Primary Tumor Resection Plus Chemotherapy versus Chemotherapy Alone for Colorectal Cancer Patients with Synchronous Bone Metastasis
Abstract
1. Introduction
2. Material and Methods
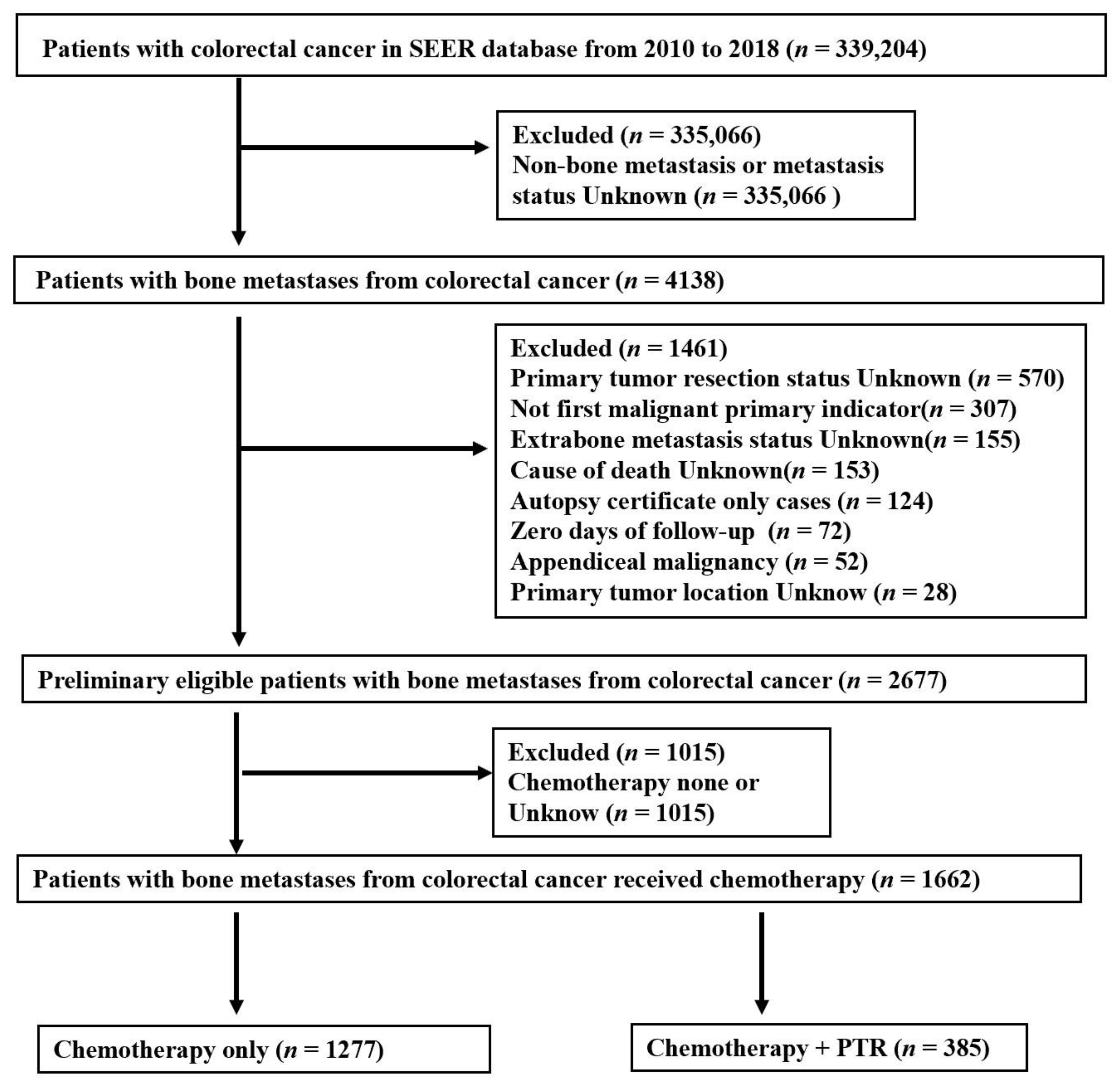
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
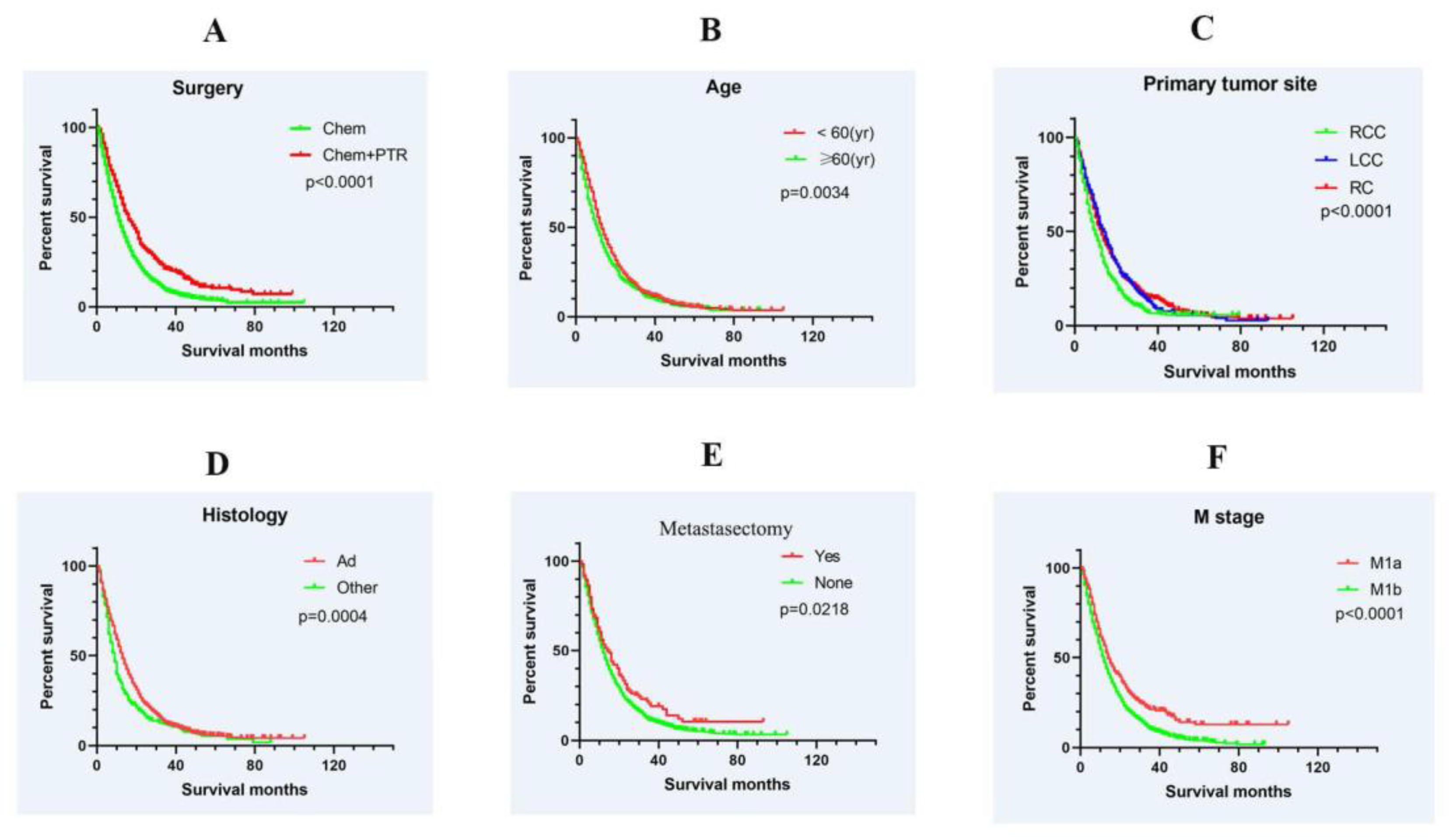
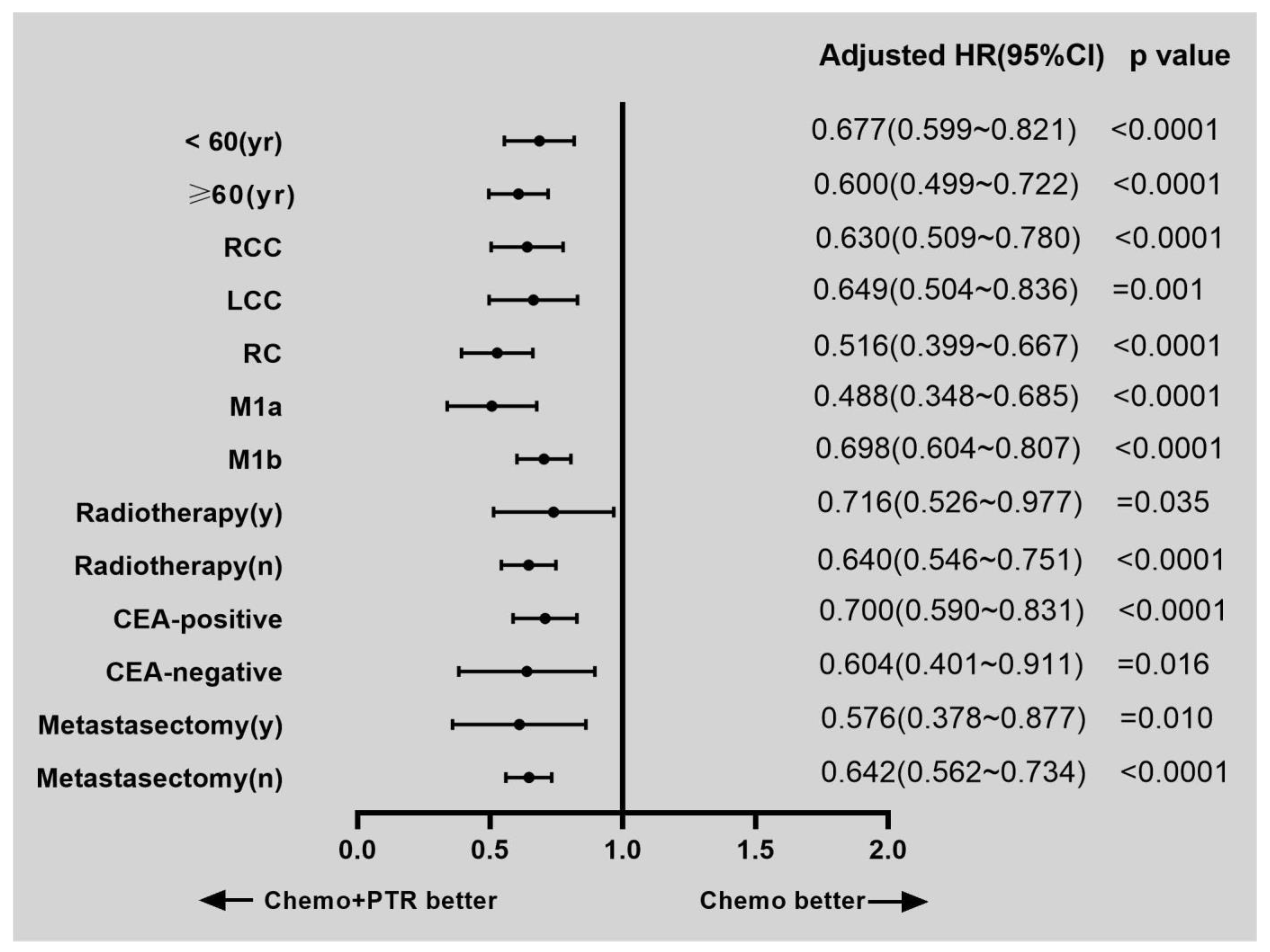
3.2. Survival and Prognostic Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Hernandez, R.K.; Wade, S.W.; Reich, A.; Pirolli, M.; Liede, A.; Lyman, G.H. Incidence of bone metastases in patients with solid tumors: Analysis of oncology electronic medical records in the United States. BMC Cancer 2018, 18, 44. [Google Scholar] [CrossRef]
- Park, H.S.; Chun, Y.J.; Kim, H.S.; Kim, J.H.; Lee, C.-K.; Beom, S.-H.; Shin, S.J.; Ahn, J.B. Clinical features and KRAS mutation in colorectal cancer with bone metastasis. Sci. Rep. 2020, 10, 21180. [Google Scholar] [CrossRef]
- Thompson, E.; Banerjee, S.; Thompson, S.; Silva, R.; Muse, A.; Arif-Tiwari, H.; Scott, A.J.; Nfonsam, V. Incidence and predictors of brain metastasis in colorectal cancer patients. Int. J. Color. Dis. 2022, 37, 153–159. [Google Scholar] [CrossRef]
- Christensen, T.D.; Jensen, S.G.; Larsen, F.O.; Nielsen, D.L. Systematic review: Incidence, risk factors, survival and treatment of bone metastases from colorectal cancer. J. Bone Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef]
- Kawamura, H.; Yamaguchi, T.; Yano, Y.; Hozumi, T.; Takaki, Y.; Matsumoto, H.; Nakano, D.; Takahashi, K. Characteristics and Prognostic Factors of Bone Metastasis in Patients with Colorectal Cancer. Dis. Colon Rectum 2018, 61, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, Z.S.; Kim, E.B.; Raje, N. Bone Disease in Multiple Myeloma: Biologic and Clinical Implications. Cells 2022, 11, 2308. [Google Scholar] [CrossRef] [PubMed]
