Changes in Nutritional Status during Induction Phase and Their Association with Fever and Minimal Residual Disease in Paediatric Acute Lymphoblastic Leukaemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Statistical Analysis
3. Treatment
4. Results
4.1. BMI Change during Induction
4.2. BMI and Fever
4.3. BMI and MRD
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- National Cancer Institute. Cancer Stat Facts: Childhood Leukemia (Ages 0–19). SEER. Available online: https://seer.cancer.gov/statfacts/html/childleuk.html (accessed on 30 March 2023).
- O’Connor, D.; Bate, J.; Wade, R.; Clack, R.; Dhir, S.; Hough, R.; Vora, A.; Goulden, N.; Samarasinghe, S. Infection-related mortality in children with acute lymphoblastic leukemia: An analysis of infectious deaths on UKALL2003. Blood 2014, 124, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.D.; Stevens, M.C.G. The influence of nutrition on clinical outcomes in children with cancer. Pediatr. Blood Cancer 2020, 67, e28117. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.; Åsberg, A.; Heyman, M.; Kanerva, J.; Harila-Saari, A.; Hasle, H.; Söderhäll, S.; Jónsson, O.G.; Lydersen, S.; Schmiegelow, K. Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr. Blood Cancer 2011, 56, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Toft, N.; Birgens, H.; Abrahamsson, J.; Griškevičius, L.; Hallböök, H.; Heyman, M.; Kalusen, T.W.; Jonsson, O.G.; Palk, K.; Pruundild, K.; et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia 2018, 32, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.S.; Heyman, M.; Möttönen, M.; Zeller, B.; Jonmundsson, G.; Hasle, H. Treatment-related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992–2001. Br. J. Haematol. 2005, 131, 50–58. [Google Scholar] [CrossRef]
- Marinella, M.A. Fever in Patients with Cancer—Infectious Disease and Antimicrobial Agents. Available online: http://www.antimicrobe.org/e13.asp (accessed on 9 December 2022).
- Schaible, U.E.; Kaufmann, S.H.E. Malnutrition and Infection: Complex Mechanisms and Global Impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.; Moulik, N.R.; Kumar, A.; Mahdi, A.A.; Kumar, A. Effect of Pre-treatment Nutritional Status, Folate and Vitamin B12 Levels on Induction Chemotherapy in Children with Acute Lymphoblastic Leukemia. Indian Pediatr. 2015, 52, 385–389. [Google Scholar] [CrossRef]
- Roy Moulik, N.; Kumar, A.; Agrawal, S.; Mahdi, A.A. Folate deficiency in north Indian children undergoing maintenance chemotherapy for acute lymphoblastic leukemia—Implications and outcome. Pediatr. Blood Cancer 2018, 65, e26730. [Google Scholar] [CrossRef]
- Chandra, R.K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 2002, 56, S73–S76. [Google Scholar] [CrossRef]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef]
- Broto, G.E.; Silva, P.R.B.; Trigo, F.C.; Victorino, V.J.; Bonifácio, K.L.; Pavanelli, W.R.; Tomiotto-Pelissier, F.; Garbim, M.R.; Oliviera, S.T.; Jumeas, J.J.; et al. Impact of the induction phase chemotherapy on cytokines and oxidative markers in peripheral and bone marrow plasma of children with acute lymphocytic leukemia. Curr. Res. Immunol. 2021, 2, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Closa, D.; Folch-Puy, E. Oxygen Free Radicals and the Systemic Inflammatory Response. IUBMB Life 2004, 56, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.E.; Lashinger, L.M.; Hursting, S.D. The growing challenge of obesity and cancer: An inflammatory issue. Ann. N. Y. Acad. Sci. 2011, 1229, 45–52. [Google Scholar] [CrossRef]
- Takele, Y.; Adem, E.; Getahun, M.; Tajebe, F.; Kiflie, A.; Hailu, A.; Raynes, J.; Mengesha, B.; Ayele, T.A.; Shkedy, Z.; et al. Malnutrition in Healthy Individuals Results in Increased Mixed Cytokine Profiles, Altered Neutrophil Subsets and Function. PLoS ONE 2016, 11, e0157919. [Google Scholar] [CrossRef]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.-M.; Abdal Dayem, A.; Cho, S.-G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Belgaumi, A.F.; Al-Bakrah, M.; Al-Mahr, M.; Al-Jefri, A.; Al-Musa, A.; Saleh, M.; Salim, M.F.; Osman, M.; Osman, L.; El-Solh, H. Dexamethasone-associated toxicity during induction chemotherapy for childhood acute lymphoblastic leukemia is augmented by concurrent use of daunomycin. Cancer 2003, 97, 2898–2903. [Google Scholar] [CrossRef]
