Analgesic Efficacy of Intraoperative Superior Hypogastric Plexus (SHP) Block during Abdominal Hysterectomy: A Systematic Review and Meta-Analysis of Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Eligibility Criteria
2.3. Databases, Search Strategy, and Study Selection Process
2.4. Data Items, Study Quality Assessment, and Data Collection Process
2.5. Data Analysis
3. Results
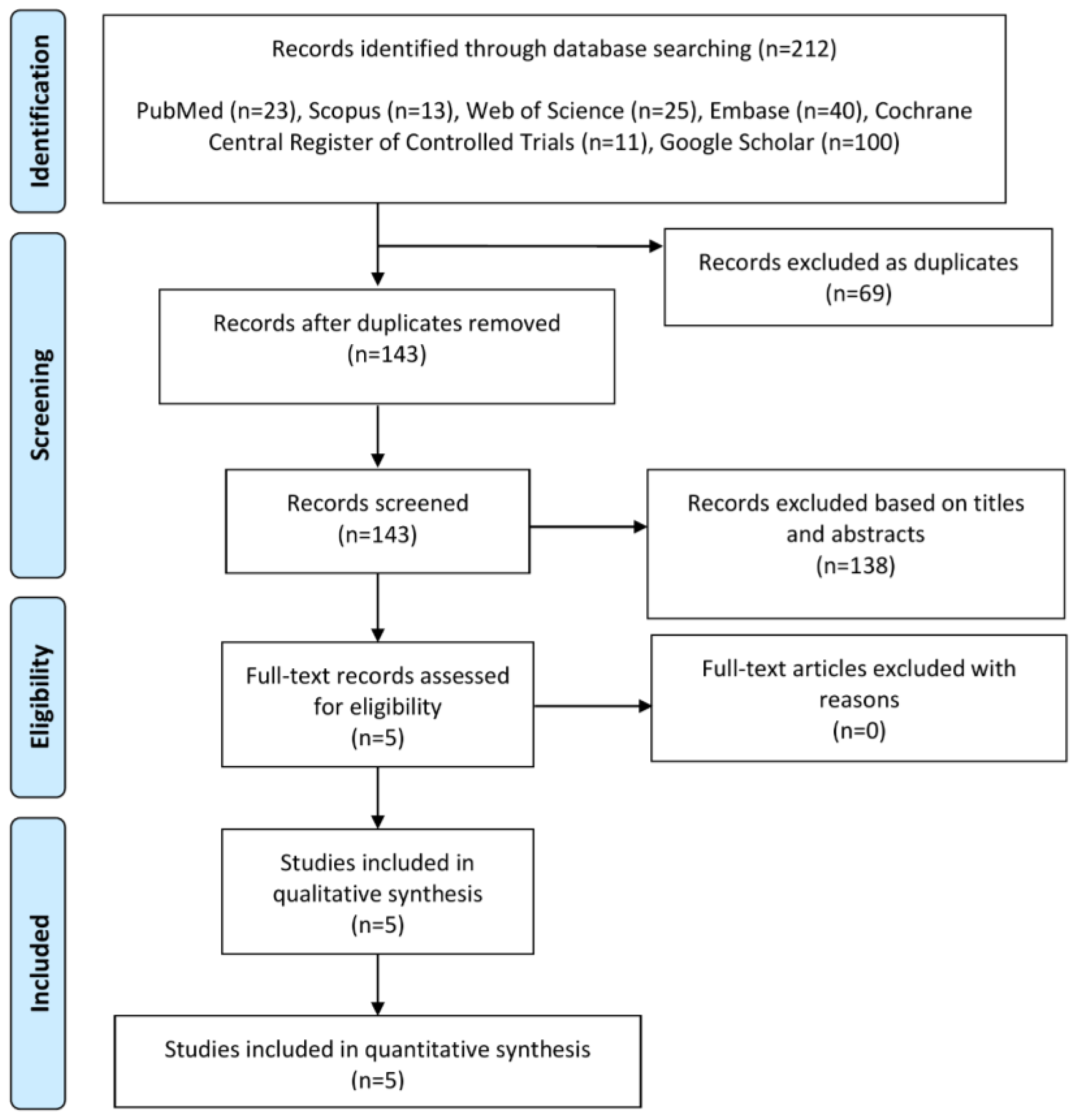
3.1. Summary of Literature Search and Included Studies
3.2. Summary of Risk of Bias of the Included Studies
3.3. Summary of Primary Endpoints
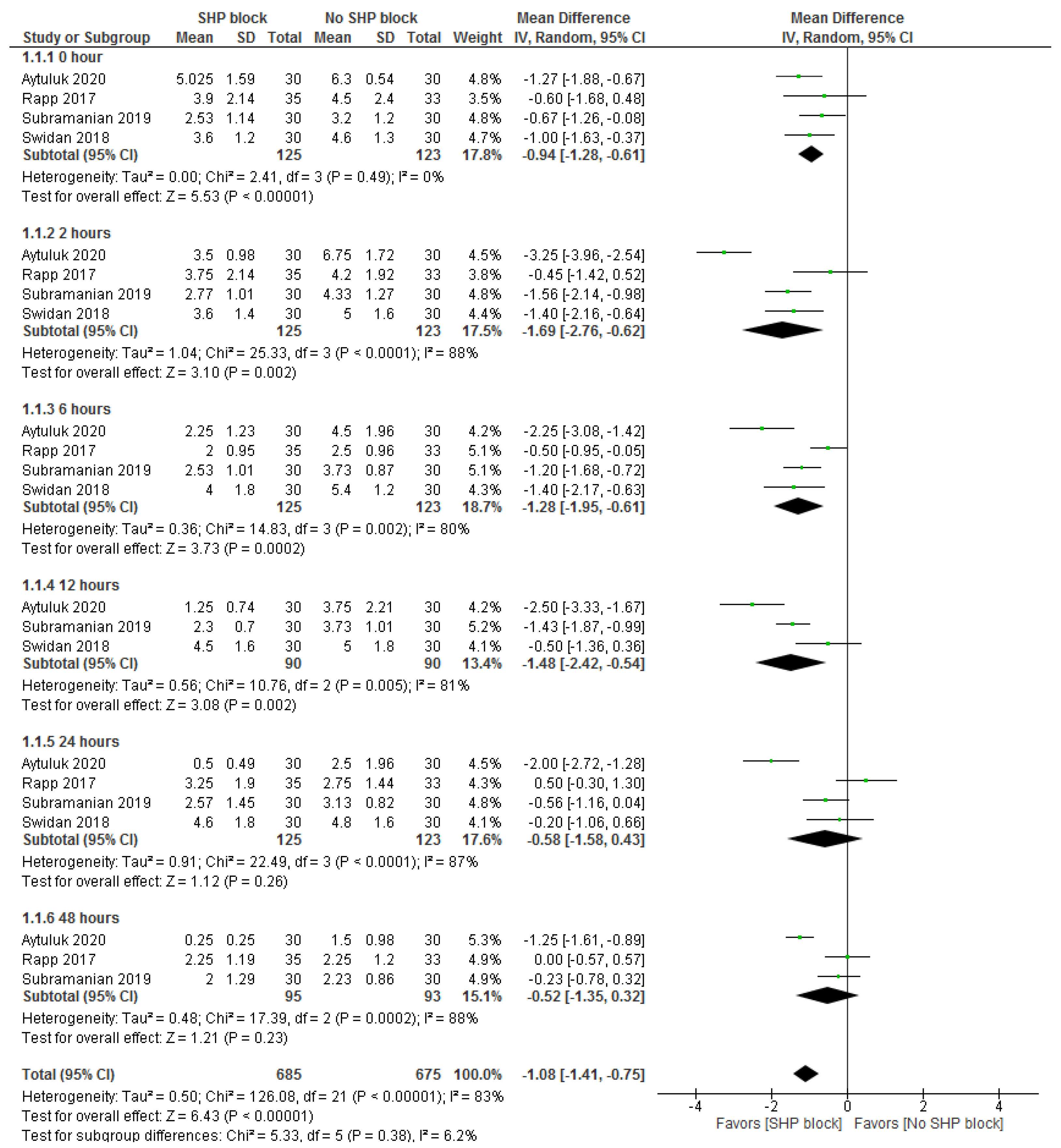