- Poultsides, G.A.; Servais, E.L.; Saltz, L.B.; Patil, S.; Kemeny, N.E.; Guillem, J.G.; Weiser, M.; Temple, L.K.F.; Wong, W.D.; Paty, P.B. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J. Clin. Oncol. 2009, 27, 3379–3384. [Google Scholar] [CrossRef]
- Alawadi, Z.; Phatak, U.R.; Hu, C.-Y.; Bailey, C.E.; You, Y.N.; Kao, L.S.; Massarweh, N.N.; Feig, B.W.; Rodriguez-Bigas, M.A.; Skibber, J.M.; et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer 2017, 123, 1124–1133. [Google Scholar] [CrossRef]
- Chen, J.-N.; Shoucair, S.; Wang, Z.; Habib, J.R.; Zhao, F.-Q.; Yu, J.; Liu, Z.; Liu, Q. Primary Tumor Resection for Rectal Cancer with Unresectable Liver Metastases: A Chance to Cut Is a Chance for Improved Survival. Front. Oncol. 2021, 11, 628715. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients with Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, F.; Malka, D.; Bourredjem, A.; Allonier, C.; Bouché, O.; Louafi, S.; Boige, V.; Mousseau, M.; Raoul, J.L.; Bedenne, L.; et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: Results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur. J. Cancer 2013, 49, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Xu, L.; Yang, W.; Xu, X.; Zheng, S. Asymptomatic Primary Tumor Resection in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 836404. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, Y.; Chen, D.; Xu, X.; Liu, F.; Zhao, F. Primary Tumor Resection Provides Survival Benefits for Patients with Synchronous Brain Metastases from Colorectal Cancer. Diagnostics 2022, 12, 1586. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Dominguez, O.; Yilmaz, S.; Steele, S.R. Stage IV Colorectal Cancer Management and Treatment. J. Clin. Med. 2023, 12, 2072. [Google Scholar] [CrossRef]
