Comparison of Type I and Type III Collagen Concentration between Oreochromis mossambicus and Oreochromis niloticus in Relation to Skin Scaffolding
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Sterilization
2.2. Microbiological Analysis
2.3. Histochemical Analysis
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Microbiological Analysis
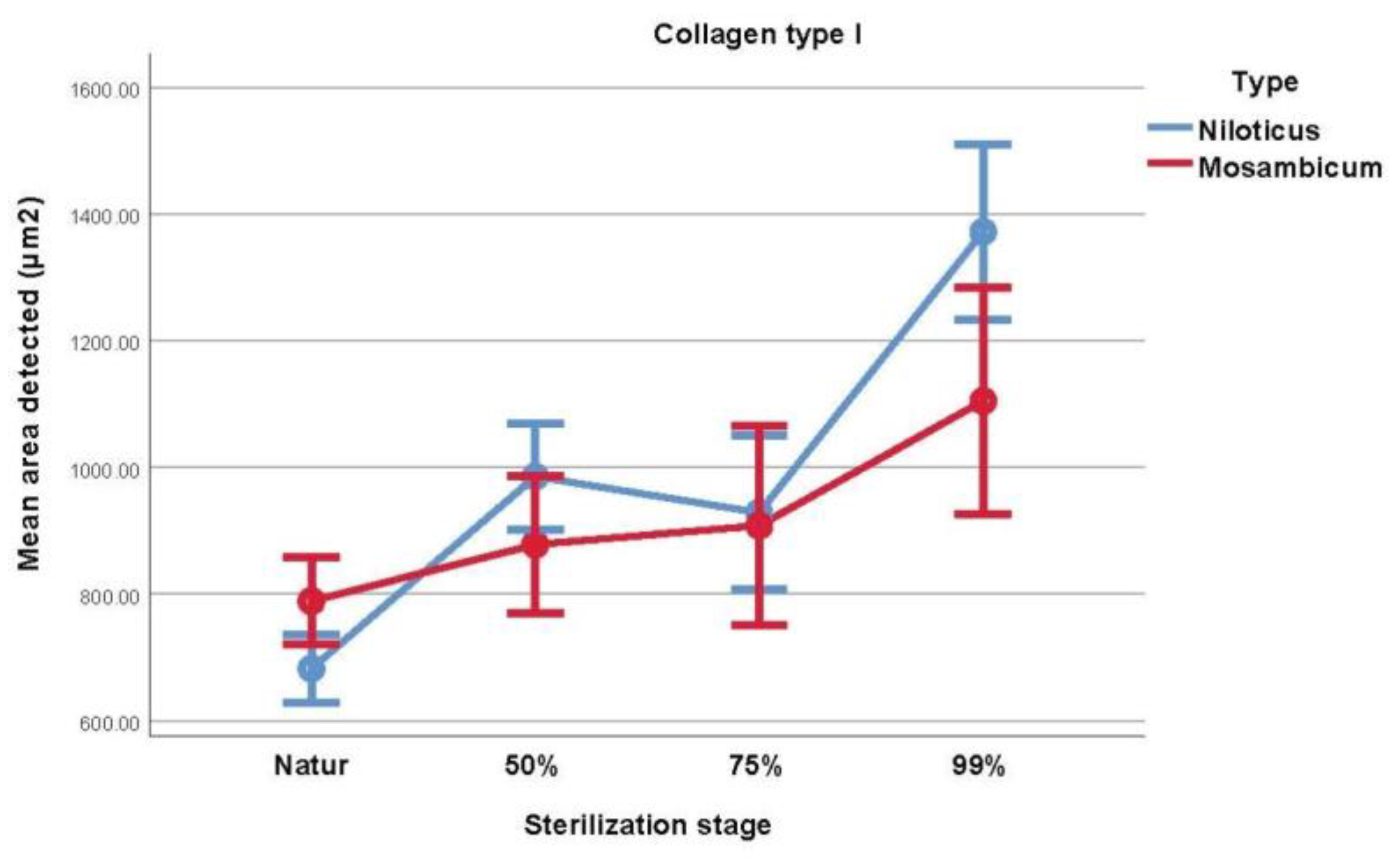
3.2. Collagen Type I Histochemical Analysis
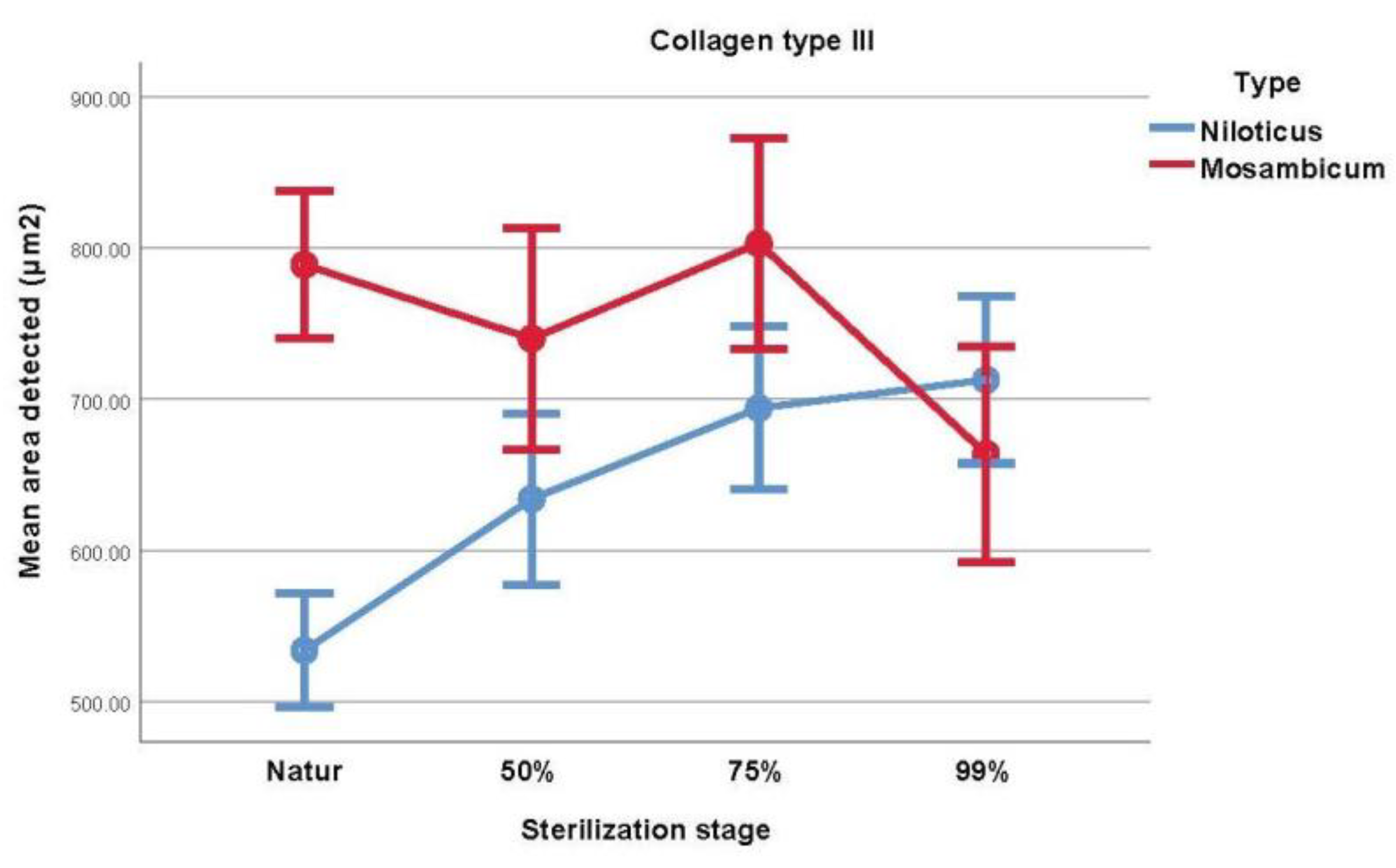
3.3. Collagen Type III Histochemical Analysis
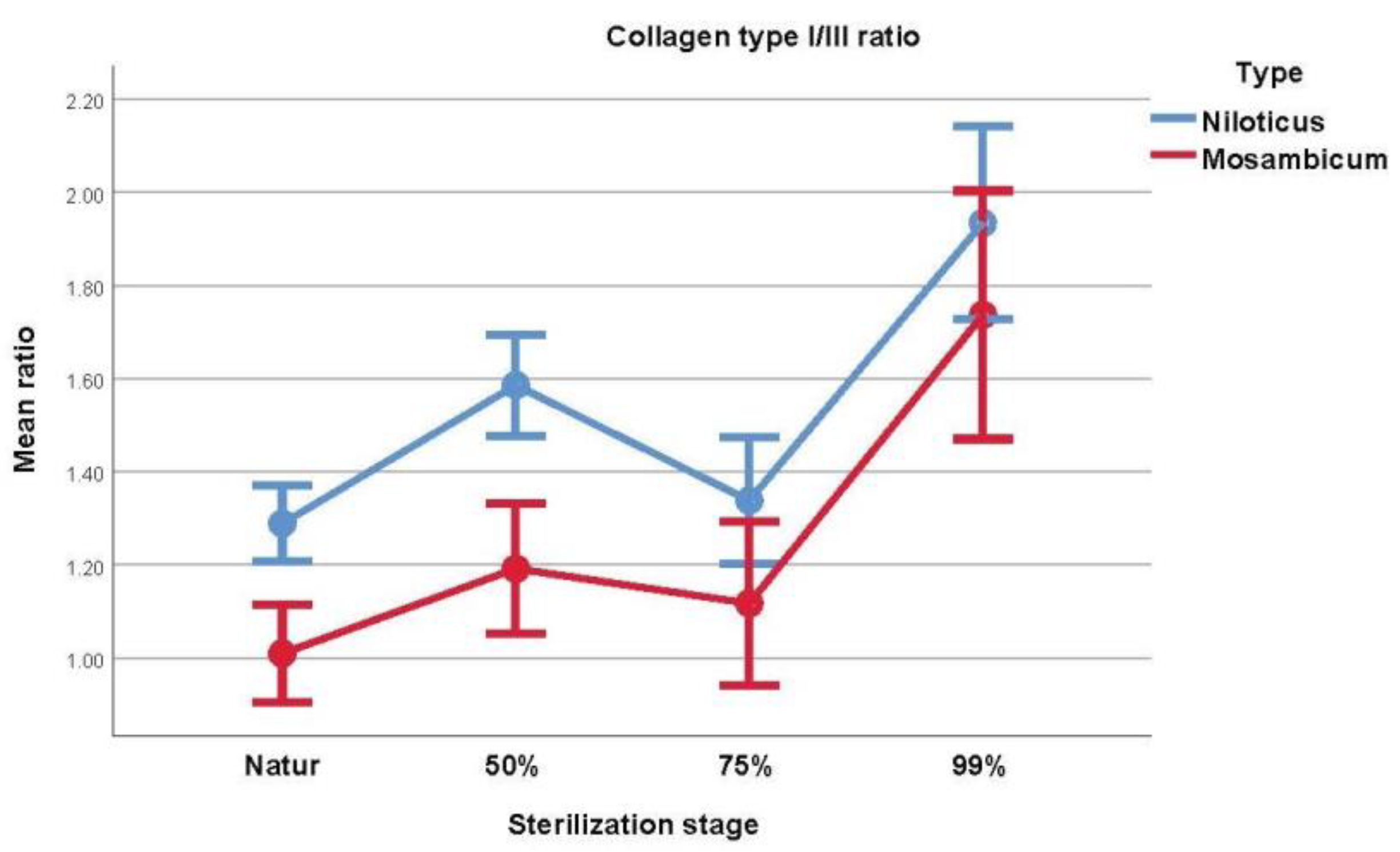
3.4. Collagen I/III Ratio
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Burns. Available online: https://www.who.int/en/news-room/fact-sheets/detail/burns (accessed on 3 January 2021).
- Burkey, B.; Besner, G.E. Burns. In Fundamentals of Pediatric Surgery; Mattei, P., Ed.; Springer International Publishing: Philadelphia, PA, USA, 2017; pp. 153–168. [Google Scholar]
- Elijah, I.E.; Komak, S.; Finnerty, C.C.; Herndon, D.N. Pediatric burns. In Pediatric Critical Care Medicine: Volume 4: Peri-Operative Care of the Critically Ill or Injured Child; Wheeler, D.S., Wong, H.R., Shanley, T.P., Eds.; Springer: London, UK, 2014; pp. 277–286. [Google Scholar]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Motamed, S.; Taghiabadi, E.; Molaei, H.; Sodeifi, N.; Hassanpour, S.E.; Shafieyan, S.; Azargashb, E.; Farajzadeh-Vajari, F.; Aghdami, N.; Bajouri, A. