Extracranial Facial Nerve Schwannoma—Histological Surprise or Therapeutic Planning?
Abstract
1. Introduction
2. Anatomical Reminder
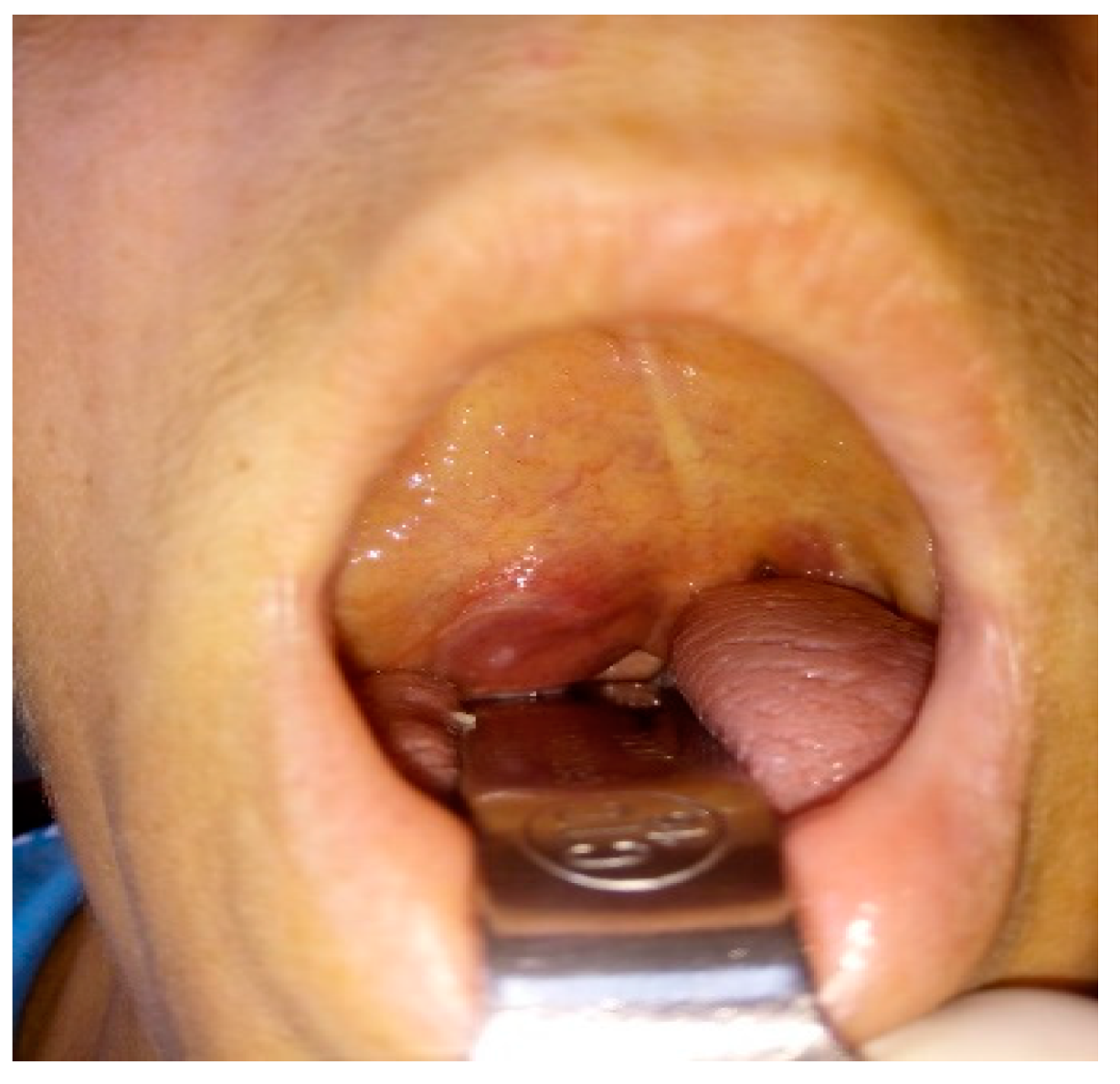
3. The Clinical Picture of Extracranial FNSs
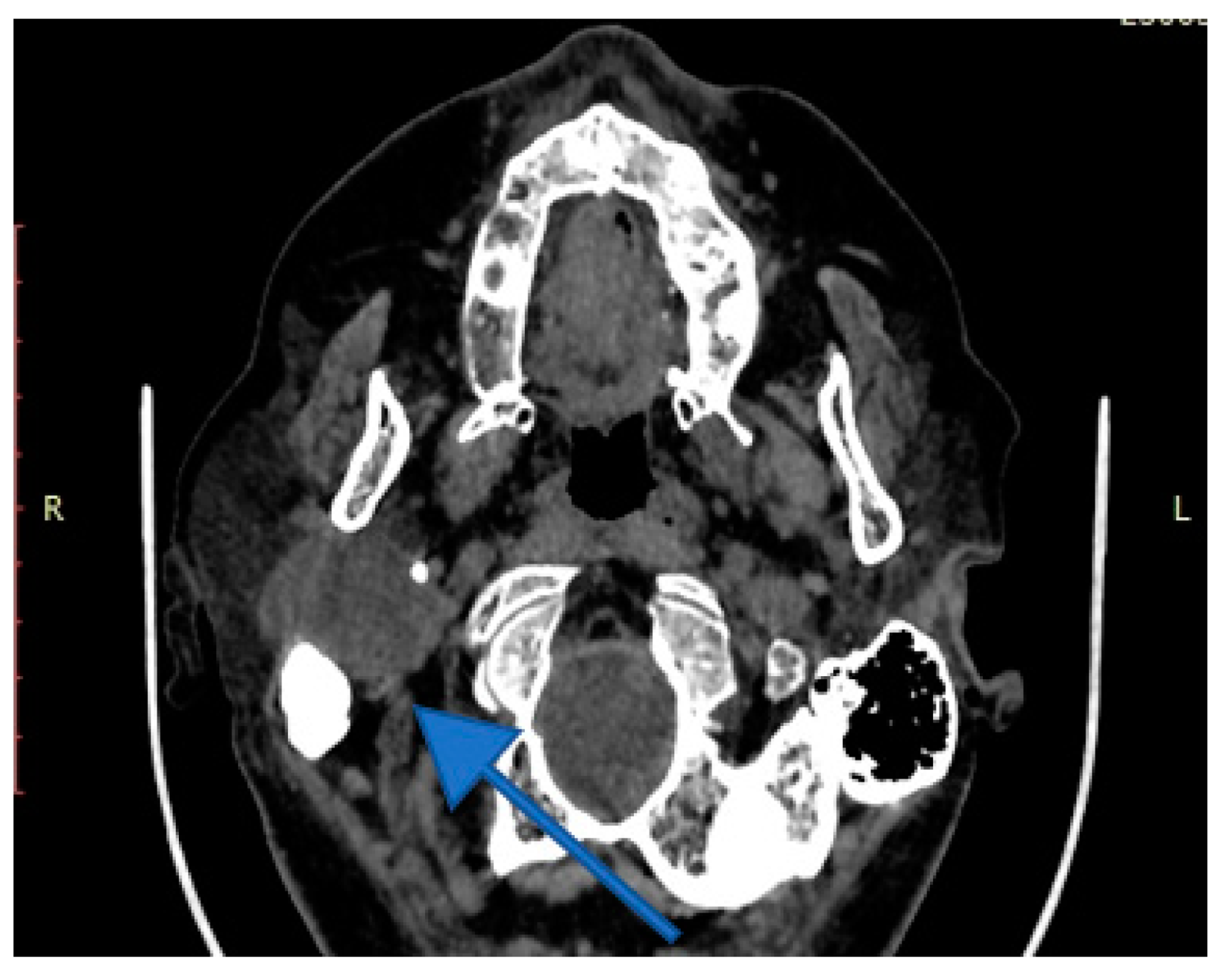
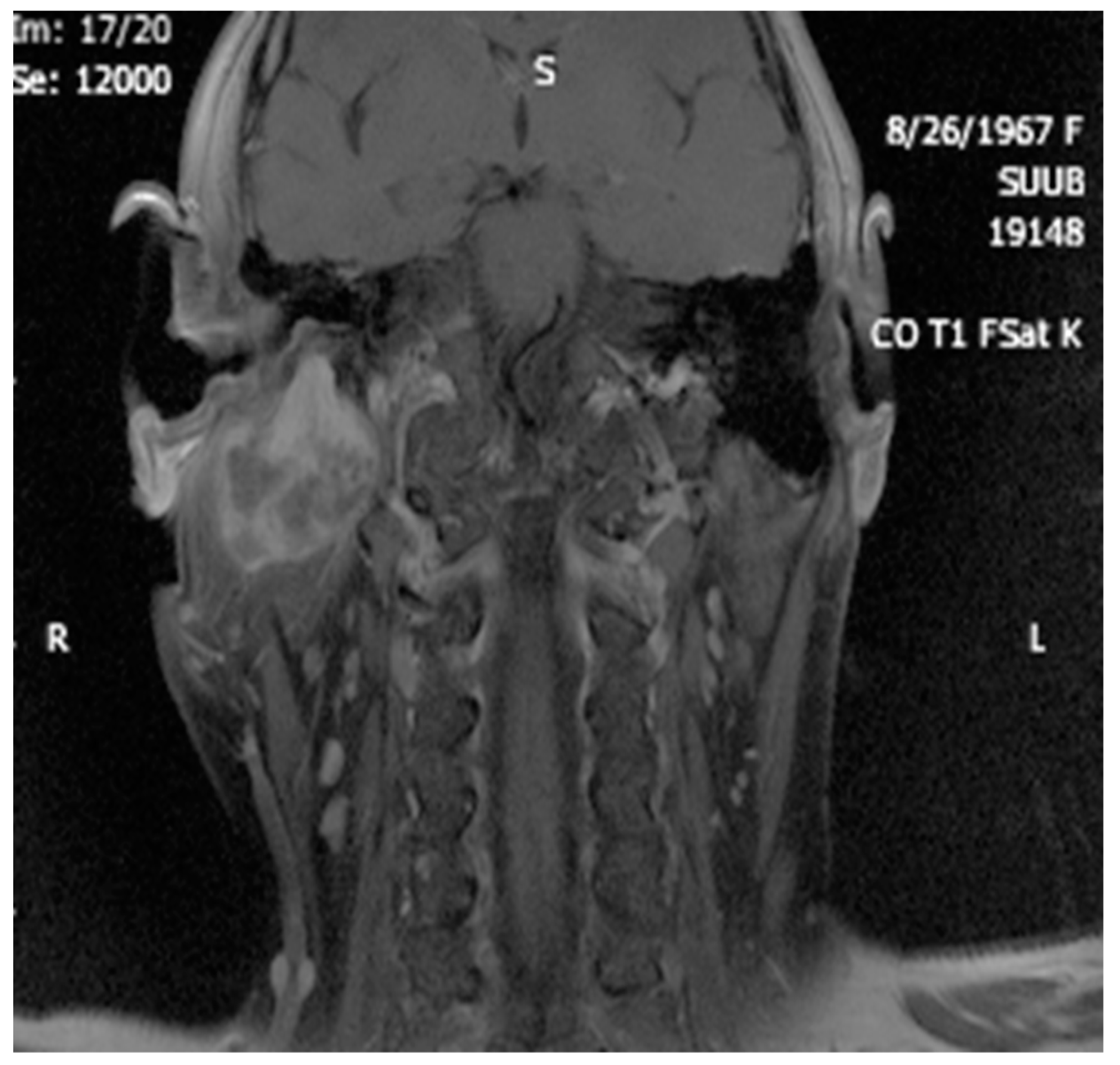
4. Imagery in Extracranial FNSs
5. Histology
6. Differential Diagnosis of the Extracranial FNS
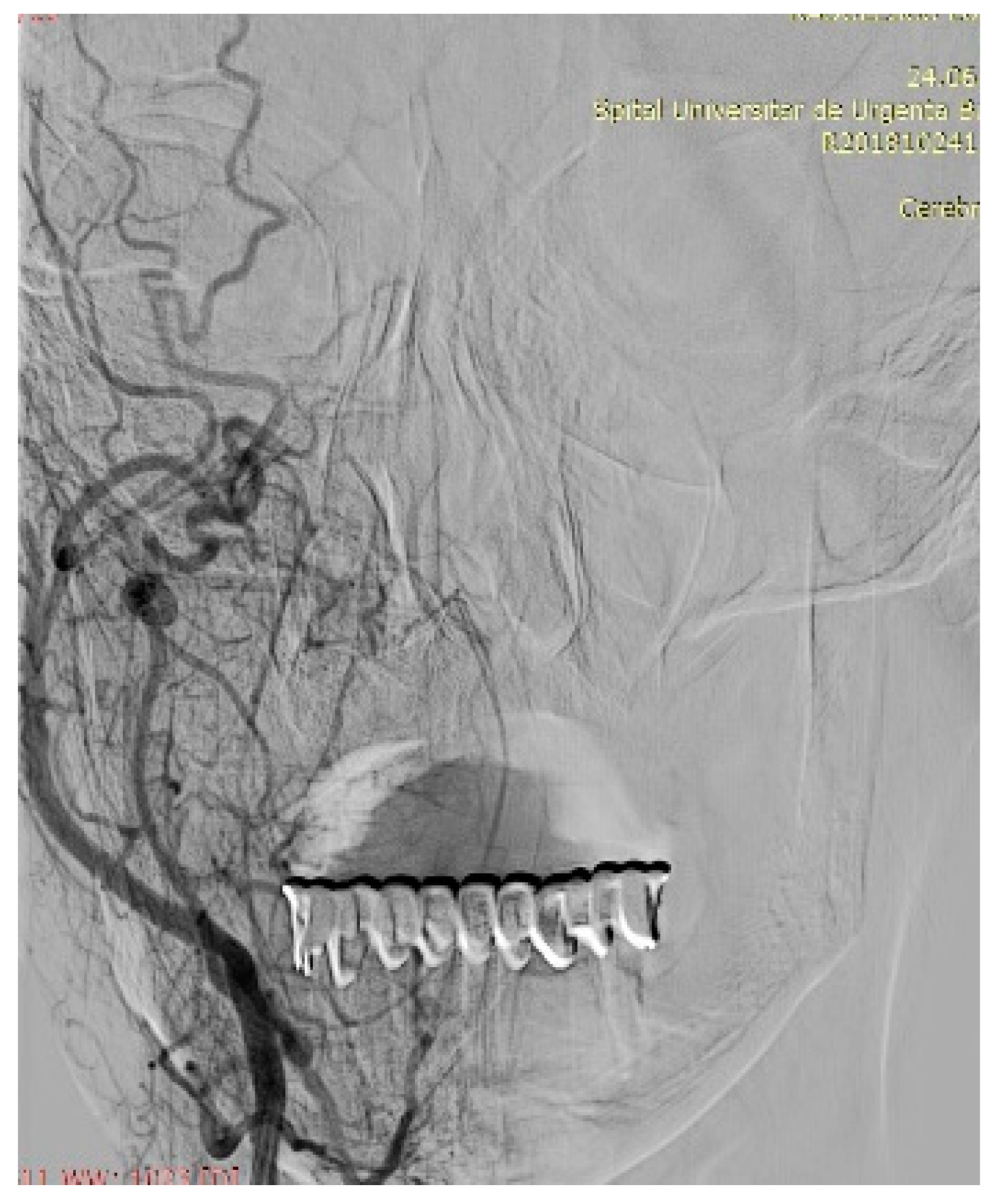
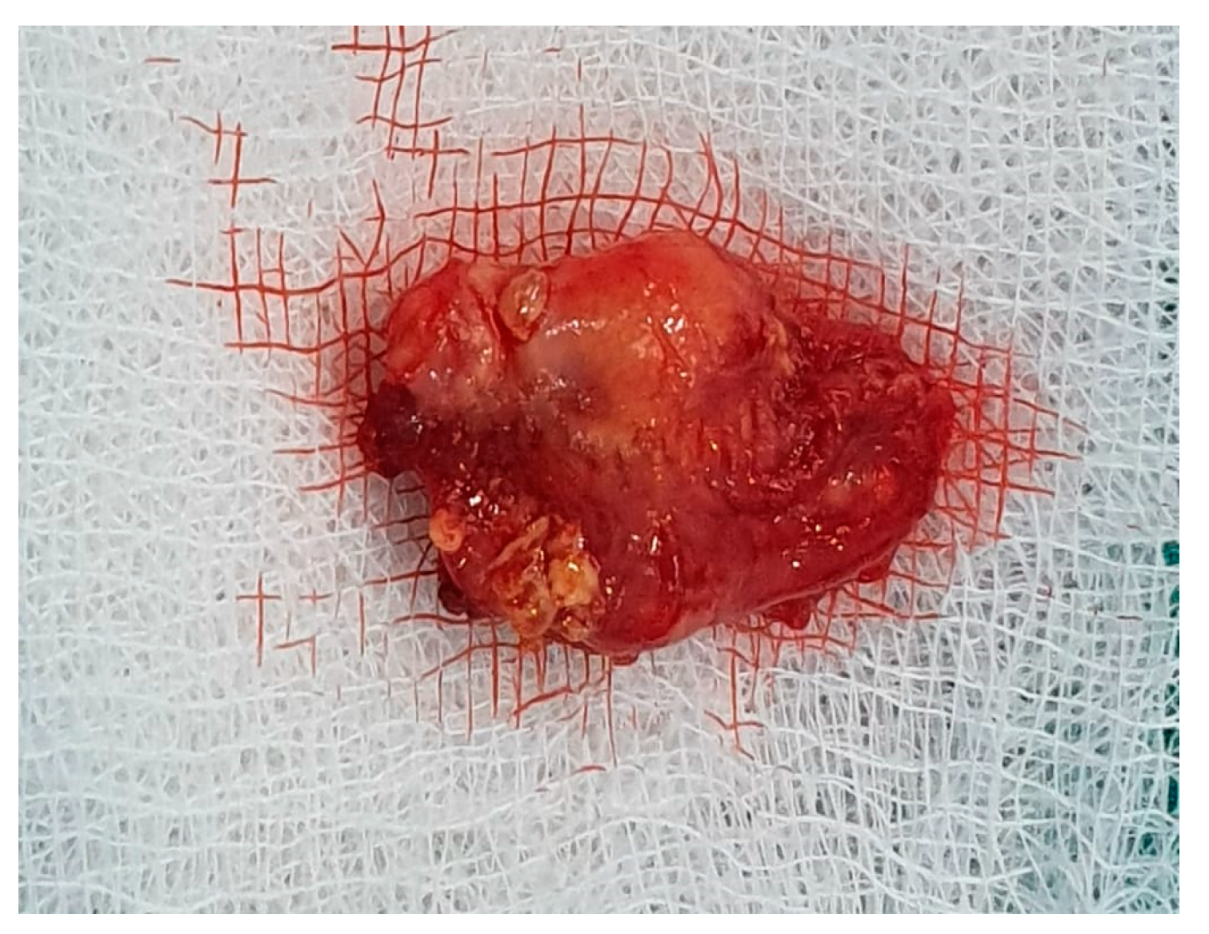
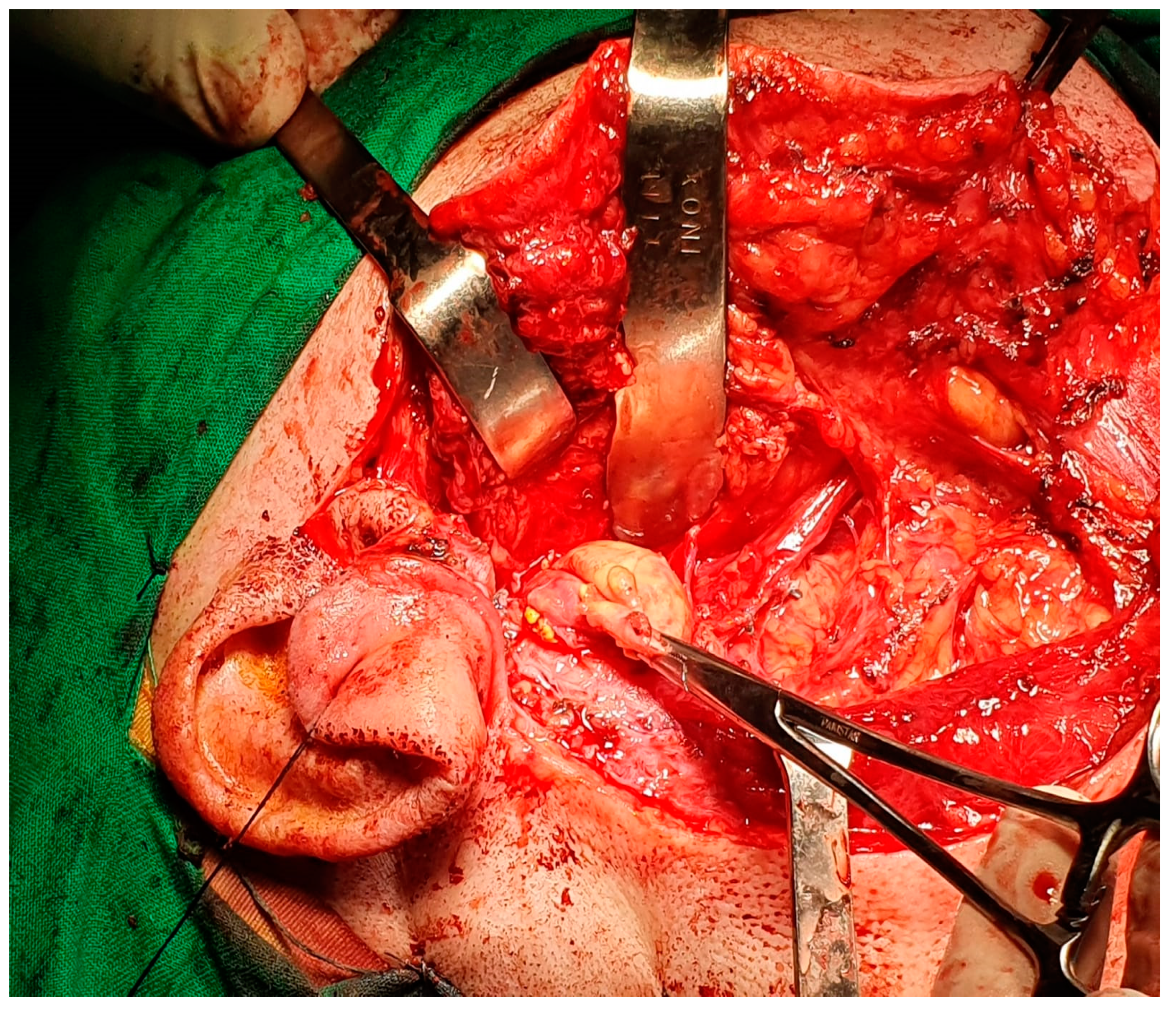
7. Therapeutic Strategies in the Management of FNS Extracranial Segment
8. Discussions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haider, M.Y.; Rahim, M.; Bashar, N.M.K.; Hossain, Z.; Islam, S.M.J. Schwannoma of the Base of the Tongue: A Case Report of a Rare Disease and Review of Literatures. Case Rep. Surg. 2020, 2020, 7942062. [Google Scholar] [CrossRef] [PubMed]
- Damar, M.; Dinç, A.E.; Eliçora, S.; Bişkin, S.; Erten, G.; Biz, S. Facial Nerve Schwannoma of Parotid Gland: Difficulties in Diagnosis and Management. Case Rep. Otolaryngol. 2016, 2016, 3939685. [Google Scholar] [CrossRef] [PubMed]
- Caughey, R.J.; May, M.; Schaitkin, B.M. Intraparotid Facial Nerve Schwannoma: Diagnosis and Management. Otolaryngol. Neck Surg. 2004, 130, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Pascual, P.M.; Maranillo, E.; Vázquez, T.; De Blas, C.S.; Lasso, J.M.; Sañudo, J.R. Extracranial Course of the Facial Nerve Revisited. Anat. Rec. 2019, 302, 599–608. [Google Scholar] [CrossRef]
