Effects of Intravascular Photobiomodulation on Insomnia, Muscle Soreness, and Biochemistry Profiles: An Eight-Year Retrospective Cohort
Abstract
1. Introduction
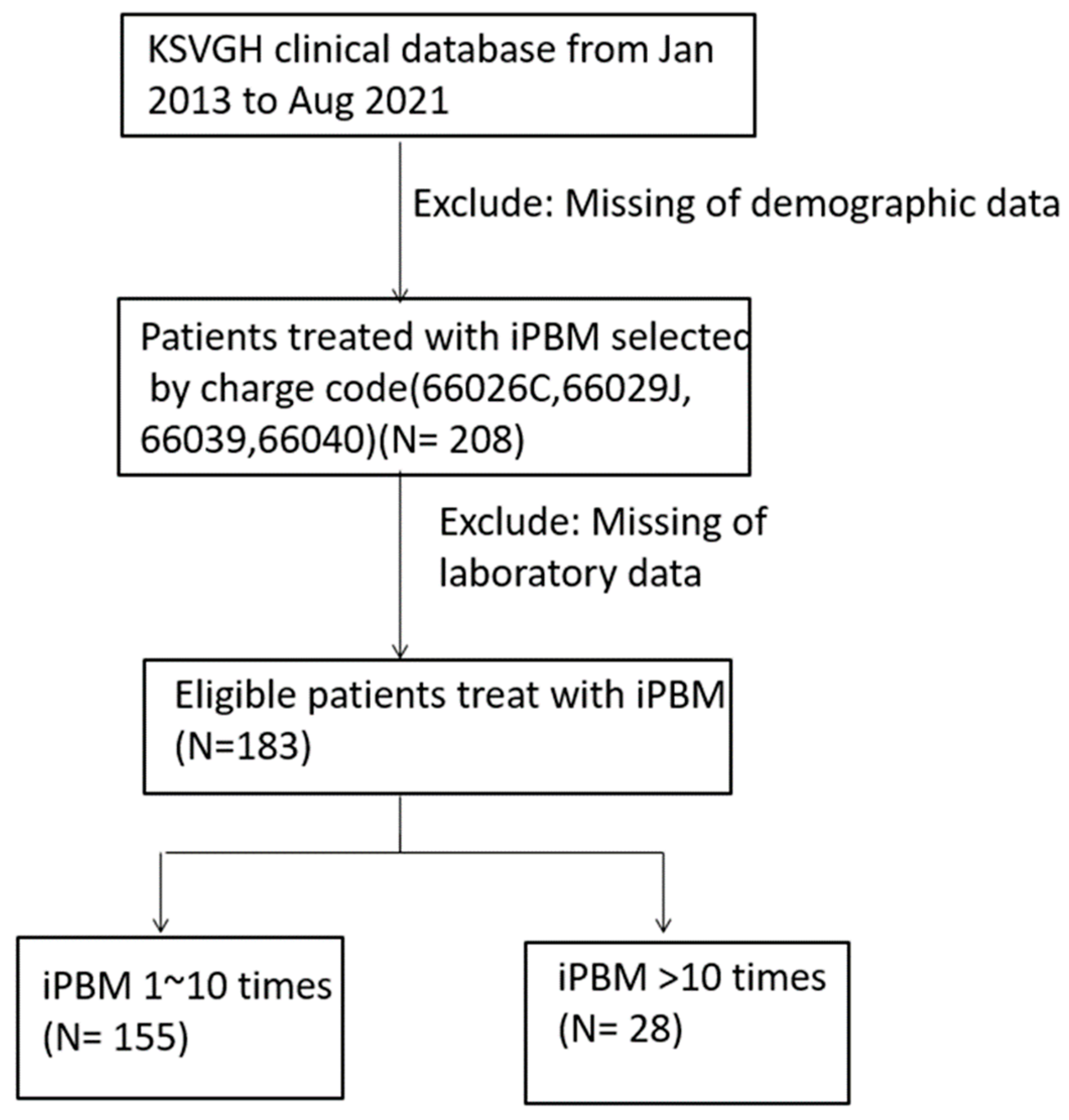
2. Methods
2.1. Participants
2.2. Intervention of iPBM
2.3. Data Collection
2.4. Pharmacotherapy Assessment
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Impact of iPBM on Laboratory Data
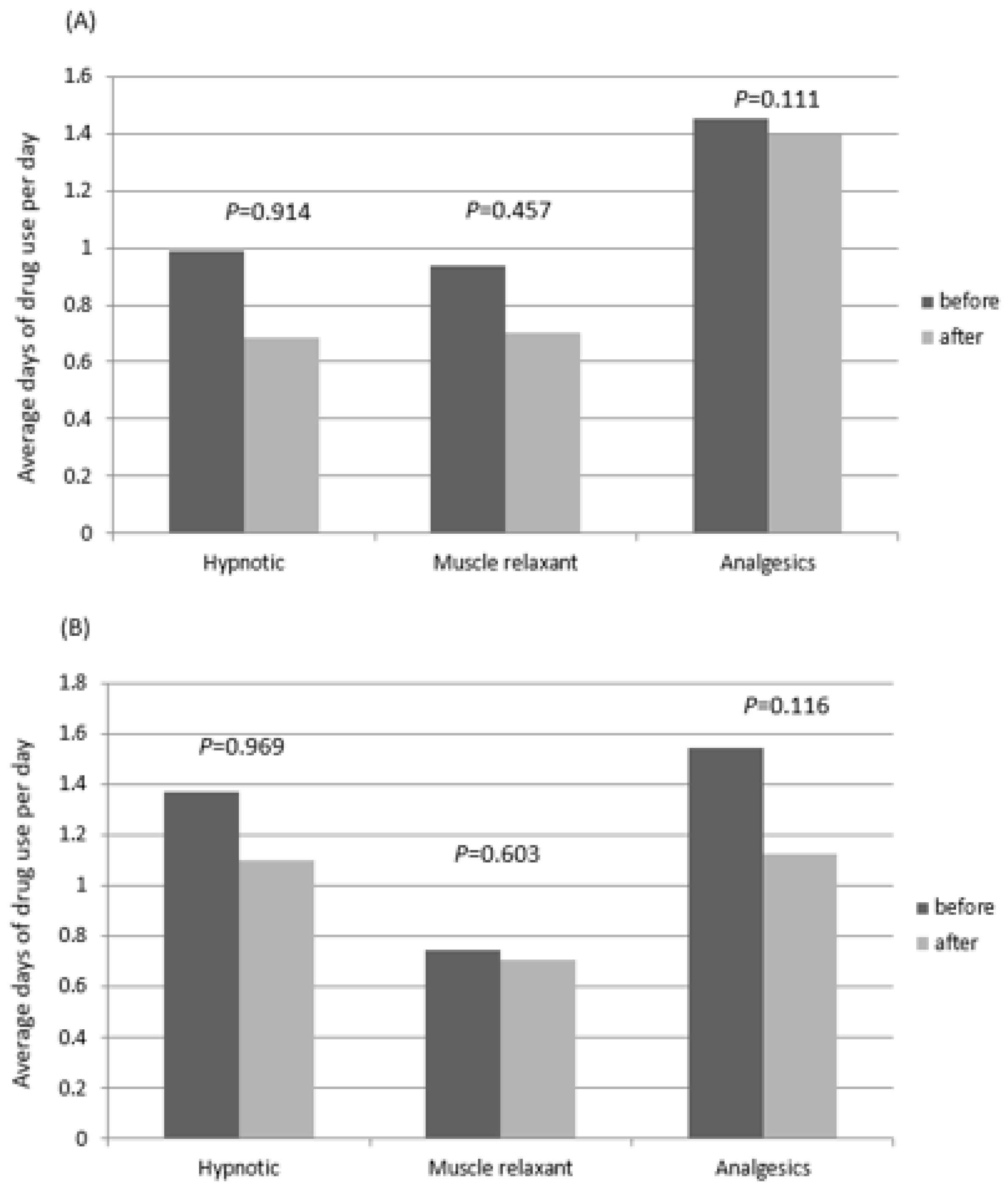
3.3. Pharmacotherapy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Morin, C.M.; Jarrin, D.C. Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med. Clin. 2022, 17, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.L.; Roehrs, T.; Roth, T. Insomnia causes, consequences, and therapeutics: An overview. Depress. Anxiety 2003, 18, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3 (Suppl. S5), S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, J.C. Muscle pain, fatigue, and fasiculations. Neurol. Clin. 1997, 15, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Mastaglia, F.L. The relationship between muscle pain and fatigue. Neuromuscul. Disord. 2012, 22 (Suppl. S3), S178–S180. [Google Scholar] [CrossRef]
- Krystal, A.D.; Prather, A.A.; Ashbrook, L.H. The assessment and management of insomnia: An update. World Psychiatry 2019, 18, 337–352. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mullings, R.; Abdi, S. The pharmacologic treatment of muscle pain. Anesthesiology 2004, 101, 495–526. [Google Scholar] [CrossRef]
- Uhl, R.L.; Roberts, T.T.; Papaliodis, D.N.; Mulligan, M.T.; Dubin, A.H. Management of chronic musculoskeletal pain. J. Am. Acad. Orthop. Surg. 2014, 22, 101–110. [Google Scholar]
- Edinoff, A.N.; Wu, N.; Ghaffar, Y.T.; Prejean, R.; Gremillion, R.; Cogburn, M.; Chami, A.A.; Kaye, A.M.; Kaye, A.D. Zolpidem: Efficacy and Side Effects for Insomnia. Health Psychol. Res. 2021, 9, 24927. [Google Scholar] [CrossRef]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Woller, S.A.; Moreno, G.L.; Hart, N.; Wellman, P.J.; Grau, J.W.; Hook, M.A. Analgesia or addiction?: Implications for morphine use after spinal cord injury. J. Neurotrauma 2012, 29, 1650–1662. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Wang, Z.Q.; Jia, J.J. Morphine Addiction and Oxidative Stress: The Potential Effects of Thioredoxin-1. Front. Pharmacol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Kirkwood, C.K. Management of insomnia. J. Am. Pharm. Assoc. (Wash) 1999, 39, 688–696. [Google Scholar] [CrossRef]