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am. J. Surg. 2017, 214, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.G.; Giatsidis, G.; Orgill, D.P.; Halvorson, E.G. Skin Substitutes and Bioscaffolds: Temporary and Permanent Coverage. Clin. Plast. Surg. 2017, 44, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Yoon, D.; Lee, H.; Lee, J.; Jo, S.; Kym, D.; Yim, H.; Hur, J.; Chun, W.; Kim, G.; et al. Wound healing ability of acellular fish skin and bovine collagen grafts for split-thickness donor sites in burn patients: Characterization of acellular grafts and clinical application. Int. J. Biol. Macromol. 2022, 205, 452–461. [Google Scholar] [CrossRef]
- Stone, R., 2nd; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Datta, P.; Das, H.; Ghosh, P.R.; Kundu, B.; Nandi, S.K. Engineering Vascularizing Electrospun Dermal Grafts by Integrating Fish Collagen and Ion-Doped Bioactive Glass. ACS Biomater. Sci. Eng. 2022, 8, 734–752. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Lin, C.; Lin, Z.; Chen, H.; Lu, W.; Lin, C.; Li, H. Approaches to cutaneous wound healing: Basics and future directions. Cell Tissue Res. 2018, 374, 217–232. [Google Scholar] [CrossRef]
- Mathangi Ramakrishnan, K.; Babu, M.; Mathivanan Jayaraman, V.; Shankar, J. Advantages of collagen based biological dressings in the management of superficial and superficial partial thickness burns in children. Ann. Burns Fire Disasters 2013, 26, 98–104. [Google Scholar]