- Yang, H.-M.; Yoo, Y.-B. Anatomy of the Facial Nerve at the Condylar Area: Measurement Study and Clinical Implications. Sci. World J. 2014, 2014, 473568. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Anson, B.J.; Budinger, J.M.; Kurth, L.R. Surgical anatomy of the facial nerve and parotid gland based upon a study of 350 cervicofacial halves. Surgery Gynecol. Obstet. 1956, 102, 385–412. [Google Scholar]
- Poutoglidis, A.; Paraskevas, G.K.; Lazaridis, N.; Georgalas, C.; Vlachtsis, K.; Markou, K.; Gougousis, S.; Fyrmpas, G.; Keramari, S.; Tsentemeidou, A.; et al. Extratemporal facial nerve branching patterns: Systematic review of 1497 cases. J. Laryngol. Otol. 2022, 136, 1170–1176. [Google Scholar] [CrossRef]
- Martínez-Pascual, P.; Pérez-Lloret, P.; Alcaide, E.M.; Sanz-García, C.; de Blas, C.S.; Sanudo, J.; Konschake, M.; Porzionato, A.; De Caro, R.; Macchi, V. Connections between postparotid terminal branches of the facial nerve: An immunohistochemistry study. Clin. Anat. 2023, 36, 28–35. [Google Scholar] [CrossRef]
