Abstract
Background and Objectives: Radiotherapy (RT) plays an important role in the treatment for locally advanced rectal cancer patients. It can bring radio exposure together with the survival benefit. Cancer survivors are generally at an increased risk for second malignancies, and survivors receiving RT may have higher risks than survivors not receiving RT. Whether the risk of an all-site second malignancy may increase after RT is still debated. This study aims to compare the second malignancy pattern in rectal cancer survivors after RT. Materials and Methods: The Surveillance, Epidemiology, and End Results (SEER) database was used for analysis. In total, 49,961 rectal cancer patients (20–84 years of age) were identified between 2000 and 2012 from 18 SEER registries. All patients underwent surgery. The occurrence of second malignancies diagnosed after rectal cancer diagnosis was compared in patients who received and did not receive RT. The standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) were used. SEER*Stat was used to generate the 95% CIs for the SIR statistics using the exact method. Results: Of the total 49,961 patients, 5582 developed second malignancies. For all-site second primary malignancies, the age-adjusted SIRs were 1.14 (95% CI 1.1–1.18) and 1.00 (95% CI 0.96–1.04) in the no RT and RT groups, respectively. In 23,192 patients from the surgery-only group, 2604 had second malignancies, and in 26,769 patients who received RT, 2978 developed second malignancies. With respect to every site, the risk of secondary prostate cancer was significantly lower in the RT group (SIR = 0.39, 95% CI 0.33–0.46) than that in the surgery-only group (SIR = 1.04, 95% CI 0.96–1.12). Moreover, the risk of thyroid cancer was significantly higher in the RT group (SIR = 2.80, 95% CI 2.2–3.51) than that in the surgery-only group (SIR = 1.29, 95% CI 0.99–1.66). Conclusions: RT may change the second malignancy pattern in rectal cancer survivors; the risk of prostate cancer decreased, and the risk of thyroid cancer increased most significantly.
1. Introduction
Colorectal cancer is the fourth most common cancer worldwide [1]. In recent years, due to the active promotion of early screening and the improvement of clinical treatment, including surgery, radiotherapy (RT), chemotherapy, and immunotherapy, the survival outcome of patients with colorectal cancer has been significantly improved [1,2]. Second primary malignancy (SPM) is a multifactorial disease that is associated with heredity, treatment, lifestyle, and environmental factors. However, it is also considered as another risk factor for death following recurrence and metastasis [3]. RT plays an important role in the treatment of rectal cancer [4]. Studies have shown that RT can effectively reduce tumor recurrence and metastasis [5,6,7,8], but whether ionizing radiation increases the occurrence of other tumors is unclear.
Ionizing radiation is a potent carcinogen, inducing malignant tumors through DNA damage. Some studies found that the exposure to ionizing radiation may cause CML. Venkata S K Manem demonstrated that the increase in second cancer risks is directly correlated with increasing values of the linear energy transfer of charged particles (including protons, alpha particles, and heavy ions like carbon and neon). On the other hand, when undergoing rectal cancer radiotherapy, radiation may have a certain coverage of the prostate area. Thus, whether it may also have a certain impact on the incidence of prostate cancer has not been studied yet. Although several studies have investigated the association between RT and SPM in rectal cancer patients, the conclusions are different [9,10]. Here, we use the SEER database to compare the effects of radiation on the incidence of second primary tumors based on the large number of population studies.
2. Materials and Methods
2.1. Data Source
The cohort for this study was extracted from the Surveillance, Epidemiology, and End Results (SEER) database using the SEER dataset for 18 SEER registries from 2000 to 2012. The SEER data cover 27.8% of the total US population [11]. The demographic composition of the SEER registries and incidence trends from the SEER data are generally considered to be representative of the US population (according to the SEER*Stat software version 8.3.6). The multiple primary standardized incidence ratio (SIR), used to track the incidence of second malignancies, includes patient information from 18 registries (Detroit, Atlanta, Seattle (Puget Sound), San Francisco (Oakland), Utah, New Mexico, Connecticut, Iowa, and Hawaii).