- Chen, C.-K.; Lin, Y.-C.; Cheng, J.-W.; Pei, Y.-C.; Liu, G.-H.; Chen, Y.-L.; Wong, A.M.-K. Effectiveness of Laser Acupuncture in Alleviating Chronic Insomnia: A Single-Blinded Randomized Controlled Trial. Evid. Based Complement Alternat. Med. 2019, 2019, 8136967. [Google Scholar] [CrossRef]
- Montazeri, K.; Farhadi, M.; Fekrazad, R.; Chaibakhsh, S.; Mahmoudian, S. Photobiomodulation therapy in mood disorders: A systematic review. Lasers Med. Sci. 2022, 37, 3343–3351. [Google Scholar] [CrossRef]
- Rahmannia, M.; Amini, A.; Chien, S.; Bayat, M. Impact of photobiomodulation on macrophages and their polarization during diabetic wound healing: A systematic review. Lasers Med. Sci. 2022, 37, 2805–2815. [Google Scholar] [CrossRef]
- Momenzadeh, S.; Abbasi, M.; Ebadifar, A.; Aryani, M.; Bayrami, J.; Nematollahi, F. The intravenous laser blood irradiation in chronic pain and fibromyalgia. J. Lasers Med. Sci. 2015, 6, 6–9. [Google Scholar]
- Avci, P.; Gupta, A.; Sadasivam, M.; Vecchio, D.; Pam, Z.; Pam, N.; Hamblin, M.R. Low-level laser (light) therapy (LLLT) in skin: Stimulating, healing, restoring. Semin. Cutan. Med. Surg. 2013, 32, 41–52. [Google Scholar]
- da Silva Leal, M.V.; Lima, M.O.; Nicolau, R.A.; de Carvallho, T.M.T.; Abreu, J.A.D.C.; Pessoa, D.R.; Arisawa, E.A.L.S. Effect of Modified Laser Transcutaneous Irradiation on Pain and Quality of Life in Patients with Diabetic Neuropathy. Photobiomodul. Photomed. Laser Surg. 2020, 38, 138–144. [Google Scholar] [CrossRef]
- Chiran, D.A.; Litscher, G.; Weber, M.; Ailioaie, L.M.; Ailioaie, C.; Litscher, D. Intravenous laser blood irradiation increases efficacy of etanercept in selected subtypes of juvenile idiopathic arthritis: An innovative clinical research approach. Evid. Based Complement. Alternat. Med. 2013, 2013, 168134. [Google Scholar] [CrossRef]
- Fu, J.C.; Wang, N.K.; Cheng, Y.Y.; Chang, S.T. The Adjuvant Therapy of Intravenous Laser Irradiation of Blood (ILIB) on Pain and Sleep Disturbance of Musculoskeletal Disorders. J. Pers. Med. 2022, 12, 1333. [Google Scholar] [CrossRef]