- Jirawatnotai, S.; Kaewpichai, K.; Tirakotai, W.; Mothong, W.; Kaewsema, A.; Sriswadpong, P. Nerve to the zygomaticus major muscle for facial reanimation surgery: A cadaveric study for branching patterns and axonal count. Asian J. Neurosurg. 2020, 15, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Stankevicius, D.; Suchomlinov, A. Variations in Facial Nerve Branches and Anatomical Landmarks for Its Trunk Identification: A Pilot Cadaveric Study in the Lithuanian Population. Cureus 2019, 11, e6100. [Google Scholar] [CrossRef]
- Piagkou, M.; Tzika, M.; Paraskevas, G.; Natsis, K. Anatomic variability in the relation between the retromandibular vein and the facial nerve: A case report, literature review and classification. Folia Morphol. 2013, 72, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Guinto, G.; Hernandez, E.; Gallardo-Ceja, D.; Gallegos-Hernandez, F.; Arechiga, N.; Guinto-Nishimura, G. Anatomical Basis of the Zygomatic-Transmandibular Approach: Operative Video. J. Neurol. Surg. Part B Skull Base 2021, 83 (Suppl. 3), e646–e647. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pinheiro-Neto, C.D.; Yang, D.; Wang, E.; Gardner, P.A.; Hirsch, B.E.; Snyderman, C.H.; Fernandez-Miranda, J.C. Comparison of Endoscopic Endonasal Approach and Lateral Microsurgical Infratemporal Fossa Approach to the Jugular Foramen: An Anatomical Study. J. Neurol. Surg. Part B Skull Base 2021, 83 (Suppl. 2), e474–e483. [Google Scholar] [CrossRef]
