A Strategy for Minimizing Circulatory Arrest Duration in Complex Aortic Arch Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Objective
2.2. Preoperative Evaluation
- The presence and size of the major intracranial collaterals (the anterior communicating artery, the posterior communicating arteries, and the junction of the vertebral arteries).
- The extension of the dissection including the involvement of supra-aortic and visceral branches and peripheral arteries.
- The size and involvement of the groin vessels.
- The size of each supra-aortic main vessel (the brachiocephalic trunk, the left carotid artery, and the left subclavian artery).
- The size of the aorta in zones 0, 1, 2, and 3.
2.3. Anesthesia
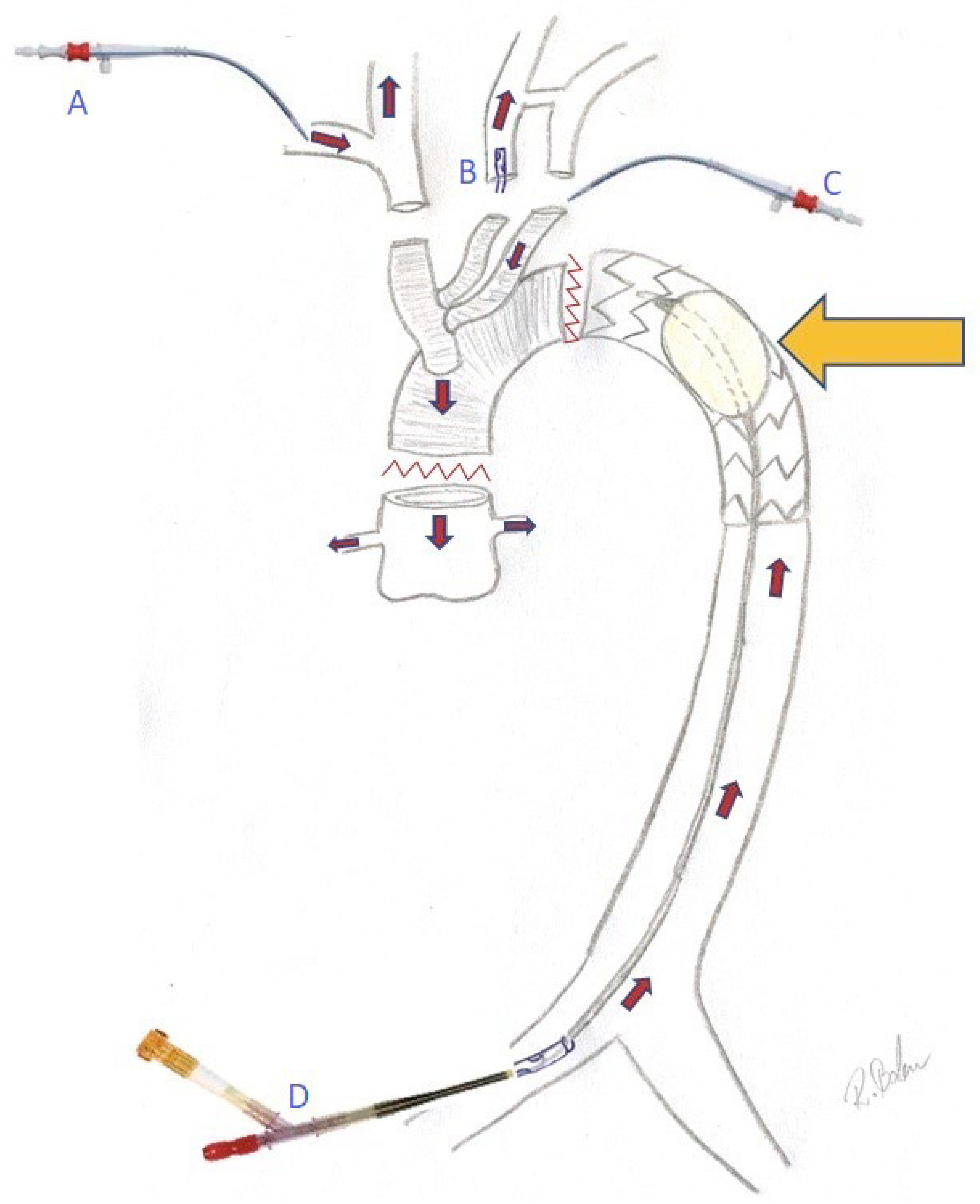
2.4. Technique
3. Results
| Age (years) | 58.2 ± 11.1 |
| Female gender n (%) | 6 (40) |
| BMI | 29.9 ± 5.7 |
| Arterial hypertension n (%) | 12 (80) |
| Diabetes mellitus n (%) | 2 (13.3) |
| COPD n (%) | 2 (13.3) |
| Peripheral arterial disease n (%) | 0 (0) |
| Dialysis n (%) | 0 (0) |
| History of stroke n (%) | 4 (26.7) |
| Ejection fraction [%] | 60 ± 0.0 |
| Coronary disease n (%) | 1 (6.7) |
| Aortic valve regurgitation n (%) | 11 (73.3) |
| Grade 1 n (%) | 2 (13.3) |
| Grade 2 n (%) | 3 (20) |
| Grade 3 n (%) | 6 (40) |
| Preoperative intubation n (%) | 0 (0) |
| Conscious n (%) | 15 (100) |
| Pericardial effusion n (%) | 4 (26.7) |
| Pericardial tamponade n (%) | 3 (20) |
| Emergency n (%) | 13 (86.7) |
| Aortic dissection type—DeBakey 1 n (%) | 12 (80) |
| Aortic dissection type—DeBakey 2 n (%) | 2 (13.3) |
| Aortic dissection type—DeBakey 3 n (%) | 1 (6.7) |
| Dissection of the brachiocephalic trunk n (%) | 13 (86.7) |
| Dissection of the left carotid artery n (%) | 10 (66.7) |
| Cerebral ischemia n (%) | 3 (20) |
| Dissection of the coronary artery n (%) | 7 (46.7) |
| Coronary ischemia n (%) | 2 (13.3) |
| Visceral ischemia n (%) | 0 (0) |
| Dissection of the femoral arteries n (%) | 2 (13.3) |
| Leg ischemia n (%) | 1 (6.7) |
| Carotid-stenting before surgery n (%) | 3 (20) |
| Unilateral cerebral perfusion n (%) | 11 (73.3) |
| Bilateral cerebral perfusion n (%) | 4 (26.7) |
| Balloon occlusion of the descending aorta n (%) | 15 (100) |
| Selective cardiac perfusion n (%) | 12 (80) |
| Bentall procedure n (%) | 7 (46.7) |
| Aortic valve reconstruction n (%) | 1 (6.7) |