- Chow, E.J.; Pihoker, C.; Friedman, D.L.; Lee, S.J.; McCune, J.S.; Wharton, C.; Roth, C.L.; Baker, S. Glucocorticoids and insulin resistance in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2013, 60, 621–626. [Google Scholar] [CrossRef]
- Lowas, S.R.; Marks, D.; Malempati, S. Prevalence of transient hyperglycemia during induction chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 52, 814–818. [Google Scholar] [CrossRef]
- Suchomlinov, A. 1990 M. Gimusių vaikų fizinės būklės ypatumai, raidos takai ir veiksniai augimo laikotarpiu (išilginis auksologinis Vilniaus miesto ir rajono vaikų tyrimas). Ph.D. Thesis, Vilniaus Universitetas, Vilnius, Lithuania, 2011. [Google Scholar]
- World Health Organization. Malnutrition. Available online: https://www.who.int/health-topics/malnutrition (accessed on 9 December 2022).
- Higienos Institutas. Pirmą kartą skelbiami visų ugdymo įstaigas lankančių vaikų ir pilnamečių mokinių kmi įvertinimo duomenys. 2021. Available online: https://www.hi.lt/news/1826/1310/Pirma-karta-skelbiami-visu-ugdymo-istaigas-lankanciu-vaiku-ir-pilnameciu-mokiniu-KMI-ivertinimo-duomenys.html (accessed on 9 December 2022).
- Orgel, E.; Genkinger, J.M.; Aggarwal, D.; Sung, L.; Nieder, M.; Ladas, E.J. Association of body mass index and survival in pediatric leukemia: A meta-analysis. Am. J. Clin. Nutr. 2016, 103, 808–817. [Google Scholar] [CrossRef]
- Athifah, A.; Hidayati, S.N.; Sulistiawati, S. Correlative Study between Nutritional Status and Remission Outcome in Childhood Acute Lymphoblastic Leukemia in Dr. Soetomo General Hospital Surabaya. Biomol. Health Sci. J. 2019, 2, 27–30. [Google Scholar] [CrossRef]
- González, H.R.; Mejía, S.A.; Ortiz, J.O.C.; Gutiérrez, A.P.O.; López, J.E.B.; Quintana, J.E.F. Malnutrition in paediatric patients with leukaemia and lymphoma: A retrospective cohort study. Ecancermedicalscience 2021, 15, 1327. [Google Scholar] [PubMed]
- Owens, J.L.; Hanson, S.J.; McArthur, J.A.; Mikhailov, T.A. The Need for Evidence Based Nutritional Guidelines for Pediatric Acute Lymphoblastic Leukemia Patients: Acute and Long-Term Following Treatment. Nutrients 2013, 5, 4333–4346. [Google Scholar] [CrossRef] [PubMed]
- Egnell, C.; Närhinen, H.; Merker, A.; Jonsson, Ó.G.; Lepik, K.; Niinimäki, R.; Schmiegelow, K.; Stabell, N.; Klug Albertsen, B.; Vaitkeviciene, G.; et al. Changes in body mass index during treatment of childhood acute lymphoblastic leukemia with the Nordic ALL2008 protocol. Eur. J. Haematol. 2022, 109, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Browne, E.K.; Zhou, Y.; Chemaitilly, W.; Panetta, J.C.; Ness, K.K.; Kaste, S.C.; Cheng, C.; Relling, M.V.; Pui, C.H.; Inaba, H. Changes in body mass index, height, and weight in children during and after therapy for acute lymphoblastic leukemia. Cancer 2018, 124, 4248–4259. [Google Scholar] [CrossRef]
- Seki, Y.; Okamoto, Y.; Kodama, Y.; Nishikawa, T.; Tanabe, T.; Nakagawa, S.; Mizota, M.; Kawano, Y. Risk Factors and the Prevention of Weight Gain during Induction Chemotherapy in Children with Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2018, 40, e334. [Google Scholar] [CrossRef]
- Weber, D.R.; Leonard, M.B.; Zemel, B.S. Body Composition Analysis in the Pediatric Population. Pediatr. Endocrinol. Rev. 2012, 10, 130–139. [Google Scholar]
- Fomon, S.; Haschke, F.; Ziegler, E.; Nelson, S. Body composition of reference children from birth to age 10 years. Am. J. Clin. Nutr. 1982, 35, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, C.T. Minimizing side effects of systemic corticosteroids in children. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 218. [Google Scholar] [CrossRef]
- Arlt, W. Disorders of the Adrenal Cortex. In Harrisons Principles of Internal Medicine, 21st ed.; Loscalzo, J., Fauci, A., Kasper, D., Hauser, S., Longo, D., Jameson, J.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2022; Available online: Accessmedicine.mhmedical.com/content.aspx?aid=1198715883 (accessed on 21 March 2023).