- Seo, B.F.; Choi, H.J.; Seo, K.J.; Jung, S.-N. Intraparotid facial nerve schwannomas. Arch. Craniofacial Surg. 2019, 20, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shi, Z.; Wang, C.; Yu, Z. Facial nerve schwannoma mimicking chronic suppurative otitis media: A case report. Medicine 2019, 98, e16844. [Google Scholar] [CrossRef] [PubMed]
- Bartindale, M.; Heiferman, J.; Joyce, C.; Balasubramanian, N.; Anderson, D.; Leonetti, J. The Natural History of Facial Schwannomas: A Meta-Analysis of Case Series. J. Neurol. Surg. Part B Skull Base 2019, 80, 458–468. [Google Scholar] [CrossRef]
- Shimizu, K.; Iwai, H.; Ikeda, K.; Sakaida, N.; Sawada, S. Intraparotid Facial Nerve Schwannoma: A Report of Five Cases and an Analysis of MR Imaging Results. AJNR Am. J. Neuroradiol. 2005, 26, 1328–1330. [Google Scholar]
- Jaiswal, A.; Mridha, A.R.; Nath, D.; Bhalla, A.S.; Thakkar, A. Intraparotid facial nerve schwannoma: A case report. World J. Clin. Cases 2015, 3, 322–326. [Google Scholar] [CrossRef]
- Yamakami, I.; Ono, J.; Yamaura, A. Sigmoid Sinus Dural Arteriovenous Malformation Resulting from Jugular Foramen Schwannoma—Case Report. Neurol. Med. Chir. 1998, 38, 43–46. [Google Scholar] [CrossRef]