3.3.1. Postsurgical Pain Score
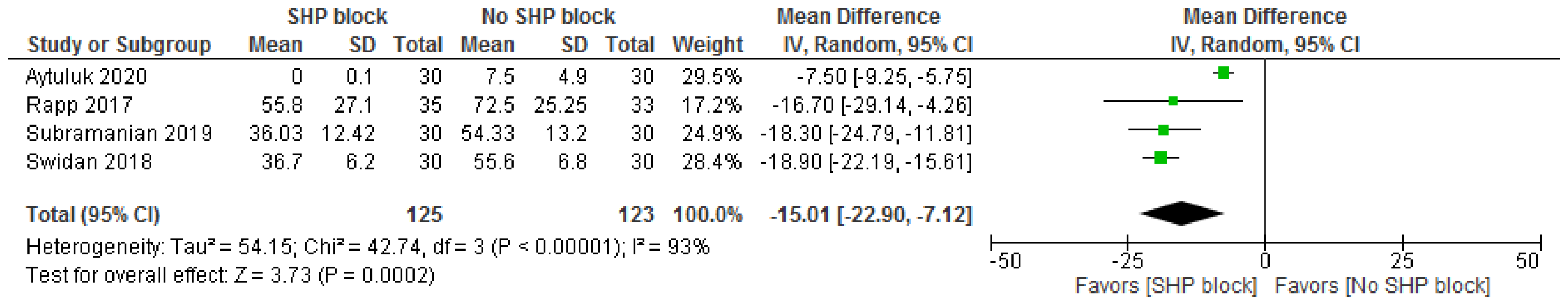
3.3.2. Postsurgical Opioid Consumption
3.4. Summary of Secondary Endpoints
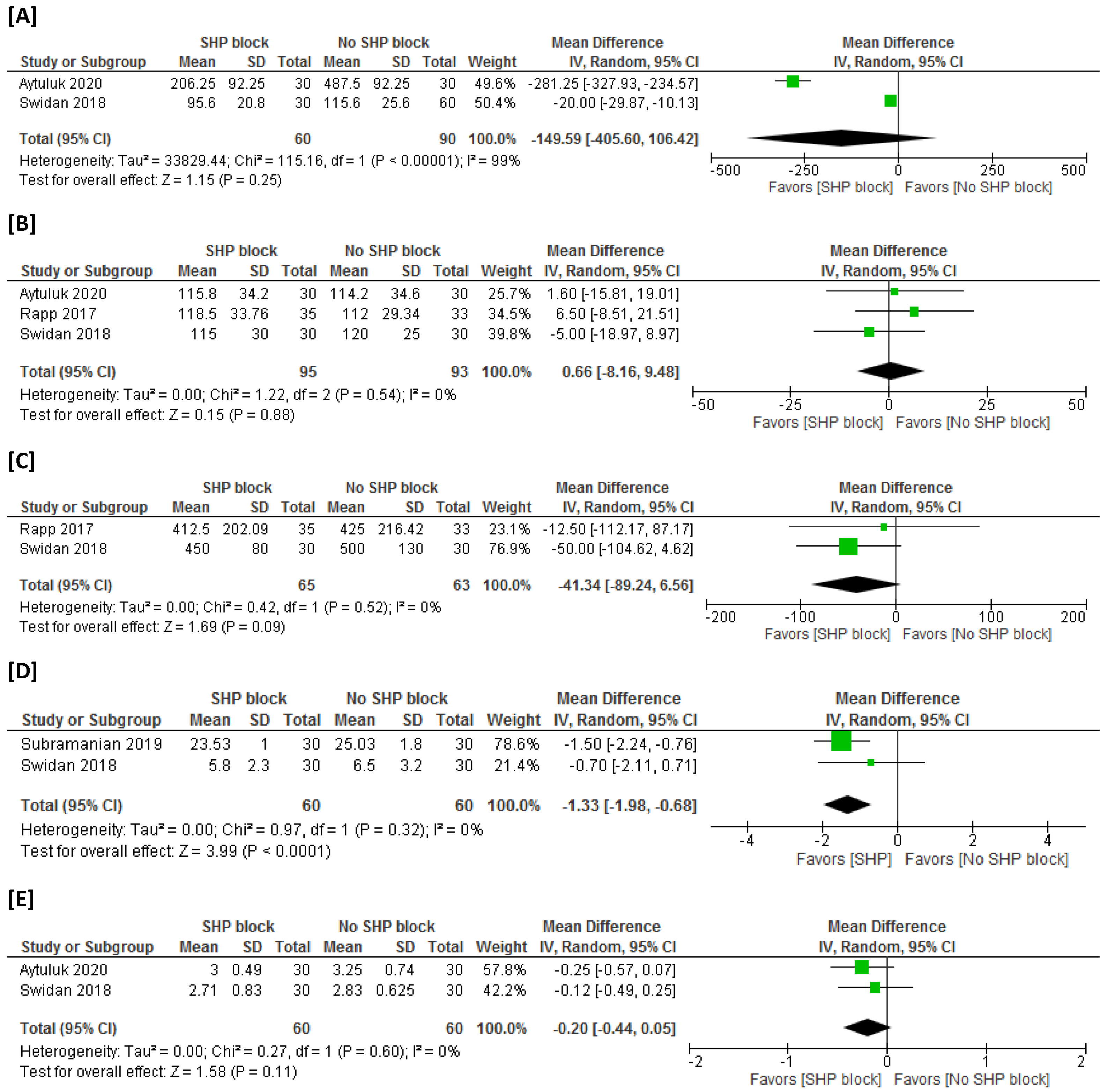
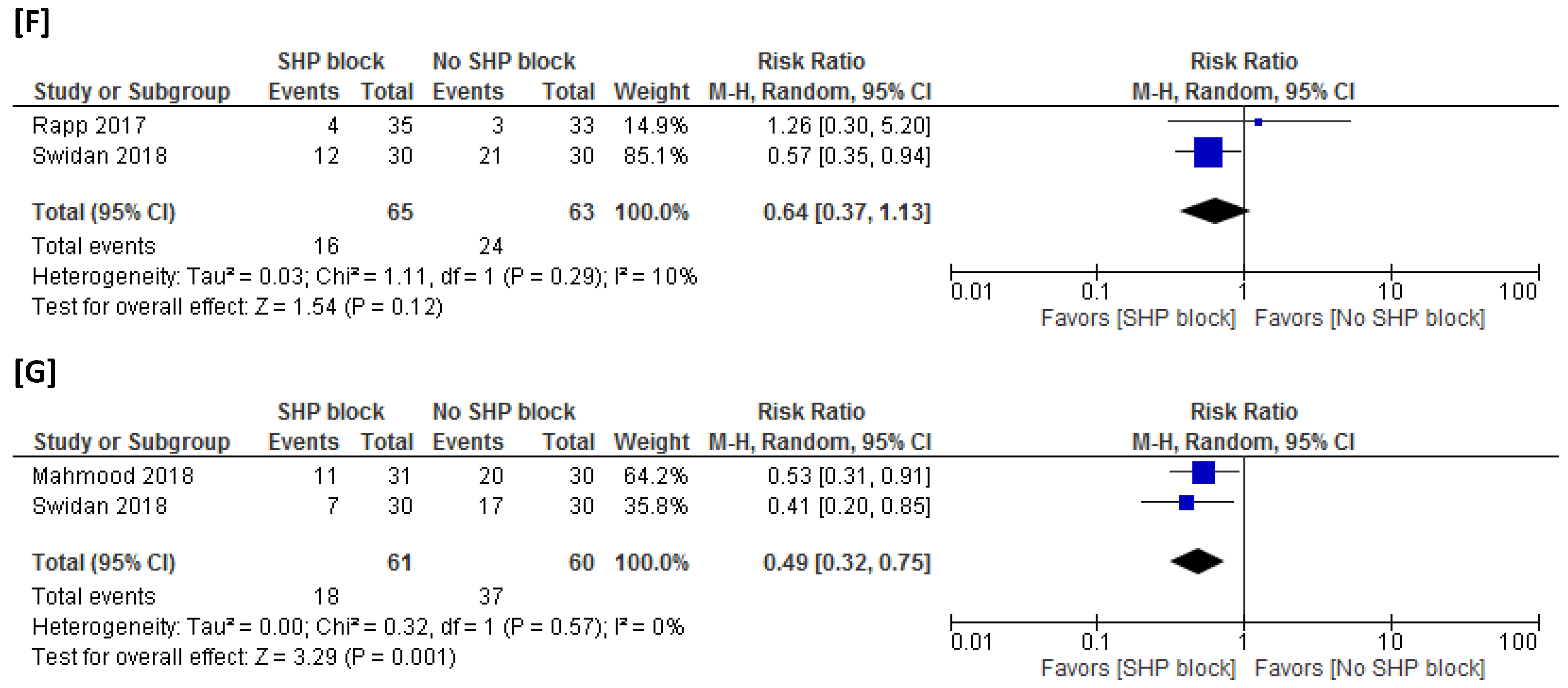
3.4.1. Postsurgical NSAID Consumption
3.4.2. Rescue Analgesic Time
3.4.3. Operation Time
3.4.4. Estimated Intraoperative Blood Loss
3.4.5. Time to First Mobilization
3.4.6. Time to First Bowel Movement and Urinary Passage
3.4.7. Length of Hospital Stay
3.4.8. Adverse Events
3.5. Leave-One-Out Sensitivity Analysis for the Primary Endpoints
