Isolated Prolongation of Activated Partial Thromboplastin Time: Not Just Bleeding Risk!
Abstract
1. Introduction
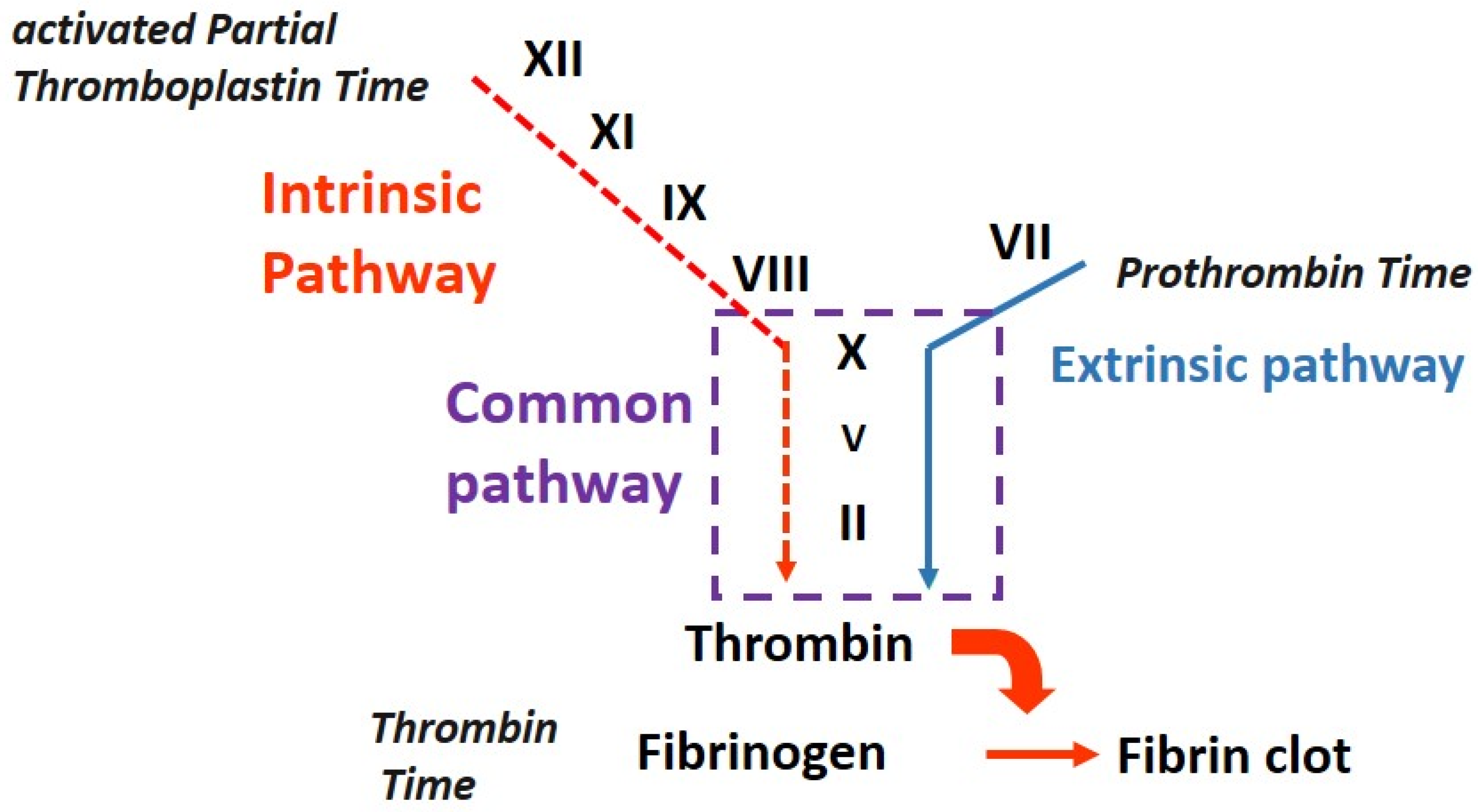
2. Activated Partial Thromboplastin Time (aPTT): Rationale, Procedure, and Aims
2.1. What Is the Rationale of aPTT?
- Congenital, for example, Factor VIII (hemophilia A) or Factor IX (hemophilia B);
- Acquired due to a neutralizing antibody (acquired hemophilia) or the effect of an anticoagulant therapy (unfractionated or low-molecular-weight heparin, LMWH, or direct oral anticoagulants, DOACs).