- Kühn, J.P.; Wagner, M.; Bozzato, A.; Linxweiler, M. Multiple schwannomas of the facial nerve mimicking cervical lymphoma: A case report. J. Med. Case Rep. 2021, 15, 436. [Google Scholar] [CrossRef]
- Simone, M.; Vesperini, E.; Viti, C.; Camaioni, A.; Lepanto, L.; Raso, F. Intraparotid facial nerve schwannoma: Two case reports and a review of the literature. Acta Otorhinolaryngol. Ital. 2018, 38, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, H.; Sasaki, R.; Fujii, K.; Sakurai, H. Intramasseteric Schwannoma Derived from the Masseteric Nerve. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2175. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.R.; Karim, F.; Rawal, Y.B. Divergent Schwannoma-Like Phenotype in a Pleomorphic Adenoma. Head Neck Pathol. 2017, 11, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Bewley, A.F.; Azhdam, A.M.; Borrelli, M. Intraparotid Facial Nerve Schwannoma Mimicking Primary Parotid Neoplasm. Ear, Nose Throat J. 2021, 100 (Suppl. 6), 881S–883S. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.H.; Faquin, W.C.; Gimenez, C.; Vadalia, B.; Frank, D.K.; Li, J.Y. Schwannoma-Like Pleomorphic Adenoma: Two Cases and a Review of the Literature. Head Neck Pathol. 2020, 14, 166–172. [Google Scholar] [CrossRef]
- Osano, H.; Ioka, Y.; Okamoto, R.; Nakai, Y.; Hayashi, H.; Tsuchiya, Y.; Yamada, S. Angioleiomyoma of the cheek: A case report. J. Oral Sci. 2015, 57, 63–66. [Google Scholar] [CrossRef]
