High Fat Diet Exaggerate Metabolic and Reproductive PCOS Features by Promoting Oxidative Stress: An Improved EV Model in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee Approval
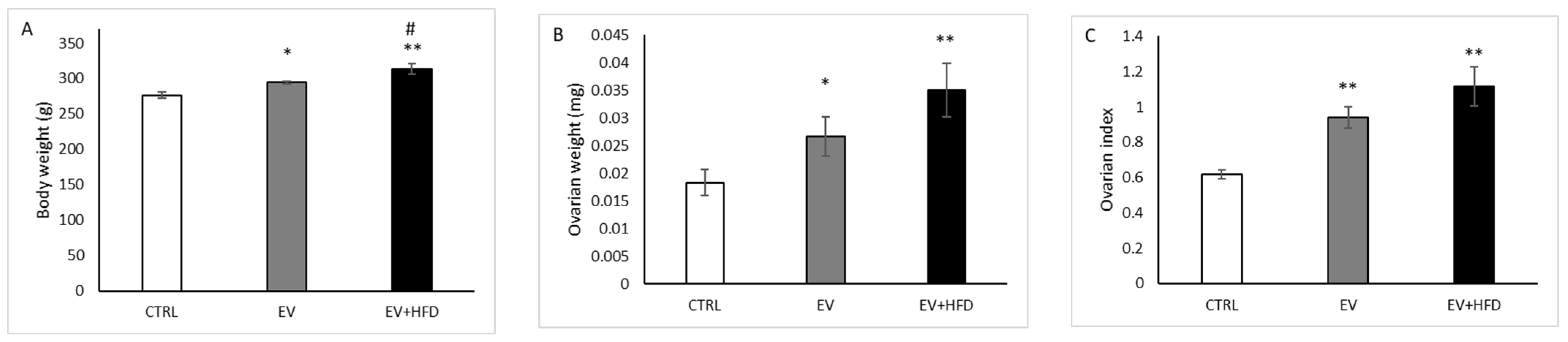
2.2. Animals
2.3. Induction of PCOS
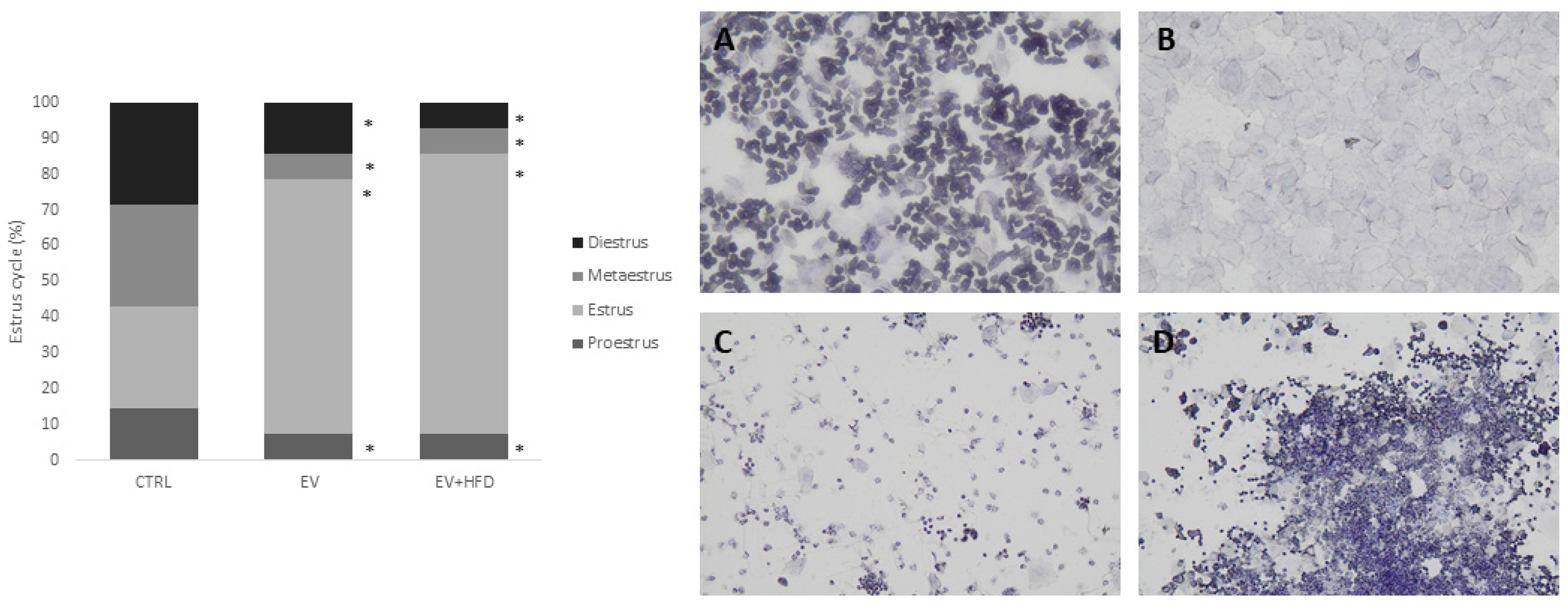
2.4. Estrus Cycle Analysis
2.5. Oral Glucose Tolerance Test (OGTT)
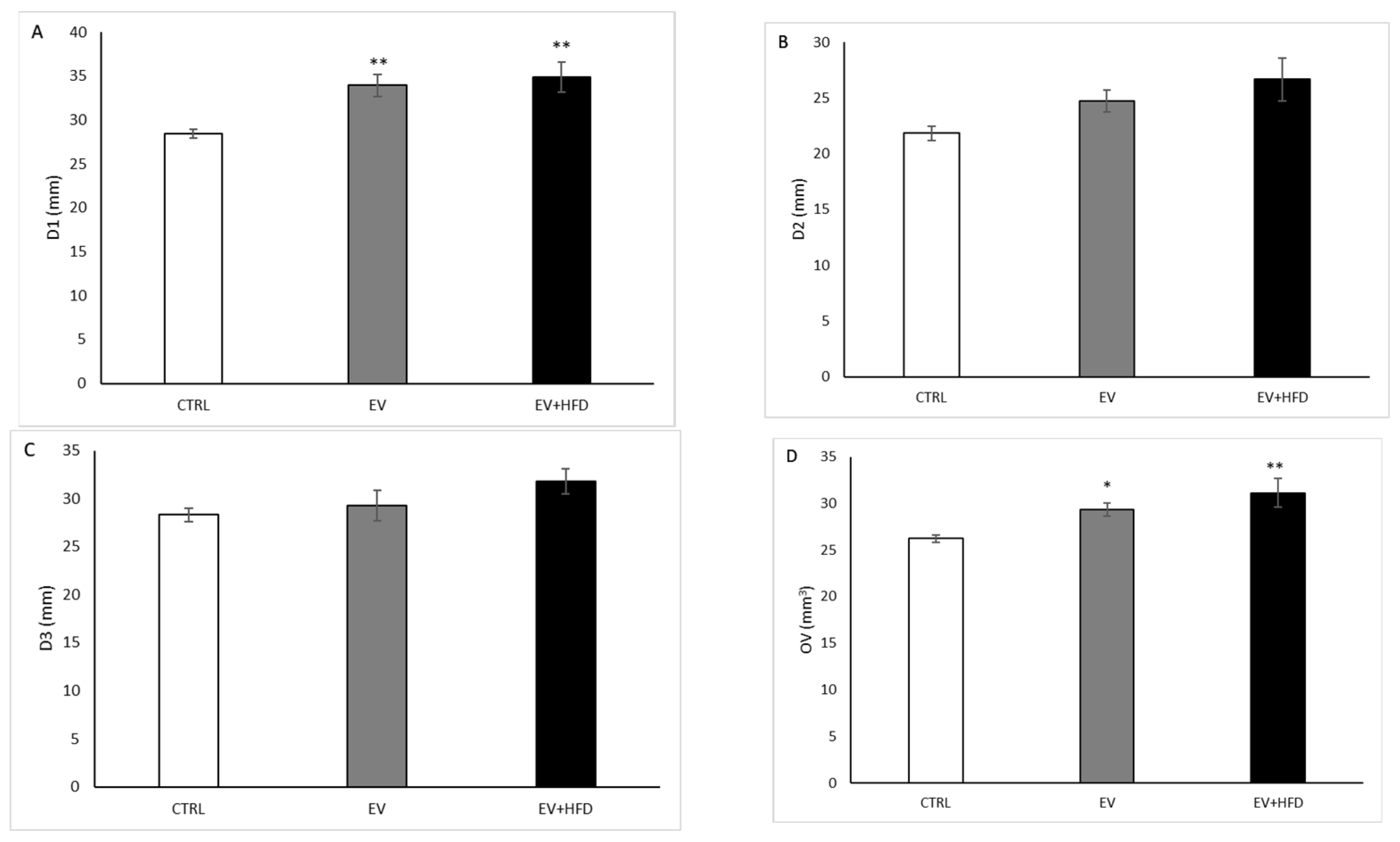
2.6. Ultrasound Examination of the Ovaries
2.7. Animal Euthanization and Sample Collection
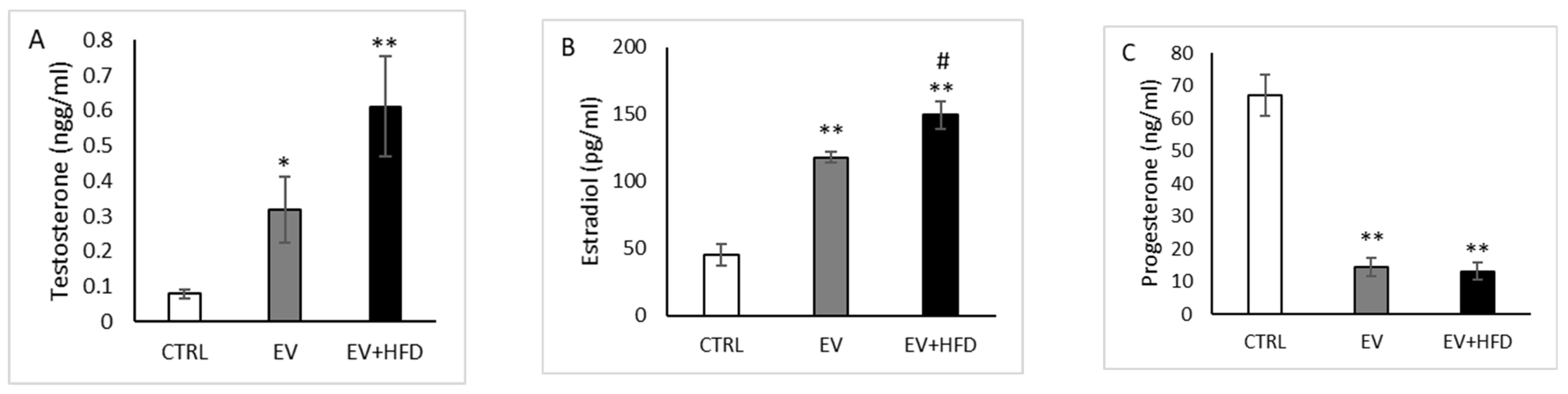
2.8. Biochemical Analyses
2.9. Oxidative Stress (OS) Parameters
2.9.1. TBARS Determination
2.9.2. Determination of NO2
2.9.3. Determination of H2O2
2.9.4. Determination of O2
2.9.5. Determination of CAT Activity
2.9.6. Determination of SOD Activity
2.9.7. Determination of GSH Concentration
2.10. Histological Analysis of Ovary
2.11. Statistical Analysis
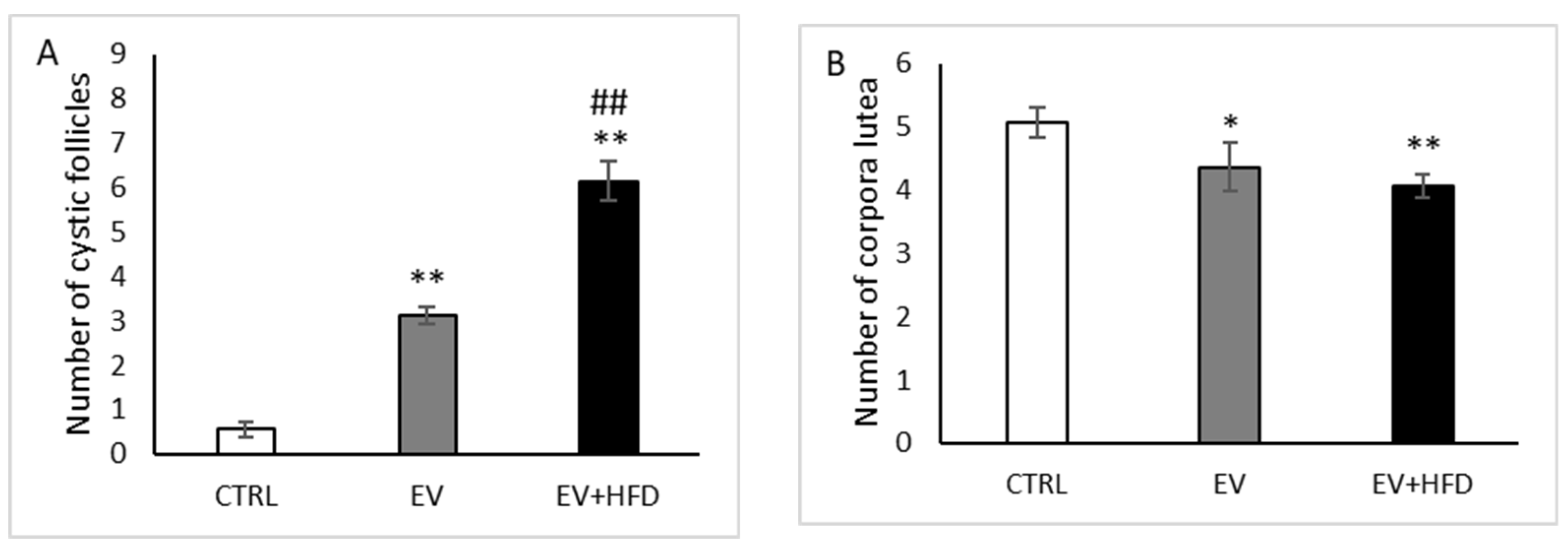
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Poly-cystic ovary syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Suturina, L.; Lizneva, D.; Davies, M.J.; Hummitzsch, K.; Irving-Rodgers, H.F.; Robertson, S.A. Is polycystic ovary syn-drome a 20th Century phenomenon? Med. Hypotheses 2019, 124, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Mohd, M.; Maqbool, M.; Dar, M.A.; Mushtaq, I. Polycystic Ovary Syndrome, a modern epidemic: An overview. JDDT 2019, 9, 641–644. [Google Scholar]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Bruno, C.; Vergani, E.; d’Abate, C.; Giacchi, E.; Silvestrini, A. Oxidative Stress and Low-Grade Inflammation in Poly-cystic Ovary Syndrome: Controversies and New Insights. Int. J. Mol. Sci. 2021, 22, 1667. [Google Scholar] [CrossRef]
- Barber, T.M. Why are women with polycystic ovary syndrome obese? Br. Med. Bull. 2022, 143, 4–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Lin, F.; Wang, Z. Endocrine Characteristics and Regulatory Mechanism of Follicular Development and Ovulation Failure in Mammalian Ovary. In Polycystic Ovarian Syndrome; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Mohammadi, M. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X. Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1085–E1092. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Shokrollahi, B.; Khademi, N.; Akbari, A. Superior effect of broccoli methanolic extract on control of oxidative damage of sperm cryopreservation and reproductive performance in rats: A comparison with vitamin C and E antioxidant. Theriogenology 2022, 181, 50–58. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Saleh Hosseini, S.M.; Amiri, A.A. Impact of Co-Administration of N-Acetylcysteine and Vitamin E on Cyclophosphamide-Induced Ovarian Toxicity in Female Rats. J. Toxicol. 2022, 2022, 9073405. [Google Scholar] [CrossRef]
- Rudic, J.; Jakovljevic, V.; Jovic, N.; Nikolic, M.; Sretenovic, J.; Mitrovic, S.; Bolevich, S.; Bolevich, S.; Mitrovic, M.; Raicevic, S.; et al. Antioxidative Effects of Standardized Aronia melanocarpa Extract on Re-productive and Metabolic Disturbances in a Rat Model of Polycystic Ovary Syndrome. Antioxidants 2022, 11, 1099. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, Q.; Long, S.L.; Zhong, Q.; Mo, Z. Mitochondrial dysfunction in polycystic ovary syndrome. DNA Cell. Biol. 2020, 39, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Maliqueo, M.; Benrick, A.; Stener-Victorin, E. Rodent models of polycystic ovary syndrome: Phenotypic presentation, patho-physiology, and the effects of different interventions. Semin. Reprod. Med. 2014, 32, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Rakic, D.; Jovic, N.; Bicanin Ilic, M.; Dimitrijevic, A.; Djordjevic, O.; Vulovic, T.; Andric, K.; Jakovljevic, V.; Joksimovic Jovic, J. Challenges in Establishing a Relevant Model of Polycystic Ovary Syndrome in Rats—A Mini Review. Serb. J. Exp. Clin. Res. 2021. [Google Scholar] [CrossRef]
- Crisosto, N.; Ladrón de Guevara, A.; Echiburú, B.; Maliqueo, M.; Cavada, G.; Codner, E.; Paez, F.; Sir-Petermann, T. Higher luteinizing hormone levels associated with antimüllerian hor-mone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil. Steril. 2019, 111, 381–388. [Google Scholar] [CrossRef]
- Walters, K.A.; Allan, C.M.; Handelsman, D.J. Rodent models for human polycystic ovary syndrome. Biol. Reprod. 2012, 86, 1–12, 149. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Friedman, S.J.; Yen, S.S.; Frautschy, S.A. Chronic inhibition of hypothalamic-pituitary-ovarian axis and body weight gain by brain-directed delivery of estradiol-17 beta in female rats. Neuroendocrinology 1989, 50, 204–210. [Google Scholar] [CrossRef]
- Zangeneh, F.Z.; Naghizadeh, M.M.; Minaee, B.; Aminee, F. PCOS & symptomatic outcome: Role of the central and peripheral nervous system in ovarian function of rats. Asian J. Pharm. Clin. Res. 2012, 5, 26–32. [Google Scholar]