- Van de Haar, J.; Ma, X.; Ooft, S.N.; van der Helm, P.W.; Hoes, L.R.; Mainardi, S.; Pinato, D.J.; Sun, K.; Salvatore, L.; Tortora, G.; et al. Codon-specific KRAS mutations predict survival benefit of trifluridine/tipiracil in metastatic colorectal cancer. Nat. Med. 2023, 29, 605–614. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Guan, X.; Ma, C.-X.; Quan, J.-C.; Zhao, Z.-X.; Chen, H.-P.; Sun, P.; Wang, S.; Lu, Z.; Ma, X.-L.; Liu, Z.; et al. A prognostic index model to individually predict clinical outcomes for colorectal cancer with synchronous bone metastasis. J. Cancer 2020, 11, 4366–4372. [Google Scholar] [CrossRef]
- Ma, N.; Chen, Z.; Liu, G.; Yue, Y.; Li, Y.; Cheng, K.; Ma, X.; Feng, Q.; Liang, J.; Zhang, T.; et al. Normalizing the Immune Macroenvironment via Debulking Surgery to Strengthen Tumor Nanovaccine Efficacy and Eliminate Metastasis. ACS Nano 2023, 17, 437–452. [Google Scholar] [CrossRef]
- Chang, G.J. Primary Tumor Resection in Colorectal Cancer with Synchronous Unresectable Metastasis: Time to End the Debate? J. Clin. Oncol. 2021, 39, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Urban, D.; Berger, R.; Lawrence, Y.R. Changing prognosis of metastatic colorectal adenocarcinoma: Differential improvement by age and tumor location. Cancer 2013, 119, 3084–3091. [Google Scholar] [CrossRef]
- Boakye, D.; Walter, V.; Jansen, L.; Martens, U.M.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Magnitude of the Age-Advancement Effect of Comorbidities in Colorectal Cancer Prognosis. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 59–68. [Google Scholar] [CrossRef]