- Harrell, J.S.; Jessup, A.; Greene, N. Changing Our Future: Obesity and the Metabolic Syndrome in Children and Adolescents. J. Cardiovasc. Nurs. 2006, 21, 322. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents. Lancet 2007, 369, 2059–2061. [Google Scholar] [CrossRef]
- Hill, R.; Hamby, T.; Johnson, D.; Boren, C.; Downs, H.; Ray, A. Prevalence and predictors of weight loss during induction therapy for childhood acute lymphoblastic leukemia. Nutrition 2021, 81, 110937. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Tucker, P.; Mushtaq, T.; Wallace, A.M.; Williams, D.M.; Hughes, I.A. Short-term effects on linear growth and bone turnover in children randomized to receive prednisolone or dexamethasone. Clin. Endocrinol. 2002, 57, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Brougham, M.; Montgomery, C.; Richardson, F.; Kelly, A.; Gibson, B.E.S. Effect of Glucocorticoid Therapy on Energy Intake in Children Treated for Acute Lymphoblastic Leukemia. J. Clin. Endocrinol. Metab. 2001, 86, 3742–3745. [Google Scholar] [CrossRef] [PubMed]
- Withycombe, J.S.; Smith, L.M.; Meza, J.L.; Merkle, C.; Faulkner, M.S.; Ritter, L.; Seibel, N.L.; Moore, K. Weight Change during Childhood Acute Lymphoblastic Leukemia Induction Therapy Predicts Obesity: A Report from the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 434–439. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2018.01055 (accessed on 15 March 2023). [CrossRef]
- Toaima, N.N.; El-Owaidy, R.H.; Zaki, D.L.; Eldin, L.B. Infections in children with simple obesity: The relation to phagocytic function and serum leptin. J. Infect. Public Health 2019, 12, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Butturini, A.M.; Dorey, F.J.; Lange, B.J.; Henry, D.W.; Gaynon, P.S.; Fu, C.; Franklin, J.; Siegel, S.E.; Seibel, N.L.; Rogers, P.C.; et al. Obesity and Outcome in Pediatric Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2007, 25, 2063–2069. [Google Scholar] [CrossRef]