2.2. Patients
The cohort included patients who were diagnosed with a first primary malignant rectal cancer (International Classification of Diseases for Oncology Third Edition histology classification codes 8140/3, 8144/2, 8144/3, 8210/2, 8210/3, 8211/2, 8211/3, 8213/2, 8213/3, 8220/2, 8220/3, 8221/2, 8221/3, 8261/3, 8262/2, 8263/3, 8480/2, 8480/3, 8481/2, 8481/3, 8490/2, and 8490/3) who were presented with their first cancer between 1 January 2000 and 31 December 2012. Patients were excluded if the first primary rectal cancer was stage IV. Patients were also excluded if they did not undergo surgery for rectal cancer.
2.3. Statistical Analyses
Second primary tumors are defined as tumors originating from other organs and tissues, and patients with second primary tumors must exclude concurrent primary tumors and ensure a minimum 2-month interval after diagnosis of primary cancer. Analyses were stratified by age at rectal cancer diagnosis (20–49 years, 50–59 years, 60–69 years, and 70–84 years), race (white, black, or other), and latency period (2–11 months, 12–59 months, 60–120 months, or >120 months from the date of rectal cancer diagnosis). Additionally, we evaluated the risk of second primary tumor and compared it to the incidence in the general population between patients who received and did not receive RT. SEER*Stat was used to generate the 95% confidence intervals (CIs) for SIR statistics using the exact method. An SIR is defined as the ratio of the number of observed cases divided by the number of expected cases. Categorical data were compared using chi-squared tests for nominal data and Jonckheere-Terpstra nonparametric tests for ordinal data. Univariate and multivariate logistic regression models were used to identify factors associated with SPM. Statistical significance was defined, and a two-sided p value <0.05 was considered statistically significant.
3. Results
3.1. Demographics of Patients with First Primary Rectal Cancer
The descriptive characteristics of first primary rectal cancer cases are summarized in Table 1. In total, 49,961 patients were selected through the selection procedure. Among these, 26,769 (54%) patients were treated with RT and surgery, and 23,192 (46%) patients only underwent surgery. The median ages were 61 and 67 years in the RT and no RT groups, respectively. Compared with the no RT group, more patients younger than 60 years of age received RT. Moreover, more patients received RT in 2008–2012 than in 2000–2004.

Table 1.
Descriptive characteristics of rectal cancer patients in 2000–2012.
3.2. Second Cancer Site Analysis between the No Radiotherapy (RT) and RT Patients
The overall incidence of SPM was 11.2% (5582/49,961), 2604/26,769 in the RT group and 2978/23,192 in the no RT group (Table 2). For all-site SPM, the age-adjusted SIRs were 1.14 (95% CI 1.1–1.18) and 1.00 (95% CI 0.96–1.04) in the no RT and RT groups, respectively. For most-site SPM, the age-adjusted SIRs were similar between the RT group and the no RT group except for the male and female genital systems and thyroid. For prostate SPM, the age-adjusted SIRs were 0.96 (95% CI 0.87–1.05) and 0.38 (95% CI 0.33–0.44) in the no RT group and the RT group, respectively (p < 0.05) (Figure 1). Furthermore, for ovary SPM, the age-adjusted SIRs were 0.79 (95% CI 0.49–1.21) and 0.44 (95% CI 0.21–0.81) in the no RT group and the RT group, respectively (p < 0.05). Meanwhile, thyroid SPM increased significantly in the RT group, and the age-adjusted SIRs were 1.82 (95% CI 1.40–2.33) and 1.06 (95% CI 0.73–1.50) in the RT group and the no RT group, respectively. Moreover, for colorectal SPM, both the RT group and the no RT group had significantly increased age-adjusted SIRs (2.55 [95% CI 2.36–2.74] in the no RT group and 1.78 [95% CI 1.62–1.95] in the RT group).

Table 2.
The second malignancy pattern between radiation group and surgery-only group.