- Sugiura, H.; Yunoki, S.; Kondo, E.; Ikoma, T.; Tanaka, J.; Yasuda, K. In Vivo Biological Responses and Bioresorption of Tilapia Scale Collagen as a Potential Biomaterial. J. Biomater. Sci. Polym. Ed. 2009, 20, 1353–1368. [Google Scholar] [CrossRef]
- Lin, C.; Ritch, R.; Lin, S.; Ni, M.-H.; Chang, Y.-C.; Lu, Y.; Lai, H.; Lin, F.-H. A new fish scale-derived scaffold for corneal regeneration. Eur. Cells Mater. 2010, 19, 50–57. [Google Scholar] [CrossRef]
- Alves, A.P.N.N.; Verde, M.E.Q.L.; Fereira Júnior, A.E.C.; Silva, P.G.B.; Feitosa, V.P.; Júnior, E.M.L.; de Miranda, M.J.B.; de Filho, M.O.M. Avaliação microscópica, estudo histoquímico e análise de propriedades tensiométricas da pele de tilápia do Nilo. Rev. Bras. Queimaduras 2015, 14, 203–210. [Google Scholar]
- Lau, C.S.; Hassanbhai, A.; Wen, F.; Wang, D.; Chanchareonsook, N.; Goh, B.T.; Yu, N.; Teoh, S. Evaluation of decellularized tilapia skin as a tissue engineering scaffold. J. Tissue Eng. Regen. Med. 2019, 13, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.P.N.N.; Júnior, E.M.L.; Piccolo, N.S.; de Miranda, M.J.B.; Verde, M.E.Q.L.; Júnior, A.E.C.F.; Silva, P.G.D.B.; Feitosa, V.P.; de Bandeira, T.J.P.G.; Mathor, M.B.; et al. Study of tensiometric properties, microbiological and collagen content in nile tilapia skin submitted to different sterilization methods. Cell Tissue Bank. 2018, 19, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.A.; Lima Júnior, E.M.; de Moraes Filho, M.O.; Fechine, F.V.; de Moraes, M.E.A.; Silva Júnior, F.R.; do Nascimento Soares, M.F.A.; Rocha, M.B.S. Use of Tilapia Skin as a Xenograft for Pediatric Burn Treatment: A Case Report. J. Burn Care Res. 2019, 40, 714–717. [Google Scholar] [CrossRef]
- Lima Júnior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.; Alves, A.P.N.N.; de Moraes, M.E.A.; Rocha, M.B.S.; Silva Júnior, F.R. Pediatric Burn Treatment Using Tilapia Skin as a Xenograft for Superficial-Partial Thickness Wounds: A Pilot Study. J. Burn Care Res. 2020, 41, 241–247. [Google Scholar] [CrossRef]
- Basavaraja, N.; Raghavendra, C.H. Hormonal sex reversal in red tilapia (Oreochromis niloticus and Oreochromis mossambicus) and inheritance of body colour in O. mossambicus and red tilapia: Implications for commercial farming. Aquac. Int. 2017, 25, 1317–1331. [Google Scholar] [CrossRef]
- Valenzuela-Rojo, D.R.; López-Cervantes, J.; Sánchez-Machado, D.I. Tilapia (Oreochromis aureus) Collagen for Medical Biomaterials, Seaweed Biomaterials, Sabyasachi Maiti, IntechOpen. Available online: https://www.intechopen.com/chapters/61471 (accessed on 2 March 2020).
- Center for Invasive Species Solutions Australia Tilapia Mossambica. Available online: https://pestsmart.org.au/toolkit-resource/biology-and-ecology-of-mozambique-tilapia-oreochromis-mossambicus/ (accessed on 2 March 2021).