- Bakar, B. The Jugular Foramen Schwannomas: Review of the Large Surgical Series. J. Korean Neurosurg. Soc. 2008, 44, 285–294. [Google Scholar] [CrossRef]
- Shivhare, P.; Shankarnarayan, L.; Jambunath, U.; Basavaraju, S.M. Benign lymphoepithelial cysts of parotid and submandibular glands in a HIV-positive patient. J. Oral Maxillofac. Pathol. 2015, 19, 107. [Google Scholar] [CrossRef]
- Galhotra, V.; Sheikh, S.; Jindal, S.; Singla, A. Segmental neurofibromatosis. Indian J. Dent. 2014, 5, 166–169. [Google Scholar] [CrossRef]
- Kader, M.I.S.A.; Abdullah, A.; Yunus, M.R.M.; Jaafar, M.N.; Kew, T.Y. Preoperative Challenges in Managing Intraparotid Schwannoma. Cureus 2022, 14, e21392. [Google Scholar] [CrossRef]
- Chowdhary, S.; Thangavel, S.; Ganesan, S.; Alexander, A. Extratemporal intraparotid facial nerve schwannoma. BMJ Case Rep. 2021, 14, e239407. [Google Scholar] [CrossRef] [PubMed]
- Sim, L.; Yeoh, X.Y.; Tan, T.E.; Zakaria, Z.; Mohamad, I. Intracapsular Enucleation of Intraparotid Facial Nerve Schwannoma with Intratemporal Extension. Medeni. Med. J. 2022, 37, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, D.; Ciufelli, M.A.; Presutti, L. Intraparotid facial nerve schwannoma: Literature review and classification proposal. J. Laryngol. Otol. 2007, 121, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Bagga, M.B.; Bhatnagar, D.; Katoch, S. Preauricular intraparotid schwannoma: A rare presentation with literature review. Contemp. Clin. Dent. 2021, 12, 191–194. [Google Scholar] [CrossRef]
- Paolucci, T.; Cardarola, A.; Colonnelli, P.; Ferracuti, G.; Gonnella, R.; Murgia, M.; Santilli, V.; Paoloni, M.; Bernetti, A.; Agostini, F.; et al. Give me a kiss! An integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur. J. Phys. Rehabil. Med. 2020, 56, 58–67. [Google Scholar] [CrossRef]
- Sasaki, A.; Miyazaki, S.; Hori, T. Extracranial Facial Nerve Schwannoma Treated by Hypo-fractionated CyberKnife Radiosurgery. Cureus 2016, 8, e797. [Google Scholar] [CrossRef]
- Cho, H.R.; Kwon, S.S.; Chung, S.; Choi, Y.J. Intraparotid Facial Nerve Schwannoma. Arch. Craniofacial Surg. 2014, 15, 28–31. [Google Scholar] [CrossRef]
- Dumitru, M.; Berghi, O.N.; Taciuc, I.-A.; Vrinceanu, D.; Manole, F.; Costache, A. Could Artificial Intelligence Prevent Intraoperative Anaphylaxis? Reference Review and Proof of Concept. Medicina 2022, 58, 1530. [Google Scholar] [CrossRef]
- Kang, G.C.; Soo, K.-C.; Lim, D.T. Extracranial Non-vestibular Head and Neck Schwannomas: A Ten-year Experience. Ann. Acad. Med. Singap. 2007, 36, 233–238. [Google Scholar] [CrossRef]
