (SD) |
|---|---|---|---|---|
| Forced-choice recognition memory test | ||||
| Figures identified, n | Old (9) | 4.89 (0.93) | 5.11 (1.27) | 0.22 (1.20) |
| Young (10) | 7.30 (1.06) # | 7.70 (1.49) # | 0.40 (1.90) | |
| Forward digit-span test | ||||
| Mean digits identified, n | Old (9) | 6.35 (0.67) | 6.39 (0.65) | 0.04 (0.57) |
| Young (10) | 6.65 (0.58) | 6.45 (0.63) | −0.21 (0.40) | |
| Odd/even test | ||||
| Reaction time, ms | Old (9) | 692.4 (105.0) | 636.5 (49.2) * | −55.8 (61.5) |
| Young (10) | 600.5 (83.4) | 587.5 (82.3) # | −13.0 (55.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Solianik, R.; Eimantas, N.; Baranauskiene, N.; Brazaitis, M. Age-Related Difference in Cognitive Performance under Severe Whole-Body Hyperthermia Parallels Cortisol and Physical Strain Responses. Medicina 2023, 59, 1665. https://doi.org/10.3390/medicina59091665
Wang J, Solianik R, Eimantas N, Baranauskiene N, Brazaitis M. Age-Related Difference in Cognitive Performance under Severe Whole-Body Hyperthermia Parallels Cortisol and Physical Strain Responses. Medicina. 2023; 59(9):1665. https://doi.org/10.3390/medicina59091665
Chicago/Turabian StyleWang, Junli, Rima Solianik, Nerijus Eimantas, Neringa Baranauskiene, and Marius Brazaitis. 2023. "Age-Related Difference in Cognitive Performance under Severe Whole-Body Hyperthermia Parallels Cortisol and Physical Strain Responses" Medicina 59, no. 9: 1665. https://doi.org/10.3390/medicina59091665
APA StyleWang, J., Solianik, R., Eimantas, N., Baranauskiene, N., & Brazaitis, M. (2023). Age-Related Difference in Cognitive Performance under Severe Whole-Body Hyperthermia Parallels Cortisol and Physical Strain Responses. Medicina, 59(9), 1665. https://doi.org/10.3390/medicina59091665