- Saenz, A.M.; Stapleton, S.; Hernandez, R.G.; Hale, G.A.; Goldenberg, N.A.; Schwartz, S.; Amankwah, E.K. Body Mass Index at Pediatric Leukemia Diagnosis and the Risks of Relapse and Mortality: Findings from a Single Institution and Meta-analysis. J. Obes. 2018, 2018, 7048078. [Google Scholar] [CrossRef]
- Hijiya, N.; Panetta, J.C.; Zhou, Y.; Kyzer, E.P.; Howard, S.C.; Jeha, S.; Razzouk, B.I.; Ribeiro, R.C.; Rubnitz, J.E.; Hudson, M.M.; et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood 2006, 108, 3997–4002. [Google Scholar] [CrossRef]
- Baillargeon, J.; Langevin, A.-M.; Lewis, M.; Estrada, J.; Mullins, J.; Pitney, A.; Ma, J.Z.; Chisholm, G.B.; Pollock, B.H. Obesity and Survival in a Cohort of Predominantly Hispanic Children with Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2006, 28, 575–578. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, R.; Tang, J.; Wu, X.; Zhu, L.; Huang, H.; Chen, H.; Xiao, M.; Luo, H.; Zheng, G.; et al. Prognostic Observational Analysis of BMI, Leptin, and Adiponectin in Children with Acute Lymphocytic Leukemia Undergoing Remission-Induction Chemotherapy. Front. Pediatr. 2022, 10, 797836. Available online: https://www.frontiersin.org/articles/10.3389/fped.2022.797836 (accessed on 9 December 2022). [CrossRef] [PubMed]
| Total Patients No. (%) | Age Groups No. (%) | p-Value 1 | |||
|---|---|---|---|---|---|
| 0–5 Years | 6–11 Years | 12–17 Years | |||
| Number of Patients (%) | 50 (100.0) | 26 (52.0) | 15 (30.0) | 9 (18.0) | |
| Median age in years (range, IQR) | 5.8 (0.4–15.8, 6.1) | 4.1 (0.4–5.9, 2.3) | 10.0 (6.7–11.6, 1.8) | 15.1 (12.2–15.8, 2.8) | |
| Gender | |||||