2.2. How Is aPTT Performed?
- An activator, a substance able to sustain an activation reaction activation of the zymogens belonging to the so called “contact pathway”; these are mostly factor XII but may also be HMWK and PK. Activators can be inorganic (kaolin or micronized silica) or organic (ellagic acid or vegetable phosphatides); they have different analytic sensitivities to detect factor deficiencies or the presence of inhibitors. Currently, kaolin is rarely used due to its opacity, which can inhibit the optical recognition of fibrin formation.
- Phospholipids, including phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphyngomyelin (SM), are incorporated into the reagent used for testing to reproduce in vitro the role of platelet in vivo.
- o
- PS serves as a surface for the assembly and activation of coagulation factors, specifically those involved in the intrinsic pathway. It provides a platform for the formation of the intrinsic tenase complex and supports the activation of Factor X, which is crucial for clot formation.
- o
- PC contributes to the overall stability and structure of the lipid vesicles used in the aPTT reagent. It helps maintain the integrity of the phospholipid membrane and aids in the presentation of other coagulation factors during the assay.
- o
- PE is involved in the formation of the phospholipid membrane used in the aPTT assay. It contributes to the overall structure and fluidity of the membrane, which are important for the proper assembly and activation of coagulation factors.
- o
- SM, like PC, is a key component of the lipid vesicles used in the aPTT reagent. It contributes to the structure and stability of the phospholipid membrane, facilitating the presentation of other necessary coagulation factors during the assay.
- Calcium chloride is used to reintroduce in the reaction calcium ions previously depleted by the anticoagulant (3.2% trisodium citrate) present in the blood.
- Citrated plasma is also used. The recommended anticoagulant for blood collection for coagulation analyses is trisodium citrate in 1 + 9 ratio with blood.
- The addition of an activator and phospholipids to citrated plasma, determining the generation of Factors XIIa and XIa.
- After incubation at 37 °C, the plasma is recalcified by adding calcium chloride; beginning from this moment, the activated partial thromboplastin time is recorded as the time in seconds needed to generate the fibrin clot.
2.3. Why Is aPTT Performed?
3. Preanalytical Cause of Prolongation of aPTT and Other Coagulation Tests
3.1. Hemolysis, Hyperbilirubinemia, and Hypertriglyceridemia
3.2. Blood/Anticoagulant Ratio and Hemoconcentration
4. Isolated, Prolonged aPTT: Prevalence and Causes
4.1. Isolated, Prolonged aPTT: A Truly Unexpected Finding?
4.2. Isolated, Prolonged aPTT: What Are the Causes?
4.2.1. Heparin Contamination
4.2.2. C-reactive Protein
4.2.3. Lupus Anticoagulants
4.2.4. Drug Interferences
4.2.5. Acquired Hemophilia A (AHA) and Acquired Von Willebrand Disease (AVWD)
4.2.6. Hemophilia A and B (HA, HB)
4.2.7. Von Willebrand Disease (VWD)
4.2.8. Factor XI Deficiency
4.2.9. Contact Pathway Factor Deficiency
5. Isolated, Prolonged aPTT: Differential Diagnosis
How can We Identify the Cause of Isolated, Prolonged aPTT?
- Heparin will be detected by both tests;
- Direct anti-IIa inhibitor will be detected only via thrombin time;
- Direct anti-Xa inhibitors will be detected only by an anti-Xa assay.