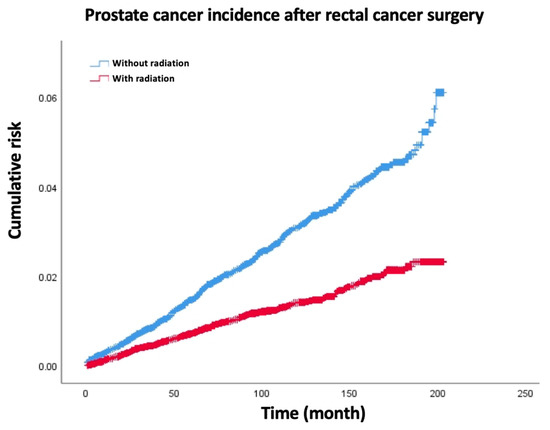
Figure 1.
The secondary prostate cancer rate decreased significantly in radiation group compared with surgery-only group of rectal cancer patients.
3.3. Latency Period Analysis of Second Primary Malignancy between the No RT and RT Patients
Previously, we found that the incidence of second primary prostate cancer in patients with primary rectal cancer in the RT group was significantly lower than that in the no RT group (Table 3). However, the incidence of thyroid cancer was higher. Furthermore, we investigated the SPM between the RT group and the no RT group in different latency periods and found that the incidence of SPM was diverse in different latency periods (Table 4).

Table 3.
The second malignancy pattern between radiation group and surgery-only group in different latencies.

Table 4.
Descriptive characteristics of prostate cancer after rectal cancer.
For all-site SPM, the median latency times from the first primary rectal cancer to the occurrence of the second malignancy of each type in the RT group and surgery-only group are all listed in Supplementary Table S1. The median latency time of the RT and surgery-only groups were 51.0 months. And the media latency time of prostate cancer in the RT group was obviously shortened compared to the surgery-only group. The age-adjusted SIRs in the 36–47-month latency were 1.3 (95% CI 1.2–1.45) and 0.83 (95% CI 0.72–0.94) in the no RT group and the RT group, respectively. Additionally, the age-adjusted SIRs in the 48–59-month latency were 1.19 (95% CI 1.06–1.33) and 0.84 (95% CI 0.73–0.96) in the no RT group and the RT group, respectively (Figure 2A). For rectal SPM, we found that in the 12–95-month latency, the no RT group had a higher SPM than the RT group (Figure 2C). However, in other latency periods, the SPM in the RT group and the no RT group was similar. For prostate cancer, the RT group had a significantly lower SPM than the no RT group in different latency periods, except for the 2–5-month latency. The biggest difference was observed in the 12–35-month latency (Figure 2B).
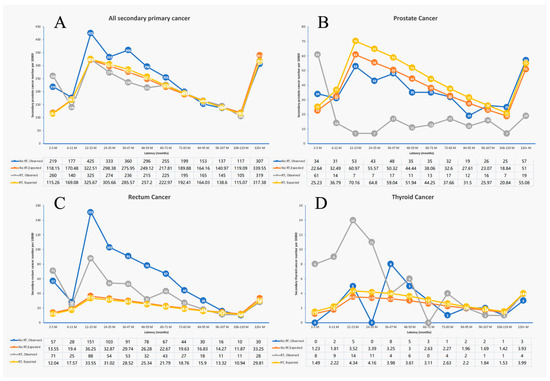
Figure 2.
The second primary cancer patient number of different latency in surgery-only group and radiation group. (A) All second primary cancer patient number; (B) second primary prostate cancer patient number; (C) second primary rectum cancer patient number; (D) second primary thyroid cancer patient number.
For thyroid SPM, the RT group had a significantly higher SPM than the no RT group mainly in the first 3 years after rectal cancer (2–35-month latency) (Figure 2D).