- Patel, R.; Shah, G. High-fat diet exposure from pre-pubertal age induces polycystic ovary syndrome (PCOS) in rats. Reproduction 2018, 155, 141–151. [Google Scholar] [CrossRef]
- Chen, X.; Huang, L.; Cui, L.; Xiao, Z.; Xiong, X.; Chen, C. Sodium-glucose cotransporter 2 inhibitor ameliorates high fat di-et-induced hypothalamic-pituitary-ovarian axis disorders. J. Physiol. 2022, 600, 4549–4568. [Google Scholar] [CrossRef]
- Pikov, V.; Sridhar, A.; Lara, H.E. High-Frequency Electrical Modulation of the Superior Ovarian Nerve as a Treatment of Poly-cystic Ovary Syndrome in the Rat. Front. Physiol. 2018, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Pak, S.C.; Lee, S.H.; Lim, S.C.; Bai, Y.H.; Jin, C.S.; Kim, J.S.; Na, C.S.; Bae, C.S.; Oh, K.S.; et al. The effect of herbal medicine on nerve growth factor in estradiol valerate-induced polycystic ovaries in rats. Am. J. Chin. Med. 2003, 31, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.S.; Perets, R.A.; Sarfert, K.S.; Bowman, J.J.; Ozark, P.A.; Whitworth, G.B.; Blythe, S.N.; Toporikova, N. High-fat high-sugar diet induces polycystic ovary syndrome in a rodent model. Biol. Reprod. 2017, 96, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Joksimovic Jovic, J.; Jovic, N.; Sretenovic, J.; Zivkovic, V.; Nikolic, M.; Rudic, J.; Milošević, V.; Ristić, N.; Andric, K.; Dimkic Tomic, T.; et al. Normotensive rats with PCOS exhibit the hypertensive pattern: Focus on oxidative stress. Reproduction 2021, 163, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Joksimovic Jovic, J.; Sretenovic, J.; Jovic, N.; Rudic, J.; Zivkovic, V.; Srejovic, I.; Mihajlovic, K.; Draginic, N.; Andjic, M.; Milinkovic, M.; et al. Cardiovascular Properties of the Androgen-Induced PCOS Model in Rats: The Role of Oxida-tive Stress. Oxid. Med. Cell. Longev. 2021, 2021, 8862878. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef]
- Auclair, C.; Voisin, E. Nitroblue tetrazolium reduction. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Beutler, E. A Manual of Biochemical Methods; Grune & Stratton: New York, NY, USA, 1975. [Google Scholar]
- Barzegar, M.H.; Khazali, H.; Kalantar, S.M.; Khoradmehr, A. Effect of Citrullus colocynthis hydro-alcoholic extract on hormonal and folliculogenesis process in estradiol valerate-induced PCOs rats model: An experimental study. Int. J. Reprod. Biomed. 2017, 15, 661–668. [Google Scholar] [CrossRef]