- Atwa, E.I. Bacteriological Study of Fish Samples Collected from Different Markets in Some Egyptian Governorates and Antimicrobial Sensitivity of Isolates. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2765–2776. [Google Scholar] [CrossRef]
- De Chaumont, F.; Dallongeville, S.; Chenouard, N.; Hervé, N.; Pop, S.; Provoost, T.; Meas-Yedid, V.; Pankajakshan, P.; LeComte, T.; Le Montagner, Y.; et al. Icy: An open bioimage informatics platform for extended reproducible research. Nat. Methods 2012, 9, 690–696. [Google Scholar] [CrossRef]
- Liu, H.; Huang, K. Structural Characteristics of Extracted Collagen from Tilapia (Oreochromis mossambicus) Bone: Effects of Ethylenediaminetetraacetic Acid Solution and Hydrochloric Acid Treatment. Int. J. Food Prop. 2016, 19, 63–75. [Google Scholar] [CrossRef]
- Menezes, M.D.L.L.R.; Ribeiro, H.L.; Flávia de Oliveira, M.; de Andrade Feitosa, J.P. Optimization of the collagen extraction from Nile tilapia skin (Oreochromis niloticus) and its hydrogel with hyaluronic acid. Colloids Surf. B Biointerfaces 2020, 189, 110852. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Li, X.; Xin, Q.; Han, W.; Shi, C.; Guo, R.; Shi, W.; Qiao, R.; Wang, X.; Zhong, J. Effect of extraction methods on the preparation of electrospun/electrosprayed microstructures of tilapia skin collagen. J. Biosci. Bioeng. 2019, 128, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Neumeister, M.W.; Levi, B. Stem Cells and Tissue Engineering: Regeneration of the Skin and Its Contents. Clin. Plast. Surg. 2017, 44, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iwase, H.; King, T.W.; Hara, H.; Cooper, D.K. Skin xenotransplantation: Historical review and clinical potential. Burns 2018, 44, 1738–1749. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef]
- Yamamoto, K.; Igawa, K.; Sugimoto, K.; Yoshizawa, Y.; Yanagiguchi, K.; Ikeda, T.; Yamada, S.; Hayashi, Y. Biological Safety of Fish (Tilapia) Collagen. BioMed Res. Int. 2014, 2014, 630757. [Google Scholar] [CrossRef]
- Li, D.; Gao, Y.; Wang, Y.; Yang, X.; He, C.; Zhu, M.; Zhang, S.; Mo, X. Evaluation of biocompatibility and immunogenicity of micro/nanofiber materials based on tilapia skin collagen. J. Biomater. Appl. 2019, 33, 1118–1127. [Google Scholar] [CrossRef]
- Tang, J.; Saito, T. Biocompatibility of Novel Type I Collagen Purified from Tilapia Fish Scale: An In Vitro Comparative Study. BioMed Res. Int. 2015, 2015, 139476. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Liu, J.; Wu, J.; Duan, Z.; Chen, M.; Meng, K.; Chen, S.; Shen, X.; Xia, G.; Zhao, M. Collagen Peptides Isolated from Salmo salar and Tilapia nilotica Skin Accelerate Wound Healing by Altering Cutaneous Microbiome Colonization via Upregulated NOD2 and BD14. J. Agric. Food Chem. 2020, 68, 1621–1633. [Google Scholar] [CrossRef]