- Mikhaylov, V.A. The use of Intravenous Laser Blood Irradiation (ILBI) at 630–640 nm to prevent vascular diseases and to increase life expectancy. Laser Ther. 2015, 24, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, A.P.C.; Moreira, M.S.; Gonçalves, F.; Aranha, A.C.C.; Cunha, S.R.; Steiner-Oliveira, C.; Eduardo, C.D.P.; Ramalho, K.M. Photobiomodulation in the Prevention of Tooth Sensitivity Caused by In-Office Dental Bleaching. A Randomized Placebo Preliminary Study. Photomed. Laser Surg. 2017, 35, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Lin, S.P.; Chang, S.T. Case report: Rapid improvement of crossed cerebellar diaschisis after intravascular laser irradiation of blood in a case of stroke. Medicine 2017, 96, e5646. [Google Scholar] [CrossRef] [PubMed]
- Keilani, M.; Crevenna, R.; Dorner, T.E. Sleep quality in subjects suffering from chronic pain. Wien. Klin. Wochenschr. 2018, 130, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Foo, H.; Mason, P. Brainstem modulation of pain during sleep and waking. Sleep Med. Rev. 2003, 7, 145–154. [Google Scholar] [CrossRef]
- Hagiwara, S.; Iwasaka, H.; Okuda, K.; Noguchi, T. GaAlAs (830 nm) low-level laser enhances peripheral endogenous opioid analgesia in rats. Lasers Surg. Med. 2007, 39, 797–802. [Google Scholar] [CrossRef]
- Davis, G.C. Endorphins and pain. Psychiatr. Clin. N. Am. 1983, 6, 473–487. [Google Scholar] [CrossRef]
- Larkin, K.A.; Martin, J.S.; Zeanah, E.H.; True, J.M.; Braith, R.W.; Borsa, P.A. Limb blood flow after class 4 laser therapy. J. Athl. Train. 2012, 47, 178–183. [Google Scholar] [CrossRef]
- Wasik, M.; Gorska, E.; Modzelewska, M.; Nowicki, K.; Jakubczak, B.; Demkow, U. The influence of low-power helium-neon laser irradiation on function of selected peripheral blood cells. J. Physiol. Pharmacol. 2007, 58 (Suppl. S5), 729–737. [Google Scholar]
- Pastore, D.; Greco, M.; Passarella, S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000, 76, 863–870. [Google Scholar] [CrossRef]
- Huang, S.F.; Tsai, Y.A.; Wu, S.B.; Wei, Y.H.; Tsai, P.Y.; Chuang, T.Y. Effects of intravascular laser irradiation of blood in mitochondria dysfunction and oxidative stress in adults with chronic spinal cord injury. Photomed. Laser Surg. 2012, 30, 579–586. [Google Scholar] [CrossRef]