- Ryu, Y.; Kim, S.W.; Kim, Y.Y.; Ku, S.Y. Animal Models for Human Polycystic Ovary Syndrome (PCOS) Focused on the Use of Indi-rect Hormonal Perturbations: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 2720. [Google Scholar] [CrossRef]
- Venegas, B.; De León Gordillo, L.Y.; Rosas, G.; Espinoza, J.A.; Morán, C.; Domínguez, R.; Morales-Ledesma, L. In rats with estradiol valerate-induced polycystic ovary syndrome, the acute blockade of ovarian β-adrenoreceptors improve ovulation. Reprod. Biol. Endocrinol. 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor-Zarate, R.; Dorfman, M.; Paredes, A.; Lara, H.E. Neonatal exposure to estradiol valerate programs ovarian sympa-thetic innervation and follicular development in the adult rat. Biol. Reprod. 2008, 78, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef]
- Begum, N.; Manipriya, K.; Veeresh, B. Role of high-fat diet on letrozole-induced polycystic ovarian syndrome in rats. Eur. J. Pharmacol. 2022, 917, 174746. [Google Scholar] [CrossRef] [PubMed]
- Barria, A.; Leyton, V.; Ojeda, S.R.; Lara, H.E. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is en-hanced in polycystic ovary syndrome: Role of sympathetic innervation. Endocrinology 1993, 133, 2696–2703. [Google Scholar] [CrossRef]
- Volk, K.M.; Pogrebna, V.V.; Roberts, J.A.; Zachry, J.E.; Blythe, S.N.; Toporikova, N. High-Fat, High-Sugar Diet Disrupts the Preovulatory Hormone Surge and Induces Cystic Ovaries in Cycling Female Rats. J. Endocr. Soc. 2017, 1, 1488–1505. [Google Scholar] [CrossRef]
- Wang, X.; Gu, L.; Zhang, Y.; Xiong, C.; Peng, Y.; Ding, X. Effects of dehydroepiandrosterone alone or in combination with a high-fat diet and antibiotic cocktail on the heterogeneous phenotypes of PCOS mouse models by regulating gut microbiota. Front. Endocrinol. 2022, 13, 1030151. [Google Scholar] [CrossRef]
- Rosenberg, S.L. The Relationship Between PCOS and Obesity: Which Comes First? Sci. J. Lander Coll. Arts Sci. 2019, 13, 5. [Google Scholar]
- Manni, L.; Holmäng, A.; Cajander, S.; Lundeberg, T.; Aloe, L.; Stener-Victorin, E. Effect of anti-NGF on ovarian expression of al-pha1- and beta2-adrenoceptors, TrkA, p75NTR, and tyrosine hydroxylase in rats with steroid-induced polycystic ovaries. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R826–R835. [Google Scholar] [CrossRef]