- Lima Júnior, E.M.; De Moraes Filho, M.O.; Costa, B.A.; Rohleder, A.V.P.; Sales Rocha, M.B.; Fechine, F.V.; Forte, A.J.; Alves, A.P.N.N.; Silva Júnior, F.R.; Martins, C.B.; et al. Innovative Burn Treatment Using Tilapia Skin as a Xenograft: A Phase II Randomized Controlled Trial. J. Burn Care Res. 2020, 41, 585–592. [Google Scholar] [CrossRef]
- Lv, K.; Wang, L.; He, X.; Li, W.; Han, L.; Qin, S. Application of Tilapia Skin Acellular Dermal Matrix to Induce Acute Skin Wound Repair in Rats. Front. Bioeng. Biotechnol. 2022, 9, 792344. [Google Scholar] [CrossRef] [PubMed]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Mater. Sci. Eng. C 2017, 80, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Zain, N.M.; Saidin, S.; Sosiawan, A. Properties of Tilapia Collagen as a Biomaterial for Tissue Engineering: A Review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 932, p. 012021. [Google Scholar] [CrossRef]
- Na, G.C. Interaction of calf skin collagen with glycerol: Linked function analysis. Biochemistry 1986, 25, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Penkova, R.; Goshev, I.; Gorinstein, S.; Nedkov, P. Stabilizing effect of glycerol on collagen type I isolated from different species. Food Chem. 1999, 66, 483–487. [Google Scholar] [CrossRef]
- Herson, M.R.; Hamilton, K.; White, J.; Alexander, D.; Poniatowski, S.; O’connor, A.J.; Werkmeister, J.A. Interaction of preservation methods and radiation sterilization in human skin processing, with particular insight on the impact of the final water content and collagen disruption. Part I: Process validation, water activity and collagen changes in tissues. Cell Tissue Bank. 2018, 19, 215–227. [Google Scholar] [CrossRef]
- Liu, C.-K. Absorption of glycerol and its effects on the physical properties of a collagen material: Leather. J. Appl. Polym. Sci. 2002, 87, 1221–1231. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Griffiths, C.; Barker, J.; Bleiker, T.; Chalmers, R.; Creamer, D. Rook’s Textbook of Dermatology, 9th ed.; Wiley-Blackwell: Chichester, UK, 2016; Volume 1–4. [Google Scholar]
- Singer, A.J.; Boyce, S.T. Burn Wound Healing and Tissue Engineering. J. Burn Care Res. 2017, 38, 605–613. [Google Scholar] [CrossRef]
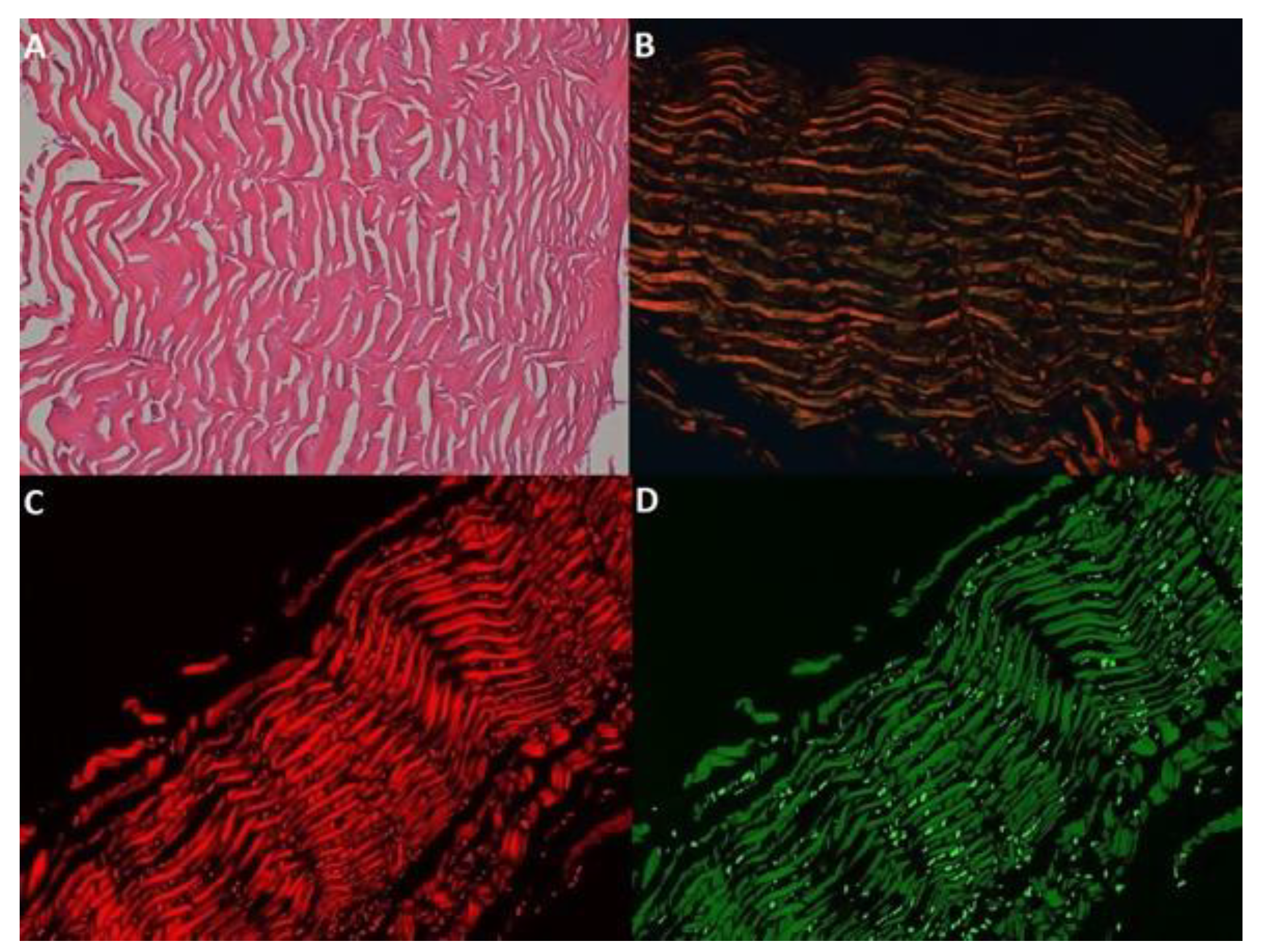
| Groups | Intervention | Time |
|---|---|---|
| 1 | Fat and muscle scrapping and 0.9% saline wash (Natural state) | N/A |
| 2 | 2 × 2% Chlorhexidine baths + Solution: 50% Glycerol–49% Saline (0.9%) 1% PMF (Penicillin + Metronidazole + Fluconazole solution) + | 2 × 30 min 24 h at 4 °C |
| 3 | Solution: 75% Glycerol–24% Saline (0.9%) 1% PMF + | 3 h at 37 °C |
| 4 | Solution: 99% Glycerol–1% PMF | 3 h at 37 °C |
| Sterilization | (I) Type | (J) Type | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval for Difference | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Natur | Niloticus | Mosambicus | −106.683 * | 43.154 | 0.017 | −193.547 | −19.818 |
| 50% Glycerol | Niloticus | Mosambicus | 107.243 | 68.461 | 0.124 | −30.562 | 245.048 |