- For an immediate mixing test, the plasma is prepared using equal volumes (1:1) of NPP and patient plasma, and aPTT is performed on it at room temperature [7]. In parallel, as a control, aPTT is performed on the NPP. Chang et al. [34] suggested that the aPTT correction in a mixing test with a 4:1 ratio of NPP and patient plasma can achieve better sensitivity and specificity, mostly in the situations in which the antibody power is relatively weak. Recently, a Chinese study [35] on 251 samples demonstrated that the aPTT mixing studies had good sensitivity and specificity in differentiating factor deficiencies from inhibitors (and in differentiating time-dependent from time-independent inhibitors) and that the combination of 1:1 and 4:1 mixing studies was able to improve the diagnostic ability compared with the 1:1 ratio alone in those cases characterized by a borderline Rosner index value (see below);
- The normality range method, in which the aPTT value measured from the mixture must fall within the normal range (a range determined by the laboratory that is effective for the specific combination of the reagent and coagulation analyzer used for the measurement). The advantages of this method are its easiness and immediacy; it has an important diagnostic power in the situations in which the patient plasma aPTT is markedly prolonged. However, it shows its weakness in situations in which aPTT is only mildly prolonged (because the mixing could determine an aPTT value in the normal range if there are both a factor deficiency and a low titer inhibitor due to inhibitor dilution) [36,37];
- The index of circulating anticoagulant (ICA) method, ICA (or Rosner index), is defined by the following formula:
- Test for lupus anticoagulants by performing the dilute Russell viper venom time (dRVVT): the presence of a lupus anticoagulant can be diagnosed by finding a ratio between a phospholipid-rich dRVVT assay (confirm) and a phospholipid-poor assay (screen) ≥1.3. A negative dRVVT test does not allow for the exclusion of lupus anticoagulants as certain antibodies of this class are only detected using an aPTT-derived assay.
- Perform specific factor assays (VIII, IX, XI, and XII), which allow for the identification of the specific deficient factor. The next step is the titration of the inhibitor (typically of the inhibitor against factor VIII in acquired hemophilia A); in a Bethesda assay, the presence of lupus anticoagulants will be able to interfere with the test [41].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paddock, M.; Chapin, J. Bleeding Diatheses: Approach to the Patient Who Bleeds or Has Abnormal Coagulation. Prim. Care 2016, 43, 637–650. [Google Scholar] [CrossRef]
- Langdell, R.D.; Wagner, R.H.; Brinkhous, K.M. Effect of anti-hemophilic factor on one-stage clotting tests: A presumptive test for hemophilia and a simple one-stage anti-hemophilic factor assay procedure. J. Lab. Clin. Med. 1953, 41, 637–647. [Google Scholar]
- Dam, H.; Venndt, H. Observations on the coagulation of blood plasma in haemophilia. Lancet 1940, 238, 70–72. [Google Scholar] [CrossRef]
- Proctor, R.R.; Rapaport, S.I. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am. J. Clin. Pathol. 1961, 36, 212–219. [Google Scholar] [CrossRef]
- Favaloro, E.; Lippi, G. (Eds.) Hemostasis and Thrombosis, Methods and Protocols; Chapter 5; Humana: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Kumar, R.; Carcao, M. Inherited abnormalities of coagulation: Hemophilia, von Willebrand disease, and beyond. Pediatr. Clin. N. Am. 2013, 60, 1419–1441. [Google Scholar] [CrossRef]
- AICE (Associazione Italiana Centri Emofilia) Quality Laboratory Working Group. Consensus Document: Procedures for Hemostasis Laboratory, Mixing Test and Measurement of Factors VIII and IX; AICE (Italian Association of Haemophilia Centres): Milan, Italy. Available online: https://aiceonline.org/?p=197082022 (accessed on 8 May 2023).