- Anele, C.C.; Askari, A.; Navaratne, L.; Patel, K.; Jenkin, J.T.; Faiz, O.D.; Latchford, A. The association of age with the clinicopathological characteristics and prognosis of colorectal cancer: A UK single-centre retrospective study. Color. Dis. 2020, 22, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Talwar, V.; Sirohi, B.; Mehta, P.; Arya, D.; Shrivastava, G.; Dahiya, A.; Pavithran, K. Primary Tumor Location as a Prognostic and Predictive Marker in Metastatic Colorectal Cancer (mCRC). Front. Oncol. 2020, 10, 964. [Google Scholar] [CrossRef] [PubMed]
- Boeckx, N.; Koukakis, R.; Op de Beeck, K.; Rolfo, C.; Van Camp, G.; Siena, S.; Tabernero, J.; Douillard, J.Y.; André, T.; Peeters, M. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: Results from two randomized first-line panitumumab studies. Ann. Oncol. 2017, 28, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, Z.; Guo, W.; Zhang, N.; Cai, Y.; Ying, J.; Xiao, M. Retrospective study of predictors of bone metastasis in colorectal cancer patients. J. Bone Oncol. 2017, 9, 25–28. [Google Scholar] [CrossRef]
- Gallois, C.; Pernot, S.; Zaanan, A.; Taieb, J. Colorectal Cancer: Why Does Side Matter? Drugs 2018, 78, 789–798. [Google Scholar] [CrossRef]
- Svensson, E.; Christiansen, C.F.; Ulrichsen, S.P.; Rørth, M.R.; Sørensen, H.T. Survival after bone metastasis by primary cancer type: A Danish population-based cohort study. BMJ Open 2017, 7, e016022. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Lugli, A.; Zlobec, I.; Berger, M.D.; Kirsch, R.; Nagtegaal, I.D. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 2021, 18, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Delattre, J.-F.; Selcen Oguz Erdogan, A.; Cohen, R.; Shi, Q.; Emile, J.-F.; Taieb, J.; Tabernero, J.; André, T.; Meyerhardt, J.A.; Nagtegaal, I.D.; et al. A comprehensive overview of tumour deposits in colorectal cancer: Towards a next TNM classification. Cancer Treat. Rev. 2022, 103, 102325. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M.; Farag, A.S.; Abdelwahed, M.S.; Hanbazazh, M.; Samman, A.; Ashmawy, D.; Abd-Elhameed, N.R.; Tharwat, M.; Othman, A.E.; Shawky, T.A.; et al. The Expression of Stem Cell Marker LGR5 and Its Coexpression with Β-Catenin in Sporadic Colorectal Carcinoma and Adenoma: A Comparative Immunohistochemical Study. Medicina 2023, 59, 1233. [Google Scholar] [CrossRef]
- Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.B.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; Liem, M.S.L.; et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): An open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023, 24, 757–771. [Google Scholar] [CrossRef]
| Variable | Total (n = 1662) | Chemo (n = 1277) | Chemo + PTR (n = 385) | p Value | |||
|---|---|---|---|---|---|---|---|
| Age (yr) | 0.007 | ||||||
| <60 | 852 | (51.3) | 678 | (53.1) | 174 | (45.2) | |
| ≥60 | 810 | (48.7) | 599 | (46.9) | 211 | (54.8) | |
| Gender | 0.409 | ||||||
| Female | 665 | (40.0) | 504 | (39.5) | 161 | (41.8) | |
| Male | 997 | (60.0) | 773 | (60.5) | 224 | (58.2) | |
| Primary tumor site | <0.0001 | ||||||
| RCC | 499 | (30.0) | 330 | (25.8) | 169 | (43.9) | |
| LCC | 404 | (24.3) | 285 | (22.3) | 119 | (30.9) | |
| RC | 759 | (45.7) | 662 | (51.8) | 97 | (25.2) | |
| Histology | 0.856 | ||||||
| Adenocarcinoma | 1395 | (83.9) | 1073 | (84.0) | 322 | (83.6) | |
| Other | 267 | (16.1) | 204 | (16.0) | 63 | (16.4) | |
| T stage | <0.0001 | ||||||
| T0/T1 | 178 | (10.7) | 171 | (13.4) | 7 | (1.8) | |
| T2 | 35 | (2.1) | 25 | (2.0) | 10 | (2.6) | |
| T3 | 379 | (22.8) | 219 | (17.1) | 160 | (41.6) | |
| T4 | 387 | (23.3) | 207 | (16.2) | 180 | (46.8) | |
| Tx | 683 | (41.1) | 655 | (51.3) | 28 | (7.3) | |
| N stage | <0.0001 | ||||||
| N0 | 505 | (30.4) | 454 | (35.6) | 51 | (13.2) | |
| N1 | 489 | (29.4) | 372 | (29.1) | 117 | (30.4) | |