- Walski, T.; Drohomirecka, A.; Bujok, J.; Czerski, A.; Wąż, G.; Trochanowska-Pauk, N.; Gorczykowski, M.; Cichoń, R.; Komorowska, M. Low-Level Light Therapy Protects Red Blood Cells Against Oxidative Stress and Hemolysis during Extracorporeal Circulation. Front. Physiol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Shval’b, P.G.; Kachinskiĭ, A.E.; Kolobaev, V.I.; Kataev, M.I. Intravenous laser irradiation of the blood in severe forms of chronic venous insufficiency. Vestn. Khirurgii Im. II Grek. 1992, 149, 78–80. [Google Scholar]
- Lin, Y.P.; Ku, C.H.; Chang, C.C.; Chang, S.T. Effects of intravascular photobiomodulation on cognitive impairment and crossed cerebellar diaschisis in patients with traumatic brain injury: A longitudinal study. Lasers Med. Sci. 2023, 38, 108. [Google Scholar] [CrossRef]
- Zimon, I.N.; Agzamov, A.I.; Choroshaev, V.A.; Kalish Iu, I.; Dalimov, I.Z. Effects of intravascular laser irradiation of blood on erythrocyte stereo-ultrastructure in the treatment of generalized suppurative peritonitis. Khirurgiia 1992, 9–10, 35–39. [Google Scholar]
- Sarycheva, T.G.; Tsybzhitova, E.B.; Popova, O.V.; Aleksandrov, O.V. Morphometry and electrophoretic mobility of red blood cells from patients with asthma in the intravenous blood laser irradiation. Klin. Lab. Diagn. 2009, 3, 13–14. [Google Scholar]
- Deryugina, A.V.; Ivashchenko, M.N.; Ignatiev, P.S.; Balalaeva, I.V.; Samodelkin, A.G. Low-level laser therapy as a modifier of erythrocytes morphokinetic parameters in hyperadrenalinemia. Lasers Med. Sci. 2019, 34, 1603–1612. [Google Scholar] [CrossRef]
- Wang, H.; Deng, J.; Tu, W.; Zhang, L.; Chen, H.; Wu, X.; Li, Y.; Sha, H. The hematologic effects of low intensity 650 nm laser irradiation on hypercholesterolemia rabbits. Am. J. Transl. Res. 2016, 8, 2293–2300. [Google Scholar]
- Suardi, N.; Sodipo, B.K.; Mustafa, M.Z.; Ali, Z. Effect of visible laser light on ATP level of anaemic red blood cell. J. Photochem. Photobiol. B 2016, 162, 703–706. [Google Scholar] [CrossRef]
- Mitchell, U.H.; Mack, G.L. Low-level laser treatment with near-infrared light increases venous nitric oxide levels acutely: A single-blind, randomized clinical trial of efficacy. Am. J. Phys. Med. Rehabil. 2013, 92, 151–156. [Google Scholar] [CrossRef]