- Kitchen, S.; Makris, M. Laboratory tests of hemostasis. In Practical Hemostasis and Thrombosis, 2nd ed.; Key, N., Makris, M., O’Shaughnessy, D., Lillecrap, D., Eds.; Blackwell Publishing Ltd.: London, UK, 2009; pp. 7–16. [Google Scholar]
- Lippi, G.; Blanckaert, N.; Bonini, P.; Green, S.; Kitchen, S.; Palicka, V.; Vassault, A.J.; Plebani, M. Haemolysis: An overview of the leading cause of unsuitable specimens in clinical laboratories. Clin. Chem. Lab. Med. 2008, 46, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, S.; Adcock, D.M.; Dauer, R.; Kristoffersen, A.H.; Lippi, G.; Mackie, I.; Marlar, R.A.; Nair, S. International Council for Standardisation in Haematology (ICSH) recommendations for collection of blood samples for coagulation testing. Int. J. Lab. Hematol. 2021, 43, 571–580. [Google Scholar] [CrossRef]
- Siegel, J.E.; Swami, V.K.; Glenn, P.; Peterson, P. Effect (or lack of it) of severe anemia on PT and APTT results. Am. J. Clin. Pathol. 1998, 110, 106–110. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Shu, K.; Chen, T.; Wang, X.; Xie, Y.; Li, S.; Zhang, Z.; Jin, S.; Jiang, M. The analysis of false prolongation of the activated partial thromboplastin time (activator: Silica): Interference of C-reactive protein. J. Clin. Lab. Anal. 2018, 32, e22571. [Google Scholar] [CrossRef]
- Erdem-Eraslan, L.; Hens, J.J.H.; van Rossum, A.P.; Frasa, M.A.M.; Keuren, J.F.W. Inter-individual variability in phospholipid-dependent interference of C-reactive protein on activated partial thromboplastin time. Br. J. Haematol. 2018, 183, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26 (Suppl. S6), 1–158. [Google Scholar] [CrossRef]
- Menegatti, M.; Peyvandi, F. Treatment of rare factor deficiencies other than hemophilia. Blood 2019, 133, 415–424. [Google Scholar] [CrossRef]
- Favaloro, E. Coagulation mixing studies: Utility, algorithmic strategies and limitations for lupus anticoagulant testing or follow up of abnormal coagulation tests. Am. J. Haematol. 2020, 95, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Tiede, A.; Zieger, B.; Lisman, T. Acquired bleeding disorders. Haemophilia 2021, 27 (Suppl. S3), 5–13. [Google Scholar] [CrossRef]
- Tripodi, A. Laboratory testing for lupus anticoagulants: A review of issues affecting results. Clin. Chem. 2007, 53, 1629–1635. [Google Scholar] [CrossRef]
- Mulliez, S.M.N.; De Keyser, F.; Verbist, C.; Vantilborgh, A.; Wijns, W.; Beukinga, I.; Devreese, K.M.J. Lupus anticoagulant-hypoprothrombinemia syndrome: Report of two cases and review of literature. Lupus 2015, 24, 736–745. [Google Scholar] [CrossRef]
- Unlu, O.; Zuily, S.; Erkan, D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur. J. Rheumatol. 2016, 3, 75–84. [Google Scholar] [CrossRef]
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef]
- Vila, P.; Hernandez, M.C.; Lopez-Fernandez, M.F.; Batlle, J. Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb. Haemost. 1994, 72, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, V.; Ames, P.R.; Glynn, J.; Iannaccone, L.; Mackie, I.J. A rapid screen for lupus anticoagulant with good discrimination from oral anticoagulants, congenital factor deficiency and heparin, is provided by comparing a sensitive and an insensitive APTT reagent. Blood Coagul. Fibrinolysis 1997, 8, 155–160. [Google Scholar] [CrossRef]
- Rasmussen, K.L.; Philips, M.; Tripodi, A.; Goetze, J.P. Unexpected, isolated activated partial thromboplastin time prolongation: A practical mini-review. Eur. J. Haematol. 2020, 104, 519–525. [Google Scholar] [CrossRef]