| N2 | 315 | (19.0) | 109 | (8.5) | 206 | (53.5) | |
| Nx | 353 | (21.2) | 342 | (26.8) | 11 | (2.9) | |
| M stage | |||||||
| M1a | 286 | (17.2) | 206 | (16.1) | 80 | (20.8) | <0.0001 |
| M1b | 1376 | (82.8) | 1071 | (83.9) | 305 | (79.2) | |
| Radiotherapy | |||||||
| Yes | 216 | (13.0) | 83 | (6.5) | 133 | (34.5) | <0.0001 |
| None | 1446 | (87.0) | 1194 | (93.5) | 252 | (65.5) | |
| CEA | |||||||
| Positive | 931 | (56.0) | 730 | (57.2) | 201 | (52.2) | <0.0001 |
| Negative | 133 | (8.0) | 83 | (6.5) | 50 | (13.0) | |
| Unknown | 598 | (36.0) | 464 | (36.3) | 134 | (34.8) | |
| Metastasectomy | |||||||
| Yes | 127 | (7.6) | 76 | (6.0) | 51 | (13.2) | <0.0001 |
| None | 1535 | (92.4) | 1201 | (94.0) | 334 | (86.8) | |
| Clinicopathologic Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Age (yr) | |||
| <60 | Reference | ||
| ≥60 | 1.186 | 1.063–1.323 | 0.002 |
| Gender | |||
| Female | Reference | ||
| Male | 0.963 | 0.861–1.076 | 0.503 |
| Primary tumor site | |||
| RCC | Reference | ||
| LCC | 0.741 | 0.638–0.860 | <0.0001 |
| RC | 0.742 | 0.653–0.843 | <0.0001 |
| Histology | |||
| Adenocarcinoma | Reference | ||
| Other | 1.294 | 1.118–1.496 | 0.001 |
| T stage | |||
| T0/T1 | Reference | ||
| T2 | 0.774 | 0.522–1.147 | 0.202 |
| T3 | 0.700 | 0.572–0.855 | <0.0001 |
| T4 | 0.846 | 0.694–1.032 | 0.099 |
| Tx | 0.943 | 0.786–1.131 | 0.527 |
| N stage | |||
| N0 | Reference | ||
| N1 | 0.989 | 0.860–1.137 | 0.875 |
| N2 | 1.111 | 0.950–1.300 | 0.188 |
| Nx | 1.198 | 1.019–1.408 | 0.029 |
| M stage | |||
| M1a | Reference | <0.0001 | |
| M1b | 1.444 | 1.240–1.682 | |
| Radiotherapy | |||
| Yes | Reference | 0.003 | |
| None | 1.278 | 1.085–1.504 | |
| CEA | |||
| Positive | Reference | ||
| Negative | 0.556 | 0.406–0.759 | <0.0001 |
| Unknown | 1.167 | 0.965–1.410 | 0.110 |
| Surgery | |||