- Mehraban, M.; Jelodar, G.; Rahmanifar, F. A combination of spearmint and flaxseed extract improved endocrine and histomor-phology of ovary in experimental PCOS. J. Ovarian Res. 2020, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Sanchez, L.A.; Knochenhauer, E.S.; Moran, C.; Lazenby, J.; Stephens, K.C.; Taylor, K.; Boots, L.R. Androgen excess in wom-en: Experience with over 1000 consecutive patients. J. Clin. Endocrinol. Metab. 2004, 89, 453–462. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef] [PubMed]
- Dunaif, A.; Segal, K.R.; Futterweit, W.; Dobrjansky, A. Profound peripheral insulin resistance, independent of obesity, in poly-cystic ovary syndrome. Diabetes 1989, 38, 1165–1174. [Google Scholar] [CrossRef]
- Rodriguez Paris, V.A.-O.; Solon-Biet, S.A.-O.X.; Senior, A.A.-O.; Edwards, M.C.; Desai, R.; Tedla, N.; Cox, M.J.; Ledger, W.L.; Gilchrist, R.B.; Simpson, S.J.; et al. Defining the Impact of Dietary Macronutrient Balance on PCOS Traits. Nat. Commun. 2020, 11, 5262. [Google Scholar] [CrossRef]
- Farshchi, H.; Rane, A.; Love, A.; Kennedy, R.L. Diet and Nutrition in Polycystic Ovary Syndrome (PCOS): Pointers for Nutritional Management. J. Obstet. Gynaecol. 2007, 27, 762–773. [Google Scholar] [CrossRef]
- Naderpoor, N.; Shorakae, S.; de Courten, B.; Misso, M.L.; Moran, L.J.; Teede, H.J. Metformin and Lifestyle Modification in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Hum. Reprod. Update 2015, 21, 560–574. [Google Scholar] [CrossRef]
- Dikmen, A.; Ergenoglu, A.M.; Yeniel, A.O.; Dilsiz, O.Y.; Ercan, G.; Yilmaz, H. Evaluation of glycemic and oxidative/antioxidative status in the estradiol valerate-induced PCOS model of rats. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 55–59. [Google Scholar] [CrossRef]
- Ghafurniyan, H.; Azarnia, M.; Nabiuni, M.; Karimzadeh, L. The Effect of Green Tea Extract on Reproductive Improvement in Estradiol Valerate-Induced Polycystic Ovarian Syndrome in Rat. Iran. J. Pharm. Res. 2015, 14, 1215–1233. [Google Scholar]
- Karimzadeh, L.; Nabiuni, M.; Kouchesfehani, H.M.; Adham, H.; Bagheri, A.; Sheikholeslami, A. Effect of bee venom on IL-6, COX-2 and VEGF levels in polycystic ovarian syndrome induced in Wistar rats by estradiol valerate. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 32. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Lundeberg, T.; Waldenström, U.; Manni, L.; Aloe, L.; Gunnarsson, S.; Janson, P.O. Effects of electro-acupuncture on nerve growth factor and ovarian morphology in rats with experimentally induced polycystic ovaries. Biol. Reprod. 