3.4. Clinical–Pathological Characteristics between Secondary Prostate Cancer of Rectal Cancer after RT or without RT
The prostate cancer SPM was significantly lower in the RT group than that in the no RT group, and in almost every latency period, we hypothesized that using RT for rectal cancer can incidentally treat the invisible prostate cancer. Thus, we tried to compare the clinical–pathological characteristics between secondary prostate cancer of rectal cancer after RT or without RT. The results show that secondary prostate cancer showed no difference when compared by age, race, and grade. Regarding the tumor stage, the no RT group had more localized stage secondary prostate cancer than the RT group, and the RT group had more regional and distant stage secondary prostate cancer than the no RT group.
4. Discussion
Overall, we observed that rectal cancer survivors had a different risk of developing a second primary cancer compared with the general US population. Currently, with more effective treatment of cancer, the second primary tumor in cancer survivors has become an urgent problem. Age, sex, and treatment methods are risk factors for its development. Additionally, RT has always been controversial on the treatment and development of malignant tumors [10,12]. It is often considered as an inducer of the second primary tumor. The purpose of using RT for rectal cancer is to reduce the risk of recurrence, which has been fully demonstrated [6,7]. In the past decade, encouraging results of preoperative RT have been observed, and preoperative RT has been frequently used in clinical practice [8,13,14]. However, studies investigating the risk of second primary cancer after rectal RT are insufficient.
In this study, we found that there were significant differences in the pattern of second primary cancer in patients who received RT or patients who did not receive RT. Specifically, patients who received RT had a lower risk of developing ovarian, prostate, and breast cancers and a higher risk of developing thyroid, lung, and bronchial cancers than patients who did not receive RT. The change in cancer risk in these patients is consistent with the results of previous studies. Lu M et al. did not find an increase in the risk of developing a second primary cancer in breast cancer patients after RT [15]. Warschkow et al. also used the SEER database to compare the difference of second primary cancer between colorectal cancer patients with and without RT. Similar to our results, they found a reduced risk of prostate cancer and an increased risk of endometrial cancer with RT [16]. Nevertheless, we found that the risk of thyroid cancer was increased in patients with RT as well, which was not observed in their results. In this study, we included people from the year 2000 onward, whereas they included people from 1973 onward. Hence, the population we included was more consistent than their population, and the RT techniques performed in our study were more advanced compared to the techniques performed in these previous studies.
Regarding the influence of RT on the second primary cancer, there are not only reports of rectal cancer, but also similar studies on other cancers. Jahreiß MC et al. found that the risk of second primary cancer in prostate cancer patients with external beam radiotherapy (EBRT) was increased and remained throughout the different EBRT eras [17]. A meta-analysis also indicated that prostate radiotherapy significantly increases the risk of subsequent rectal cancer [18]. Combined with our study results, we found that in a variety of cancers, the proportion of patients with second primary thyroid cancer is prone to increase after RT, which may be consistent with the current scientific reports that RT tends to increase the incidence of thyroid cancer. In our study, we found a significant reduction in the incidence of prostate cancer after RT, and we hypothesized that this might be due to the tumor cells in the prostate being vulnerable to death during rectal radiation. Of course, pelvic radiation can more or less affect the physiological function or cellular viability of the pelvic reproductive organs (the testicle and ovary), causing a decrease in androgen and/or estrogen levels [19,20,21], which may be the main factor for the occurrence and development of hormone-dependent tumors (such as prostate cancer and breast cancer). Therefore, the dynamic detection of sex hormone levels during radiotherapy will help to further explain this issue.
In the recent development of RT technology, the use of intensity-modulated radiation therapy and other innovations leads to a more accurate RT dose in the target area, with a smaller dose and less damage to the surrounding tissues. Thus, patients who were recently diagnosed with rectal cancer and received RT have a lower risk of second primary cancer than previous patients. With the use of more precise radiotherapy equipment, the changes in the type of second primary tumors may also be worth our forward-looking observation.
In our study, it can be seen that if divided into a young group and an elderly group of people who are 60 years of age or older, the proportion of young patients in the surgery combined with radiotherapy group is higher. However, after conducting a subgroup analysis, both the young and elderly groups have similar conclusions. Therefore, age may not be an important factor.