| 75% Glycerol | Niloticus | Mosambicus | 20.591 | 99.001 | 0.836 | −178.687 | 219.869 |
| 99% Glycerol | Niloticus | Mosambicus | 267.085 * | 112.275 | 0.022 | 41.087 | 493.083 |
| Type | Sterilization Stage | Mean Difference | Std. Error | Sig. | 95% Confidence Interval for Difference | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Niloticus | Natur | 50% Glycerol | −303.084 * | 43.812 | <0.001 | −391.273 | −214.894 |
| 75% Glycerol | −246.303 * | 63.687 | <0.001 | −374.498 | −118.107 | ||
| 99% Glycerol | −689.578 * | 71.680 | <0.001 | −833.862 | −545.294 | ||
| 50% Glycerol | 75% Glycerol | 56.781 | 66.035 | 0.394 | −76.141 | 189.703 | |
| 99% Glycerol | −386.494 * | 77.439 | <0.001 | −542.371 | −230.618 | ||
| 75% Glycerol | 99% Glycerol | −443.275 * | 87.093 | <0.001 | −618.584 | −267.967 | |
| Mossambicus | Natur | 50% Glycerol | −89.158 | 56.562 | 0.122 | −203.011 | 24.695 |
| 75% Glycerol | −119.029 | 82.220 | 0.154 | −284.529 | 46.471 | ||
| 99% Glycerol | −315.810 * | 92.538 | 0.001 | −502.080 | −129.540 | ||
| 50% Glycerol | 75% Glycerol | −29.871 | 85.251 | 0.728 | −201.473 | 141.731 | |
| 99% Glycerol | −226.652 * | 99.973 | 0.028 | −427.887 | −25.416 | ||
| 75% Glycerol | 99% Glycerol | −196.781 | 112.436 | 0.087 | −423.103 | 29.542 | |
| Sterilization | (I) Type | (J) Type | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval for Difference | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Natur | Niloticus | Mosambicus | −255.257 * | 30.623 | <0.001 | −316.899 | −193.616 |
| 50% Glycerol | Niloticus | Mosambicus | −106.039 * | 45.942 | 0.026 | −198.515 | −13.563 |
| 75% Glycerol | Niloticus | Mosambicus | −109.035 * | 43.777 | 0.016 | −197.153 | −20.917 |
| 99% Glycerol | Niloticus | Mosambicus | 49.084 | 44.883 | 0.280 | −41.261 | 139.428 |
| Type | Sterilization Stage | Mean Difference | Std. Error | Sig. | 95% Confidence Interval for Difference | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Niloticus | Natur | 50% Glycerol | −100.379 * | 28.407 | 0.001 | −157.559 | −43.199 |
| 75% Glycerol | −160.451 * | 34.668 | <0.001 | −230.233 | −90.669 | ||
| 99% Glycerol | −179.153 * | 33.035 | <0.001 | −245.648 | −112.657 | ||
| 50% Glycerol | 75% Glycerol | −60.072 | 31.775 | 0.065 | −124.032 | 3.887 | |
| 99% Glycerol | −78.774 * | 27.496 | 0.006 | −134.121 | −23.427 | ||
| 75% Glycerol | 99% Glycerol | −18.702 | 23.347 | 0.427 | −65.697 | 28.293 | |
| Mossambicus | Natur | 50% Glycerol | 48.840 | 36.673 | 0.190 | −24.979 | 122.659 |
| 75% Glycerol | −14.229 | 44.756 | 0.752 | −104.317 | 75.860 | ||
| 99% Glycerol | 125.188 * | 42.648 | 0.005 | 39.343 | 211.033 | ||
| 50% Glycerol | 75% Glycerol | −63.069 | 41.021 | 0.131 | −145.640 | 19.503 | |
| 99% Glycerol | 76.348 * | 35.497 | 0.037 | 4.896 | 147.801 | ||
| 75% Glycerol | 99% Glycerol | 139.417* | 30.141 | <0.001 | 78.747 | 200.087 | |