- Sakai, Y.; Matsuyama, Y.; Nakamura, H.; Katayama, Y.; Imagama, S.; Ito, Z.; Okamoto, A.; Ishiguro, N. The effect of muscle relaxant on the paraspinal muscle blood flow: A randomized controlled trial in patients with chronic low back pain. Spine 2008, 33, 581–587. [Google Scholar] [CrossRef]
- Shimoda, K.; Shide, K.; Kamezaki, K.; Okamura, T.; Harada, N.; Kinukawa, N.; Ohyashiki, K.; Niho, Y.; Mizoguchi, H.; Omine, M.; et al. The effect of anabolic steroids on anemia in myelofibrosis with myeloid metaplasia: Retrospective analysis of 39 patients in Japan. Int. J. Hematol. 2007, 85, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; Ahmad, M.; Mehjabeen Jahan, N.; Sajid, M.I. Phytochemical screening and assessment of analgesic, Anti-inflammatory and hematological properties of the fruit of Berberis baluchistanica. Pak. J. Pharm. Sci. 2017, 30 (Suppl. S3), 1007–1012. [Google Scholar] [PubMed]
- Lee, J. Nitric oxide in the kidney: Its physiological role and pathophysiological implications. Electrolyte Blood Press. 2008, 6, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.Y.; de Sousa, M.V.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef]
- Wu, P.Y.; Penn, I.W.; Lin, P.H.; Wang, J.C.; Chuang, E.; Wu, S.H.; Chuang, T.Y. Effects of Intravenous Laser Irradiation of Blood on Pain, Function and Depression of Fibromyalgia Patients. Gen. Med. Open Access 2018, 6, 2. [Google Scholar]
- Xiao, X.; Chu, X.; Ni, J. Effect of intravascular laser irradiation of blood and traditional Chinese medical therapy on immune function in senile cerebral infarction patients of kidney deficiency type. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000, 20, 264–266. [Google Scholar]
- Chang, Y.L.; Chang, S.T. The effects of intravascular photobiomodulation on sleep disturbance caused by Guillain-Barré syndrome after Astrazeneca vaccine inoculation: Case report and literature review. Medicine 2022, 101, e28758. [Google Scholar] [CrossRef]
- Srećković, S.; Petrović, M.J.; Petrović, N.; Vukosavljević, M. Comparison of primary medicament therapy effects and primary argon laser trabeculoplasty on regulation of intraocular pressure and stability of perimetry findings in open angle glaucoma. Vojnosanit. Pregl. 2011, 68, 225–230. [Google Scholar] [CrossRef]