| Aortic arch replacement n (%) | 15 (100) |
| Reconstruction of the aortic root and ST junction n (%) | 7 (46.7) |
| E-vita prosthesis n (%) | 14 (93.3) |
| AMDS prosthesis n (%) | 1 (6.7) |
| Intraoperative angiography n (%) | 3 (20) |
| Intraoperative carotid-stenting n (%) | 2 (13.3) |
| Body temperature [°C] | 28.9 ± 2.3; 30 (27–31) |
| Aortic cross-clamp time [min] | 91.6 ± 26.9; 90 (72–114) |
| CPB time [min] | 237.0 ± 55.6; 229 (205–259) |
| Lower body circulatory arrest time [min] | 8.1 ± 4.2; 8 (5–11) |
| Duration of surgery [min] | 397 ± 121.9; 387 (292–446) |
4. Discussion
- Perfusion of the heart after completion of aortic root surgery
- Balloon occlusion of the descending aorta and perfusion of the lower body
- Surgery in moderate hypothermia
- Proximalization of the distal anastomotic line from zone III to zone 0 and extra-anatomic reconstruction of the perfusion of the head vessels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rüffer, A.; Klopsch, C.; Münch, F.; Gottschalk, U.; Mir, T.S.; Weil, J.; Reichenspurner, H.C.; Cesnjevar, R.A. Aortic arch repair: Let it beat! Thorac. Cardiovasc. Surg. 2012, 60, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, H.; Shi, Y.; Liu, Y.; Sun, X. Early outcome of aortic balloon occlusion during total aortic arch replacement with the frozen elephant trunk technique for aortic dissection. Interact Cardiovasc. Thorac. Surg. 2020, 30, 91–98. [Google Scholar] [CrossRef]
- Fujii, M.; Watanabe, H.; Otsu, M.; Sugahara, Y. Incorrect frozen elephant trunk deployment into the false lumen of a patient with complicated type B acute dissection. Eur. J. Cardio-Thorac. Surg. 2019, 55, 1222–1224. [Google Scholar] [CrossRef]
- Ise, H.; Kitahara, H.; Oyama, K.; Takahashi, K.; Kanda, H.; Fujii, S.; Kunisawa, T.; Kamiya, H. Hypothermic circulatory arrest induced coagulopathy: Rotational thromboelastometry analysis. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y.; Guo, H.; Yu, C.; Qian, X.; Wang, W.; Sun, X. Aortic balloon occlusion technique versus moderate hypothermic circulatory arrest with antegrade cerebral perfusion in total arch replacement and frozen elephant trunk for acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2021, 161, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.E.-S.; Risteski, P.; Ay, M.; Papadopoulos, N.; Moritz, A.; Zierer, A. Moderate Hypothermic Circulatory Arrest (≥28 °C) with Selective Antegrade Cerebral Perfusion for Total Arch Replacement with Frozen Elephant Trunk Technique. Thorac. Cardiovasc. Surg. 2019, 67, 345–350. [Google Scholar]