| Sterilization | (I) Type | (J) Type | Mean Difference (I–J) | Std. Error | Sig. | 95% Confidence Interval for Difference | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Natur | Niloticus | Mosambicus | 0.280 * | 0.066 | <0.001 | 0.147 | 0.412 |
| 50% Glycerol | Niloticus | Mosambicus | 0.394 * | 0.088 | <0.001 | 0.217 | 0.571 |
| 75% Glycerol | Niloticus | Mosambicus | 0.221 | 0.110 | 0.050 | 0.000 | 0.443 |
| 99% Glycerol | Niloticus | Mosambicus | 0.198 | 0.168 | 0.244 | −0.140 | 0.535 |
| Type | Sterilization Stage | Mean Difference | Std. Error | Sig. | 95% Confidence Interval for Difference | ||
|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||
| Niloticus | Natur | 50% Glycerol | −0.297 * | 0.072 | <0.001 | −0.441 | −0.153 |
| 75% Glycerol | −0.049 | 0.069 | 0.480 | −0.189 | 0.090 | ||
| 99% Glycerol | −0.646 * | 0.115 | <0.001 | −0.877 | −0.415 | ||
| 50% Glycerol | 75% Glycerol | 0.248 * | 0.087 | 0.006 | 0.073 | 0.422 | |
| 99% Glycerol | −0.349 * | 0.112 | 0.003 | −0.575 | −0.123 | ||
| 75% Glycerol | 99% Glycerol | −0.597 * | 0.122 | <0.001 | −0.842 | −0.351 | |
| Mossambicus | Natur | 50% Glycerol | −0.182 | 0.092 | 0.054 | −0.368 | 0.003 |
| 75% Glycerol | −0.108 | 0.089 | 0.234 | −0.288 | 0.072 | ||
| 99% Glycerol | −0.728 * | 0.148 | <0.001 | −1.026 | −0.429 | ||
| 50% Glycerol | 75% Glycerol | 0.075 | 0.112 | 0.508 | −0.151 | 0.300 | |
| 99% Glycerol | −0.545 * | 0.145 | <0.001 | −0.837 | −0.253 | ||
| 75% Glycerol | 99% Glycerol | −0.620 * | 0.157 | <0.001 | −0.937 | −0.303 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciornei, B.; Vaduva, A.; David, V.L.; Popescu, D.; Vulcanescu, D.D.; Adam, O.; Avram, C.R.; Pacurari, A.C.; Boia, E.S. Comparison of Type I and Type III Collagen Concentration between Oreochromis mossambicus and Oreochromis niloticus in Relation to Skin Scaffolding. Medicina 2023, 59, 1002. https://doi.org/10.3390/medicina59061002
Ciornei B, Vaduva A, David VL, Popescu D, Vulcanescu DD, Adam O, Avram CR, Pacurari AC, Boia ES. Comparison of Type I and Type III Collagen Concentration between Oreochromis mossambicus and Oreochromis niloticus in Relation to Skin Scaffolding. Medicina. 2023; 59(6):1002. https://doi.org/10.3390/medicina59061002
Chicago/Turabian StyleCiornei, Bogdan, Adrian Vaduva, Vlad Laurentiu David, Diana Popescu, Dan Dumitru Vulcanescu, Ovidiu Adam, Cecilia Roberta Avram, Alina Cornelia Pacurari, and Eugen Sorin Boia. 2023. "Comparison of Type I and Type III Collagen Concentration between Oreochromis mossambicus and Oreochromis niloticus in Relation to Skin Scaffolding" Medicina 59, no. 6: 1002. https://doi.org/10.3390/medicina59061002
APA StyleCiornei, B., Vaduva, A., David, V. L., Popescu, D., Vulcanescu, D. D., Adam, O., Avram, C. R., Pacurari, A. C., & Boia, E. S. (2023). Comparison of Type I and Type III Collagen Concentration between Oreochromis mossambicus and Oreochromis niloticus in Relation to Skin Scaffolding. Medicina, 59(6), 1002. https://doi.org/10.3390/medicina59061002