| Variable | Patients with iPBM (N = 183) |
|---|---|
| Male | 86 (47%) |
| Age | 60.8 ± 14.9 |
| BMI | 26.4 ± 16.2 |
| DM | 59 (32%) |
| HTN | 21 (12%) |
| Hyperlipidemia | 33 (18%) |
| Depression | 6 (3.3%) |
| Insomnia | 18 (10%) |
| PSTD | 0 (0%) |
| WBC | 11.3 ± 5.9 |
| HGB | 12.1 ± 2.1 |
| HCT | 36.1 ± 5.9 |
| PLT | 310.0 ± 120.7 |
| Cr | 1.2 ± 1.4 |
| GPT | 40.3 ± 36.3 |
| Variable | iPBM Times | Before | After | p-Value |
|---|---|---|---|---|
| WBC | <10 | 11.1 ± 5.9 | 6.7 ± 2.2 | 0.010 |
| >10 | 12.5 ± 5.7 | 7.9 ± 3.9 | 0.221 | |
| HGB | <10 | 12.3 ± 2.0 | 13.1 ± 1.5 | <0.001 |
| >10 | 11.1 ± 1.9 | 12.0 ± 1.8 | 0.046 | |
| HCT | <10 | 36.6 ± 5.8 | 39.2 ± 4.4 | <0.001 |
| >10 | 32.9 ± 5.8 | 35.7 ± 5.0 | 0.029 | |
| Cr | <10 | 1.1 ± 0.8 | 0.9 ± 0.4 | <0.001 |
| >10 | 2.0 ± 3.2 | 1.7 ± 2.8 | <0.001 | |
| GPT | <10 | 40.6 ± 36.4 | 23.2 ± 14.3 | <0.001 |
| >10 | 48.5 ± 54.0 | 18.8 ± 7.8 | 0.878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-P.; Ding, R.-S.; Yin, C.-H.; Chen, Y.-S.; Chen, J.-S.; Chang, S.-T. Effects of Intravascular Photobiomodulation on Insomnia, Muscle Soreness, and Biochemistry Profiles: An Eight-Year Retrospective Cohort. Medicina 2023, 59, 1006. https://doi.org/10.3390/medicina59061006
Lin Y-P, Ding R-S, Yin C-H, Chen Y-S, Chen J-S, Chang S-T. Effects of Intravascular Photobiomodulation on Insomnia, Muscle Soreness, and Biochemistry Profiles: An Eight-Year Retrospective Cohort. Medicina. 2023; 59(6):1006. https://doi.org/10.3390/medicina59061006
Chicago/Turabian StyleLin, Yen-Po, Ruei-Sian Ding, Chun-Hao Yin, Yao-Shen Chen, Jin-Shuen Chen, and Shin-Tsu Chang. 2023. "Effects of Intravascular Photobiomodulation on Insomnia, Muscle Soreness, and Biochemistry Profiles: An Eight-Year Retrospective Cohort" Medicina 59, no. 6: 1006. https://doi.org/10.3390/medicina59061006
APA StyleLin, Y.-P., Ding, R.-S., Yin, C.-H., Chen, Y.-S., Chen, J.-S., & Chang, S.-T. (2023). Effects of Intravascular Photobiomodulation on Insomnia, Muscle Soreness, and Biochemistry Profiles: An Eight-Year Retrospective Cohort. Medicina, 59(6), 1006. https://doi.org/10.3390/medicina59061006