2000, 63, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Brawer, J.R.; Munoz, M.; Farookhi, R. Development of the polycystic ovarian condition (PCO) in the estradiol valerate-treated rat. Biol. Reprod. 1986, 35, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Gálvez, A.; Leyton, V.; Aravena, G.; Fiedler, J.L.; Bustamante, D.; Lara, H.E. Stress promotes development of ovarian cysts in rats: The possible role of sympathetic nerve activation. Endocrine 1998, 8, 309–315. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Alvarado, W.; Venegas, B.; Domínguez, R.; Morales-Ledesma, L. Pharmacological sympathetic denervation pre-vents the development of polycystic ovarian syndrome in rats injected with estradiol valerate. Reprod. Biol. Endocrinol. 2018, 16, 86. [Google Scholar] [CrossRef]
- Nteeba, J.; Ganesan, S.; Keating, A.F. Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol. Reprod. 2014, 91, 86. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R. A high-fat diet increases body weight and circulating estradiol concentrations but does not improve bone structural properties in ovariectomized mice. Nutr. Res. 2016, 36, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Akamine, E.H.; Marçal, A.C.; Camporez, J.P.; Hoshida, M.S.; Caperuto, L.C.; Bevilacqua, E.; Carvalho, C.R. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J. Endocrinol. 2010, 206, 65–74. [Google Scholar] [CrossRef]
- Negrón, A.L.; Radovick, S. High-Fat Diet Alters LH Secretion and Pulse Frequency in Female Mice in an Estrous Cy-cle-Dependent Manner. Endocrinology 2020, 161, bqaa146. [Google Scholar] [CrossRef]
- Herbison, A.E. A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front. Neuroendocrinol. 2020, 57, 100837. [Google Scholar] [CrossRef]
- He, W.; Li, X.; Adekunbi, D.; Liu, Y.; Long, H.; Wang, L.; Lyu, Q.; Kuang, Y.; O’Byrne, K.T. Hypothalamic effects of progesterone on regulation of the pulsatile and surge release of lute-inising hormone in female rats. Sci. Rep. 2017, 7, 8096. [Google Scholar] [CrossRef] [PubMed]
- Barath, B.; Varga, A.; Matrai, A.A.; Deak-Pocsai, K.; Nemeth, N.; Deak, A. Estradiol Valerate Affects Hematological and Hem-orheological Parameters in Rats. Metabolites 2022, 12, 602. [Google Scholar] [CrossRef]