| Non-PTR | Reference | ||
| PTR | 0.642 | 0.562–0.734 | <0.0001 |
| Metastasectomy | |||
| Yes | Reference | ||
| None | 1.297 | 1.052–1.598 | 0.015 |
| Clinicopathologic Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Age (yr) | |||
| <60 | Reference | ||
| ≥60 | 1.171 | 1.047–1.309 | 0.006 |
| Primary tumor site | |||
| RCC | Reference | ||
| LCC | 0.750 | 0.644–0.874 | <0.0001 |
| RC | 0.694 | 0.605–0.795 | <0.0001 |
| Histology | |||
| Adenocarcinoma | Reference | ||
| Other | 1.333 | 1.148–1.548 | <0.0001 |
| M stage | |||
| M1a | Reference | ||
| M1b | 1.425 | 1.220–1.664 | <0.0001 |
| Metastasectomy | |||
| Yes | Reference | ||
| None | 1.284 | 1.015–1.623 | 0.037 |
| Surgery | |||
| Non-PTR | Reference | ||
| PTR | 0.586 | 0.497–0.691 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, X.; Zhong, C.; Yuan, Y. Primary Tumor Resection Plus Chemotherapy versus Chemotherapy Alone for Colorectal Cancer Patients with Synchronous Bone Metastasis. Medicina 2023, 59, 1384. https://doi.org/10.3390/medicina59081384
Li Y, Cheng X, Zhong C, Yuan Y. Primary Tumor Resection Plus Chemotherapy versus Chemotherapy Alone for Colorectal Cancer Patients with Synchronous Bone Metastasis. Medicina. 2023; 59(8):1384. https://doi.org/10.3390/medicina59081384
Chicago/Turabian StyleLi, Yanqing, Xiaofei Cheng, Chenhan Zhong, and Ying Yuan. 2023. "Primary Tumor Resection Plus Chemotherapy versus Chemotherapy Alone for Colorectal Cancer Patients with Synchronous Bone Metastasis" Medicina 59, no. 8: 1384. https://doi.org/10.3390/medicina59081384
APA StyleLi, Y., Cheng, X., Zhong, C., & Yuan, Y. (2023). Primary Tumor Resection Plus Chemotherapy versus Chemotherapy Alone for Colorectal Cancer Patients with Synchronous Bone Metastasis. Medicina, 59(8), 1384. https://doi.org/10.3390/medicina59081384