- Cuker, A.; Siegal, D.M.; Crowther, M.A.; Garcia, D.A. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J. Am. Coll. Cardiol. 2014, 64, 1128–1139. [Google Scholar] [CrossRef]
- Tagariello, G.; Radossi, P.; Salviato, R.; Zardo, M.; De Valentin, L.; Basso, M.; Castaman, G. Clinical relevance of isolated prolongation of the activated partial thromboplastin time in a cohort of adults undergoing surgical procedures. Blood Transf. 2017, 15, 557–561. [Google Scholar] [CrossRef]
- Munro, J.; Booth, A.; Nicholl, J. Routine preoperative testing: A systematic review of the evidence. Health Technol. Assess. 1997, 1, 1–62. [Google Scholar] [CrossRef]
- Rocca, B.; Fox, K.A.A.; Ajjan, R.A.; Andreotti, F.; Baigent, C.; Collet, J.-P.; Grove, E.L.; Halvorsen, S.; Huber, K.; Morais, J.; et al. Antithrombotic therapy and body mass: An expert position paper of the ESC Working Group on Thrombosis. Eur. Heart J. 2018, 39, 1672f–1686f. [Google Scholar] [CrossRef]
- Watson, H.; Davidson, S.; Keeling, D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: Second edition. Br. J. Haematol. 2012, 159, 528–540. [Google Scholar] [CrossRef]
- Federici, A.B.; Königs, C.; James, A.H. Contemporary issues in the management of von Willebrand disease. Thromb. Haemost. 2016, 116 (Suppl. 1), S18–S25. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Favaloro, E.J. Interference in coagulation testing: Focus on spurious hemolysis, icterus, and lipemia. Semin. Thromb. Hemost. 2013, 39, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, G.; Orellana, D. Mixing tests: Diagnostic aides in the investigation of prolonged prothrombin times and activated partial thromboplastin times. Semin. Thromb. Hemost. 2013, 39, 283–290. [Google Scholar] [CrossRef]
- Kershaw, G. Detection and Measurement of Factor Inhibitors. Methods Mol. Biol. 2017, 1646, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Tillema, V.; Scherr, D. A “percent correction” formula for evaluation of mixing studies. Am. J. Clin. Pathol. 2002, 117, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ling, L.; Huang, X.; Mi, J.; Liao, J.; Jia, J.; Wang, X.; Zhou, J. Evaluation of Activated Partial Thromboplastin Time Mixing Studies Using Several Methods. Arch. Pathol. Lab. Med. 2022, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Ule Priebbenow, V.; Pasalic, L.; Favaloro, E.J. Development and implementation of an expert rule set for automated reflex testing and validation of routine coagulation tests in a large pathology network. Int. J. Lab. Hematol. 2019, 41, 642–649. [Google Scholar] [CrossRef]
- Blennerhassett, R.; Favaloro, E.J.; Pasalic, L. Coagulation studies: Achieving the right mix in a large laboratory network. Pathology 2019, 51, 718–722. [Google Scholar] [CrossRef]
- Rosner, E.; Pauzner, R.; Lusky, A.; Modan, M.; Many, A. Detection and quantitative evaluation of lupus circulating anticoagulant activity. Thromb. Haemost. 1987, 57, 144–147. [Google Scholar] [CrossRef]
- Benzon, H.T.; Park, M.; McCarthy, R.J.; Kendall, M.C.; Lindholm, P.F. Mixing studies in patients with prolonged activated partial thromboplastin time or prothrombin time. Anesth. Analg. 2019, 128, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Riano, I.; Prasongdee, K. A Rare Cause of Isolated Prolonged Activated Partial Thromboplastin Time: An Overview of Prekallikrein Deficiency and the Contact System. J. Investig. Med. High. Impact. Case. Rep. 2021, 9, 23247096211012187. [Google Scholar] [CrossRef]
- Kershaw, G.; Favaloro, E.J. Laboratory identification of factor inhibitors: An update. Pathology 2012, 44, 293–302. [Google Scholar] [CrossRef]