- Noroozzadeh, M.; Behboudi-Gandevani, S.; Zadeh-Vakili, A.; Ramezani Tehrani, F. Hormone-induced rat model of polycystic ovary syndrome: A systematic review. Life Sci. 2017, 191, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Dăneasă, A.; Cucolaş, C.; Lenghel, L.M.; Olteanu, D.; Orăsan, R.; Filip, G.A. Letrozole vs. estradiol valerate induced PCOS in rats: Glycemic, oxidative and inflammatory status assessment. Reproduction 2016, 151, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, F.; Ramezani, M.; Piravar, Z. Effects of Stachys sylvatica hydroalcoholic extract on the ovary and hypophy-sis-gonadal axis in a rat with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2020, 25, 4. [Google Scholar] [CrossRef]
- Komal, F.; Khan, M.K.; Imran, M.; Ahmad, M.H.; Anwar, H.; Ashfaq, U.A.; Ahmad, N.; Masroor, A.; Ahmad, R.S.; Nadeem, M.; et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in PCOS rat model. J. Transl. Med. 2020, 18, 349. [Google Scholar] [CrossRef]
- Kim, H.J.; Adams, J.M.; Gudmundsson, J.A.; Arason, G.; Pau, C.T.; Welt, C.K. Polycystic ovary morphology: Age-based ultrasound criteria. Fertil. Steril. 2017, 108, 548–553. [Google Scholar] [CrossRef]
- Le, N.S.V.; Le, M.T.; Nguyen, N.D.; Tran, N.Q.T.; Nguyen, Q.H.V.; Cao, T.N. A Cross-Sectional Study on Potential Ovarian Volume and Related Factors in Women with Polycystic Ovary Syndrome from Infertile Couples. Int. J. Womens Health 2021, 13, 793–801. [Google Scholar] [CrossRef]
- Wang, M.X.; Yin, Q.; Xu, X. A Rat Model of Polycystic Ovary Syndrome with Insulin Resistance Induced by Letrozole Combined with High Fat Diet. Med. Sci. Monit. 2020, 26, e922136. [Google Scholar] [CrossRef]
- Ibrahim, Y.F.; Alorabi, M.; Abdelzaher, W.Y.; Toni, N.D.; Thabet, K.; Hegazy, A.; Bahaa, H.A.; Batiha, G.E.; Welson, N.N.; Morsy, M.A.; et al. Diacerein ameliorates letrozole-induced polycystic ovarian syndrome in rats. Biomed. Pharmacother. 2022, 149, 112870. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005, 3, 43. [Google Scholar] [CrossRef]
- Sulaiman, M.A.H.; Al-Farsi, Y.M.; Al-Khaduri, M.M.; Saleh, J.; Waly, M.I. Polycystic ovarian syndrome is linked to increased oxida-tive stress in Omani women. Int. J. Womens Health 2018, 10, 763–771. [Google Scholar] [CrossRef]