| Male | 28 (56.0) | 16 (61.5) | 8 (53.3) | 4 (44.4) | 0.652 |
| Female | 22 (44.0) | 10 (38.5) | 7 (46.7) | 5 (55.6) | |
| Immunophenotype | |||||
| B-ALL | 46 (92.0) | 25 (96.2) | 12 (80.0) | 9 (100.0) | 0.158 |
| T-ALL | 4 (8.0) | 1 (3.8) | 3 (20.0) | 0 (0.0) | |
| Cytogenetics (B-ALL) 2 | |||||
| Normal | 2 (4.3) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 0.367 |
| Favourable risk | 26 (56.5) | 14 (56.0) | 7 (58.3) | 5 (55.6) | |
| High risk | 2 (4.3) | 1 (4.0) | 0 (0.0) | 1 (11.1) | |
| B-other | 16 (34.8) | 10 (40.0) | 3 (25.0) | 3 (33.3) | |
| Leukocyte count | |||||
| <50 × 109/L | 42 (84.0) | 22 (84.6) | 13 (86.7) | 7 (77.8) | 0.770 |
| ≥50 × 109/L | 8 (16.0) | 4 (15.4) | 2 (13.3) | 2 (22.2) | |
| Induction type 3 | |||||
| A | 25 (51.0) | 21 (84.0) | 4 (26.7) | 0 (0.0) | <0.001 |
| B | 24 (49.0) | 4 (16.0) | 11 (73.3) | 9 (100.0) | |
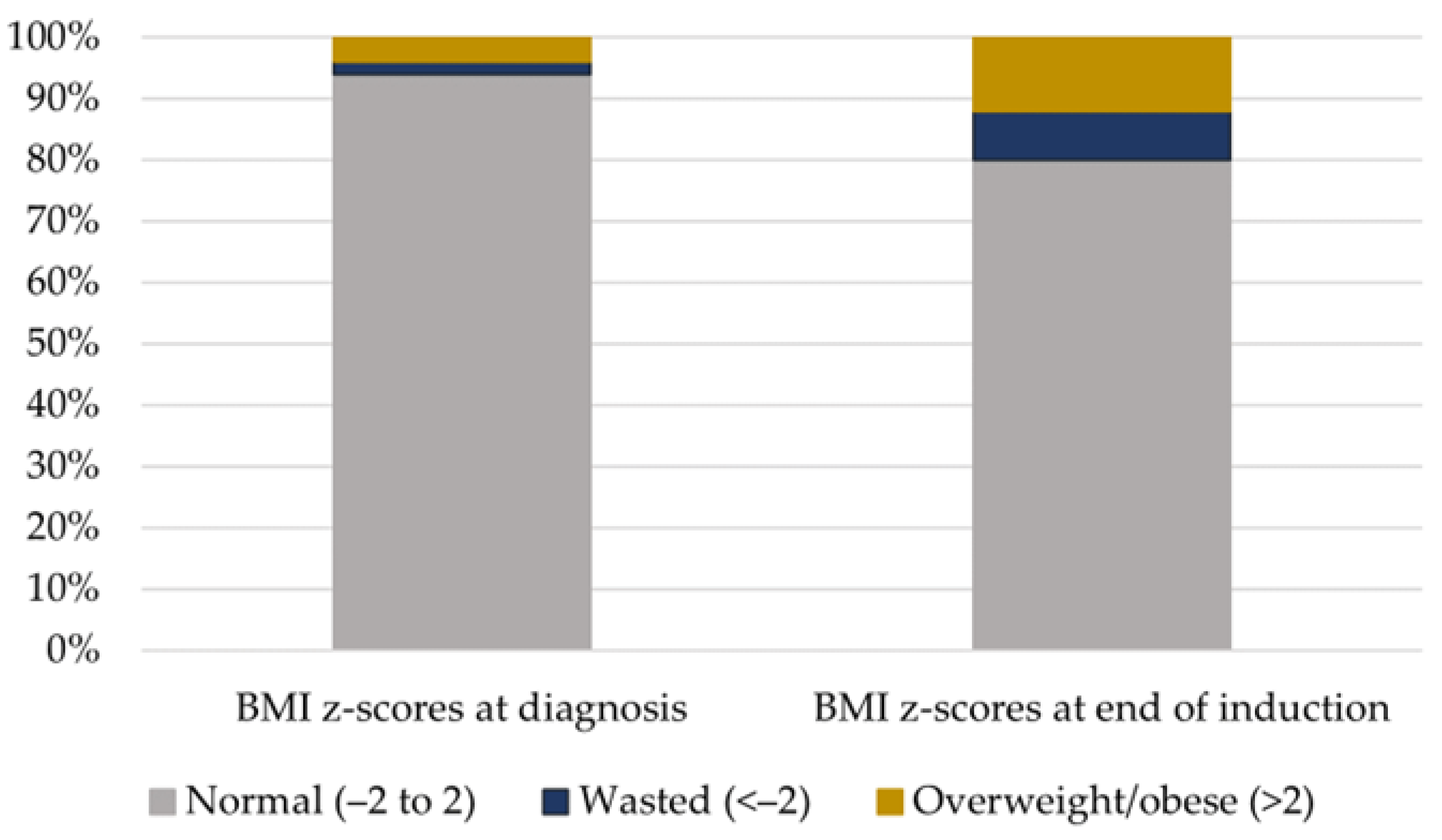
| BMI z-scores at diagnosis | |||||
| Normal (−2 to 2) | 47 (94.0) | 24 (92.3) | 14 (93.3) | 9 (100.0) | 0.509 |
| Underweight (<−2) | 1 (2.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) | |
| Overweight/obese (>2) | 2 (4.0) | 2 (7.7) | 0 (0.0) | 0 (0.0) | |
| BMI z-scores at end of induction | |||||
| Normal (−2 to 2) | 40 (80.0) | 18 (69.2) | 14 (93.3) | 8 (88.9) | 0.172 |
| Underweight (<−2) | 4 (8.0) | 2 (7.7) | 1 (6.7) | 1 (11.1) | |
| Overweight/obese (>2) | 6 (12.0) | 6 (23.1) | 0 (0.0) | 0 (0.0) | |
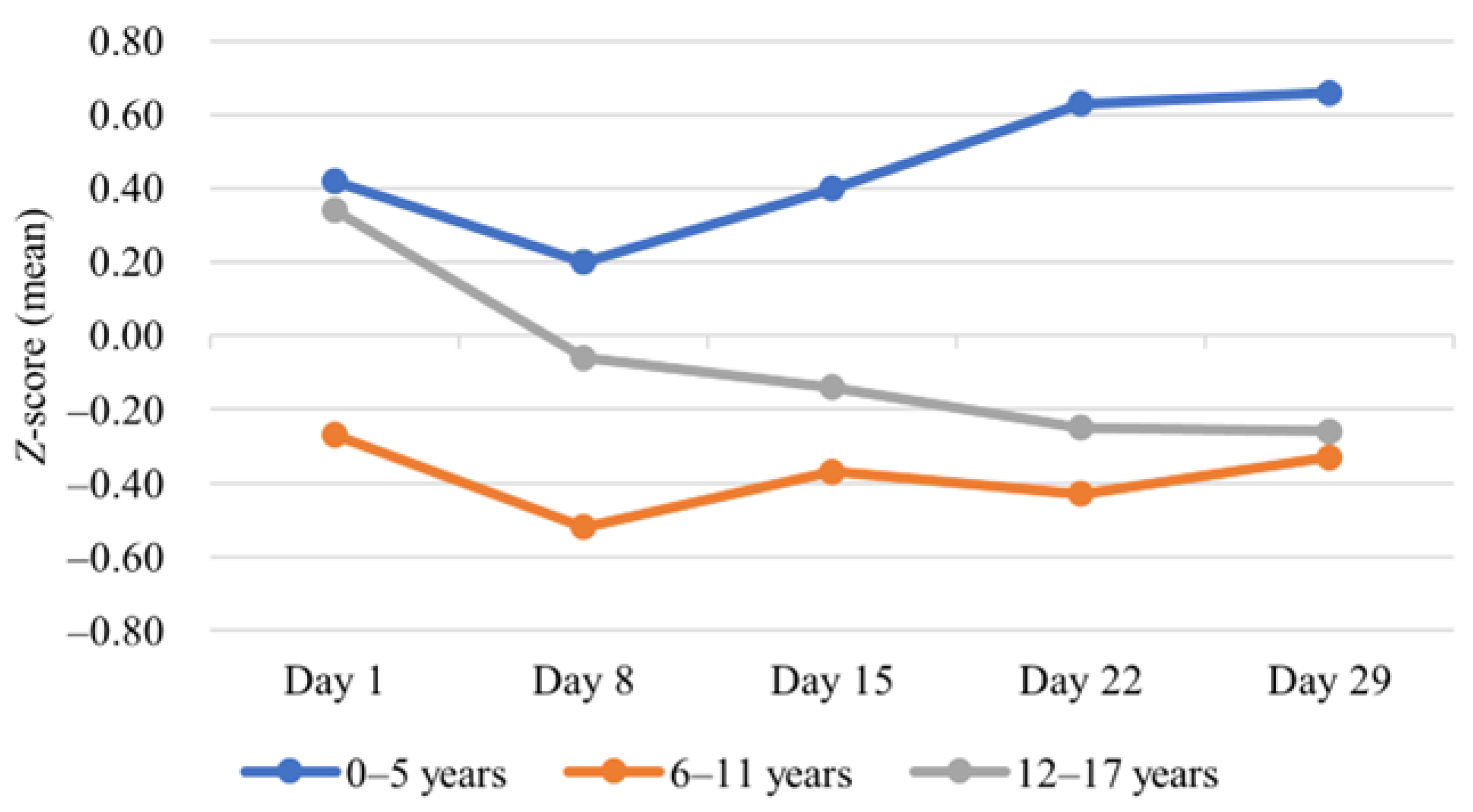
| Change in mean BMI z-score | |||||
| BMI z-scores at diagnosis (mean (SD)) | 0.2 (1.2) | 0.4 (1.1) | −0.3 (1.3) | 0.3 (1.0) | 0.181 |
| BMI z-scores at day 29 (mean (SD)) | 0.2 (1.5) | 0.7 (1.7) | −0.3 (1.3) | −0.3 (1.0) | 0.074 |
| p-Value 4 | 0.996 | 0.273 | 0.856 | 0.005 | |
| Fever at diagnosis | |||||
| <38 °C | 31 (62.0) | 15 (57.7) | 11 (73.3) | 5 (55.6) | 0.554 |