Our study has several limitations. Most importantly, with insufficient patient information, such as a family history of smoking and smoking history, selection bias is possibly observed. Moreover, some of the rectal cancer patients may have Lynch syndrome. Hence, they have an increased risk of developing secondary colorectal cancer and gastric cancer. The SEER database only provided limited treatment information on radiation and chemotherapy. Thus, we were unable to perform more analyses to investigate the association between second malignancy and the radiation regimen or the chemotherapeutic regimen. The information about tumor recurrence or treatment failure is not recorded or publicly available in the SEER database, so we are currently unable to conduct a statistical analysis on the tumor recurrence information.
5. Conclusions
In conclusion, this study demonstrates that patients with primary rectal cancer have a changed risk for the development of SPMs after RT. It would be beneficial to establish a risk profile for the development of SPMs. Potential risk factors can be identified by studying patients with SPMs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina59081463/s1, Table S1: the median latency of second primary cancer from first primary rectal cancer (months).
Author Contributions
X.Y. designed the research and wrote the manuscript; Y.T. and R.M. analyzed the results and made the figures; P.L. and Y.Y. analyzed the data and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Zhejiang Provincial Natural Science Foundation of China under a grant (LQ21H160024 to YN TAN), the Zhejiang Provincial Medicine and Technology Projects Grant (2020RC063 to YN TAN), and the National Natural Science Foundation of China (no. 81872481 to YY, no.82102708 to YN TAN).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Limited Use Agreement for Surveillance, Epidemiology, and End Results (SEER) Program (https://seer.cancer.gov, accessed on 1 May 2021) SEER*Stat Database: released in April 2019, based on the November 2018 submission. The data can be used publicly.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Abbreviations
| SEER | Surveillance, Epidemiology, and End Results |
| SPM | Second primary malignancy |
| IMRT | Intensity-modulated radiation therapy |
| RT | Radiotherapy |
| SIR | Standardized incidence ratios |
References
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Yang, J.; Wu, F.; An, H.; Gan, H. Incidence and risk outcomes of second primary malignancy of patients with post-operative colorectal cancer. Int. J. Colorectal. Dis. 2023, 38, 88. [Google Scholar] [CrossRef]
- Wang, T.-H.; Liu, C.-J.; Chao, T.-F.; Chen, T.-J.; Hu, Y.-W. Second primary malignancy risk after radiotherapy in rectal cancer survivors. World J. Gastroenterol. 2018, 24, 4586–4595. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, S.M.; Huntzinger, C.J.; Melcher, T.; Olcott, P.D.; Voronenko, Y.; Bartlett-Roberto, J.; Mazin, S. Biology-guided radiotherapy: Redefining the role of radiotherapy in metastatic cancer. Br. J. Radiol. 2021, 94, 20200873. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Y.; He, P.; Shao, B.; Liu, F.; Xiang, Z.; Yang, T.; Zeng, Y.; He, T.; Ma, J.; et al. Effective Combinations of Immunotherapy and Radiotherapy for Cancer Treatment. Front. Oncol. 2022, 12, 809304. [Google Scholar] [CrossRef]
- Diefenhardt, M.; Ludmir, E.B.; Hofheinz, R.-D.; Ghadimi, M.; Minsky, B.D.; Rödel, C.; Fokas, E. Association of Sex with Toxic Effects, Treatment Adherence, and Oncologic Outcomes in the CAO/ARO/AIO-94 and CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trials of Rectal Cancer. JAMA Oncol. 2020, 6, 294–296. [Google Scholar] [CrossRef]