- Malvindi, P.G.; Alfonsi, J.; Berretta, P.; Cefarelli, M.; Gatta, E.; Di Eusanio, M. Normothermic frozen elephant trunk: Our experience and literature review. Cardiovasc. Diagn. Ther. 2022, 12, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Goebel, N.; Holder, S.A.; Huether, F.; Bail, D.H.L.; Franke, U.F.W. Left Subclavian Artery Sacrifice in Acute Aortic Dissection Repair using the Frozen Elephant Trunk. Thorac. Cardiovasc. Surg. 2022, 70, 623–629. [Google Scholar] [CrossRef] [PubMed]
| Cardiac ischemic event n (%) | 0 (0) |
| Pacemaker n (%) | 0 (0) |
| Respiration time [min] | 114.5 ± 189.7; 72 (10–105) |
| Reintubation n (%) | 1 (6.7) |
| Pneumonia n (%) | 3 (20) |
| Cerebral ischemic event (including preoperative ischemia) n (%) | 7 (46.7) |
| Hemiplegia n (%) | 5 (33.3) |
| Arm weakness n (%) | 2 (13.3) |
| Cerebral thrombectomy n (%) | 1 (6.7) |
| TEVAR n (%) | 1 (6.7) |
| Permanent dialysis n (%) | 2 (13.3) |
| Sternal wound infection n (%) | 0 (0) |
| ICU stay (days) | 18.3 ± 13.7; 9 (8–31) |
| Hospital stay (days) | 23.8 ± 11.7; 20 (15–33) |
| 30-day mortality n (%) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balan, R.; Soso, P.; Massoudy, P.; Proschek, T.; Kurre, W.; Mogilansky, C. A Strategy for Minimizing Circulatory Arrest Duration in Complex Aortic Arch Procedures. Medicina 2023, 59, 1007. https://doi.org/10.3390/medicina59061007
Balan R, Soso P, Massoudy P, Proschek T, Kurre W, Mogilansky C. A Strategy for Minimizing Circulatory Arrest Duration in Complex Aortic Arch Procedures. Medicina. 2023; 59(6):1007. https://doi.org/10.3390/medicina59061007
Chicago/Turabian StyleBalan, Robert, Petar Soso, Parwis Massoudy, Till Proschek, Wiebke Kurre, and Christian Mogilansky. 2023. "A Strategy for Minimizing Circulatory Arrest Duration in Complex Aortic Arch Procedures" Medicina 59, no. 6: 1007. https://doi.org/10.3390/medicina59061007
APA StyleBalan, R., Soso, P., Massoudy, P., Proschek, T., Kurre, W., & Mogilansky, C. (2023). A Strategy for Minimizing Circulatory Arrest Duration in Complex Aortic Arch Procedures. Medicina, 59(6), 1007. https://doi.org/10.3390/medicina59061007