- Rigante, M.; Petrelli, L.; de Corso, E.; Paludetti, G. Intracapsular microenucleation technique in a case of intraparotid facial nerve schwannoma. Technical notes for a conservative approach. Acta Otorhinolaryngol. Ital. 2015, 35, 49–52. [Google Scholar]



















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrinceanu, D.; Dumitru, M.; Popa-Cherecheanu, M.; Marinescu, A.N.; Patrascu, O.-M.; Bobirca, F. Extracranial Facial Nerve Schwannoma—Histological Surprise or Therapeutic Planning? Medicina 2023, 59, 1167. https://doi.org/10.3390/medicina59061167
Vrinceanu D, Dumitru M, Popa-Cherecheanu M, Marinescu AN, Patrascu O-M, Bobirca F. Extracranial Facial Nerve Schwannoma—Histological Surprise or Therapeutic Planning? Medicina. 2023; 59(6):1167. https://doi.org/10.3390/medicina59061167
Chicago/Turabian StyleVrinceanu, Daniela, Mihai Dumitru, Matei Popa-Cherecheanu, Andreea Nicoleta Marinescu, Oana-Maria Patrascu, and Florin Bobirca. 2023. "Extracranial Facial Nerve Schwannoma—Histological Surprise or Therapeutic Planning?" Medicina 59, no. 6: 1167. https://doi.org/10.3390/medicina59061167
APA StyleVrinceanu, D., Dumitru, M., Popa-Cherecheanu, M., Marinescu, A. N., Patrascu, O.-M., & Bobirca, F. (2023). Extracranial Facial Nerve Schwannoma—Histological Surprise or Therapeutic Planning? Medicina, 59(6), 1167. https://doi.org/10.3390/medicina59061167