- Ghowsi, M.; Khazali, H.; Sisakhtnezhad, S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: An experimental study. Int. J. Reprod. Biomed 2018, 16, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, S.; Ramprasath, T.; Saravanan, B.; Vasudevan, V.; Sasikumar, S.; Selvam, G.S. Chrysin attenuates high-fat-diet-induced myocardial oxidative stress via upregulating eNOS and Nrf2 target genes in rats. Mol. Cell. Biochem. 2021, 476, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 14784. [Google Scholar] [CrossRef] [PubMed]

| Parameter | CTRL | EV | EV + HFD |
|---|---|---|---|
| TBARS | 0.90 ± 0.00 | 0.91 ± 0.02 | 1.00 ± 0.02 **,# |
| NO2− | 2091.63 ± 65.64 | 2235.33 ± 30.71 * | 2418.95 ± 8.75 **,## |
| O2 | 2.14 ± 0.46 | 2.86 ± 0.22 | 4.61 ± 0.80 **,# |
| H2O2 | 8468.85 ± 459.89 | 4994.45 ± 398.99 ** | 4263.38 ± 54.26 ** |
| SOD | 25.78 ± 3.27 | 23.06 ± 3.27 | 12.21 ± 2.78 *,# |
| CAT | 338.71 ± 14.22 | 223.50 ± 24.89 ** | 249.79 ± 25.81 * |
| GSH | 65,706.28 ± 1412.98 | 84,207.19 ± 5253.47 * | 62,903.11 ± 7800.83 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakic, D.; Joksimovic Jovic, J.; Jakovljevic, V.; Zivkovic, V.; Nikolic, M.; Sretenovic, J.; Nikolic, M.; Jovic, N.; Bicanin Ilic, M.; Arsenijevic, P.; et al. High Fat Diet Exaggerate Metabolic and Reproductive PCOS Features by Promoting Oxidative Stress: An Improved EV Model in Rats. Medicina 2023, 59, 1104. https://doi.org/10.3390/medicina59061104
Rakic D, Joksimovic Jovic J, Jakovljevic V, Zivkovic V, Nikolic M, Sretenovic J, Nikolic M, Jovic N, Bicanin Ilic M, Arsenijevic P, et al. High Fat Diet Exaggerate Metabolic and Reproductive PCOS Features by Promoting Oxidative Stress: An Improved EV Model in Rats. Medicina. 2023; 59(6):1104. https://doi.org/10.3390/medicina59061104
Chicago/Turabian StyleRakic, Dejana, Jovana Joksimovic Jovic, Vladimir Jakovljevic, Vladimir Zivkovic, Maja Nikolic, Jasmina Sretenovic, Marina Nikolic, Nikola Jovic, Marija Bicanin Ilic, Petar Arsenijevic, and et al. 2023. "High Fat Diet Exaggerate Metabolic and Reproductive PCOS Features by Promoting Oxidative Stress: An Improved EV Model in Rats" Medicina 59, no. 6: 1104. https://doi.org/10.3390/medicina59061104
APA StyleRakic, D., Joksimovic Jovic, J., Jakovljevic, V., Zivkovic, V., Nikolic, M., Sretenovic, J., Nikolic, M., Jovic, N., Bicanin Ilic, M., Arsenijevic, P., Dimitrijevic, A., Vulovic, T., Ristic, N., Bulatovic, K., Bolevich, S., Stijak, L., & Pantovic, S. (2023). High Fat Diet Exaggerate Metabolic and Reproductive PCOS Features by Promoting Oxidative Stress: An Improved EV Model in Rats. Medicina, 59(6), 1104. https://doi.org/10.3390/medicina59061104