- Hong, T.S.; Ryan, D.P. Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer-The New Standard of Care? JAMA Oncol. 2018, 4, e180070. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, A.; Arolfo, S.; Allaix, M.E.; Munoz, F.; Cassoni, P.; Monagheddu, C.; Ricardi, U.; Ciccone, G.; Morino, M. Results of neoadjuvant short-course radiation therapy followed by transanal endoscopic microsurgery for t1-t2 n0 extraperitoneal rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, A.J.M.; Hugen, N.; Elferink, M.A.G.; Feuth, T.; Poortmans, P.M.P.; Nagtegaal, I.D.; de Wilt, J.H.W. Incidence of second tumors after treatment with or without radiation for rectal cancer. Ann. Oncol. 2017, 28, 535–540. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, T.; Liu, C.; Wang, W.; Zhai, J.; Han, X.; Nie, C.; Ren, X.; Zhu, X.; Xiang, G.; et al. Risk and Prognosis of Second Primary Cancers among Ovarian Cancer Patients, Based on SEER Database. Cancer Investig. 2022, 40, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, J.; Hu, J.; Hu, W.; Qiu, X.; Huang, Q.; Kong, L.; Lu, J.J. Carbon-ion radiotherapy in the treatment of radiation-induced second primary malignancies. Ann. Transl. Med. 2022, 10, 1200. [Google Scholar] [CrossRef]
- Avgousti, R.; Antypas, C.; Armpilia, C.; Simopoulou, F.; Liakouli, Z.; Karaiskos, P.; Kouloulias, V.; Kyrodimos, E.; Moulopoulos, L.A.; Zygogianni, A. Adaptive radiation therapy: When, how and what are the benefits that literature provides? Cancer Radiother. 2022, 26, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Pointer, K.B.; Pitroda, S.P.; Weichselbaum, R.R. Radiotherapy and immunotherapy: Open questions and future strategies. Trends Cancer 2022, 8, 9–20. [Google Scholar] [CrossRef]
- Lu, M.; Liu, H.; Zheng, B.; Sun, S.; Chen, C. Links between Breast and Thyroid Cancer: Hormones, Genetic Susceptibility and Medical Interventions. Cancers 2022, 14, 5117. [Google Scholar] [CrossRef] [PubMed]
- Warschkow, R.; Güller, U.; Cerny, T.; Schmied, B.M.; Plasswilm, L.; Putora, P.M. Secondary malignancies after rectal cancer resection with and without radiation therapy: A propensity-adjusted, population-based SEER analysis. Radiother. Oncol. 2017, 123, 139–146. [Google Scholar] [CrossRef]
- Jahreiß, M.-C.; Heemsbergen, W.D.; van Santvoort, B.; Hoogeman, M.; Dirkx, M.; Pos, F.J.; Janssen, T.; Dekker, A.; Vanneste, B.; Minken, A.; et al. Impact of Advanced Radiotherapy on Second Primary Cancer Risk in Prostate Cancer Survivors: A Nationwide Cohort Study. Front. Oncol. 2021, 11, 771956. [Google Scholar] [CrossRef]
- Nugent, T.S.; Low, E.Z.; Fahy, M.R.; Donlon, N.E.; McCormick, P.H.; Mehigan, B.J.; Cunningham, M.; Gillham, C.; Kavanagh, D.O.; Kelly, M.E.; et al. Prostate radiotherapy and the risk of secondary rectal cancer-a meta-analysis. Int. J. Colorectal. Dis. 2022, 37, 437–447. [Google Scholar] [CrossRef]
- Bruheim, K.; Svartberg, J.; Carlsen, E.; Dueland, S.; Haug, E.; Skovlund, E.; Tveit, K.M.; Guren, M.G. Radiotherapy for rectal cancer is associated with reduced serum testosterone and increased FSH and LH. Int. J. Radiat Oncol. Biol. Phys. 2008, 70, 722–727. [Google Scholar] [CrossRef]
- Dueland, S.; Guren, M.G.; Olsen, D.R.; Poulsen, J.P.; Tveit, K.M. Radiation therapy induced changes in male sex hormone levels in rectal cancer patients. Radiother. Oncol. 2003, 68, 249–253. [Google Scholar] [CrossRef]
- Hermann, R.M.; Henkel, K.; Christiansen, H.; Vorwerk, H.; Hille, A.; Hess, C.F.; Schmidberger, H. Testicular dose and hormonal changes after radiotherapy of rectal cancer. Radiother. Oncol. 2005, 75, 83–88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).