| ≥38 °C | 19 (38.0) | 11 (42.3) | 4 (26.7) | 4 (44.4) | |
| Fever during induction treatment | |||||
| <38 °C | 38 (76.0) | 22 (84.6) | 9 (60.0) | 7 (77.8) | 0.222 |
| ≥38 °C | 12 (24.0) | 4 (15.4) | 6 (40.0) | 2 (22.2) | |
| MRD on day 29 5 | |||||
| MRD negative | 19 (38.8) | 8 (30.8) | 8 (53.3) | 3 (37.5) | 0.408 |
| MRD positive | 30 (61.2) | 18 (69.2) | 7 (46.7) | 5 (62.5) | |
| 0–5 Years (n = 26) | 6–11 Years (n = 15) | 12–17 Years (n = 9) | ||||
|---|---|---|---|---|---|---|
| BMI z-Scores at Diagnosis (Median (Range)) | p-Value * | BMI z-Scores at Diagnosis (Median (Range)) | p-Value * | BMI z-Scores at Diagnosis (Median (Range)) | p-Value * | |
| Fever at diagnosis | ||||||
| <38 °C (n = 31) | −0.2 (−1.4, 1.3) | 0.001 | 0.5 (−1.6, 0.9) | 0.896 | 0.3 (−0.3, 0.8) | 0.697 |
| ≥38 °C (n = 19) | 1.5 (−0.7, 3.1) | 0.0 (−3.6, 1.2) | 0.7 (−1.2, 1.9) | |||
| MRD at day 29 | ||||||
| Negative (n = 19) | 0.2 (−0.7, 1.5) | 0.680 | −0.4 (−3.6, 1.2) | 0.354 | 0.3 (−1.2, 1.9) | 0.803 |
| Positive (n = 30) | 0.1 (−1.4, 3.1) | 0.5 (−1.6, 0.9) | 0.4 (−0.1, 1.6) | |||
| Authors | Number of Patients | Age Range | Diagnosis | Outcomes |
|---|---|---|---|---|
| Sun et al. [47] | 82 | 1–10 | ALL | Abnormally high BMI was associated with higher MRD measured on days 19 and 46. |
| Saenz et al. [44] | Cohort study: 181 Meta-analysis: 916 | 2–20 | ALL, AML, CML | Overweight/obese patients ≥10 years showed a trend toward increased risk of relapse, which was not observed among children <10 years in the single-centre cohort study, although these results were not statistically significant. The meta-analysis revealed a statistically significant increase in mortality risk for overweight/obese patients. |