- Kazmi, M.A.; Pickering, W.; Smith, M.P.; Holland, L.J.; Savidge, G.F. Acquired haemophilia A: Errors in the diagnosis. Blood Coagul. Fibrinolysis 1998, 9, 623–628. [Google Scholar] [CrossRef]
- Kershaw, G.; Jayakodi, D.; Dunkley, S. Laboratory identification of factor inhibitors: The perspective of a large tertiary hemophilia center. Semin. Thromb. Hemost. 2009, 35, 760–768. [Google Scholar] [CrossRef]
- Lippi, G.; Favaloro, E.J.; Montagnana, M.; Manzato, F.; Guidi, G.C.; Franchini, M. Inherited and acquired factor V deficiency. Blood Coagul. Fibrinolysis 2011, 22, 160–166. [Google Scholar] [CrossRef] [PubMed]
| Cause |
|---|
| Hemolysis |
| Hyperbilirubinemia |
| Hypertriglyceridemia |
| Blood/anticoagulant ratio |
| Hemoconcentration |
| Clinical Condition | Ancillary Laboratory Data (Refer to Section 2.2) | Note | Reference |
|---|---|---|---|
| High C-reactive protein | Normal prothrombin time | Variable sensitivity among aPTT reagents | [12,13] |
| Factor VIII deficiency (hemophilia A) | Normal thrombin time Corrected immediate mixing test | Possible positive bleeding history | [14] |
| Factor IX deficiency (hemophilia B) | Normal thrombin time Corrected immediate mixing test | Possible positive bleeding history | [14] |
| Factor XI deficiency | Normal thrombin time Corrected immediate mixing test | Possible positive bleeding history | [15] |
| Factor XII deficiency | Normal thrombin time Corrected immediate mixing test | No bleeds | [15] |
| Pre kallicreine deficiency | Normal thrombin time Corrected both immediate and incubated mixing tests, as well as aPTT incubated up to 60 min | No bleeds | [16] |
| Factor XII and high molecular kininogens deficiency | Normal thrombin time Corrected immediate mixing test | No bleeds | [16] |
| FVIII inhibitor | Normal thrombin time Immediate mixing test may be corrected Incubated mixing test usually uncorrected | Possible positive bleeding history | [17] |
| Lupus anticoagulant (LA) | Normal thrombin time Uncorrected mixing test, immediate and incubated | See text | [18,19,20,21,22,23] |
| Unfractionated heparin | Uncorrected mixing test Prolonged thrombin time | Positive history for the drug | [24] |
| Low molecular weight heparin/fondaparinux | aPTT prolonged only at therapeutic doses Presence of antiXa activity | Positive history for the drug | [25] |
| Immediate Correction | Correction after Incubation | Conclusion |
|---|---|---|
| Yes | Yes | Factor deficiency |
| No or Partial | No | Factor inhibitor |
| No | No | Lupus anticoagulant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, R.C.; Molinari, A.C.; Leotta, M.; Martini, T. Isolated Prolongation of Activated Partial Thromboplastin Time: Not Just Bleeding Risk! Medicina 2023, 59, 1169. https://doi.org/10.3390/medicina59061169
Santoro RC, Molinari AC, Leotta M, Martini T. Isolated Prolongation of Activated Partial Thromboplastin Time: Not Just Bleeding Risk! Medicina. 2023; 59(6):1169. https://doi.org/10.3390/medicina59061169
Chicago/Turabian StyleSantoro, Rita Carlotta, Angelo Claudio Molinari, Marzia Leotta, and Tiziano Martini. 2023. "Isolated Prolongation of Activated Partial Thromboplastin Time: Not Just Bleeding Risk!" Medicina 59, no. 6: 1169. https://doi.org/10.3390/medicina59061169
APA StyleSantoro, R. C., Molinari, A. C., Leotta, M., & Martini, T. (2023). Isolated Prolongation of Activated Partial Thromboplastin Time: Not Just Bleeding Risk! Medicina, 59(6), 1169. https://doi.org/10.3390/medicina59061169