| Butturini et al. [43] | 4260 | 2–20 | ALL | The study revealed a higher chance of relapse in patients ≥10 years, who were obese at diagnosis, but no such trend in patients <10 years was noticed. |
| Hijiya et al. [45] | 621 | 1.0–18.8 | ALL | Event-free survival, overall survival, relapse incidence, and toxicities did not differ among different BMI categories. |
| Baillargeon et al. [46] | 322 | 2–18 | ALL | Obesity at diagnosis was not associated with decreased overall survival and event-free survival. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gustaitė, S.; Everatt, V.; Kairienė, I.; Vaišnorė, R.; Rascon, J.; Vaitkevičienė, G.E. Changes in Nutritional Status during Induction Phase and Their Association with Fever and Minimal Residual Disease in Paediatric Acute Lymphoblastic Leukaemia. Medicina 2023, 59, 1008. https://doi.org/10.3390/medicina59061008
Gustaitė S, Everatt V, Kairienė I, Vaišnorė R, Rascon J, Vaitkevičienė GE. Changes in Nutritional Status during Induction Phase and Their Association with Fever and Minimal Residual Disease in Paediatric Acute Lymphoblastic Leukaemia. Medicina. 2023; 59(6):1008. https://doi.org/10.3390/medicina59061008
Chicago/Turabian StyleGustaitė, Sigita, Veronika Everatt, Ignė Kairienė, Ramunė Vaišnorė, Jelena Rascon, and Goda Elizabeta Vaitkevičienė. 2023. "Changes in Nutritional Status during Induction Phase and Their Association with Fever and Minimal Residual Disease in Paediatric Acute Lymphoblastic Leukaemia" Medicina 59, no. 6: 1008. https://doi.org/10.3390/medicina59061008
APA StyleGustaitė, S., Everatt, V., Kairienė, I., Vaišnorė, R., Rascon, J., & Vaitkevičienė, G. E. (2023). Changes in Nutritional Status during Induction Phase and Their Association with Fever and Minimal Residual Disease in Paediatric Acute Lymphoblastic Leukaemia. Medicina, 59(6), 1008. https://doi.org/10.3390/medicina59061008