3.6. Publication Bias Analysis for the Primary Endpoints
4. Discussion
4.1. Summary of Results
4.2. Explanation of Results and Clinical Implications
4.3. Comparsion with Previous Meta-Analysis Reports
4.4. Strengths and Weaknesses
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aytuluk, H.G.; Kale, A.; Astepe, B.S.; Basol, G.; Balci, C.; Colak, T. Superior Hypogastric Plexus Blocks for Postoperative Pain Management in Abdominal Hysterectomies. Clin. J. Pain 2020, 36, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Nassif, G.J.; Miller, T.E. Evolving the management of acute perioperative pain towards opioid free protocols: A narrative review. Curr. Med. Res. Opin. 2019, 35, 2129–2136. [Google Scholar] [CrossRef]
- Rapp, H.; Ledin Eriksson, S.; Smith, P. Superior hypogastric plexus block as a new method of pain relief after abdominal hysterectomy: Double-blind, randomised clinical trial of efficacy. Bjog 2017, 124, 270–276. [Google Scholar] [CrossRef]
- Gebhart, G.F.; Bielefeldt, K. Physiology of Visceral Pain. Compr. Physiol. 2016, 6, 1609–1633. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.M., Jr. Basic neuroanatomy for understanding pelvic pain. J. Am. Assoc. Gynecol. Laparosc. 1999, 6, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Schwartz, R.; Herman, J.; Berger, A.A.; Lee, D.; Lee, C.; Zamarripa, A.M.; Slovek, A.; Habib, K.; Manchikanti, L.; et al. A Comprehensive Update of the Superior Hypogastric Block for the Management of Chronic Pelvic Pain. Curr. Pain Headache Rep. 2021, 25, 13. [Google Scholar] [CrossRef]
- Swidan, E.; Abdelzaam, E. Efficacy of superior hypogastric plexus block with bupivacaine 0.5% for post total abdominal hysterectomy pain relief. Egypt. J. Fertil. Steril. 2017, 21, 23–29. [Google Scholar] [CrossRef]
- Mahmood, K.; Jafri, S.A.U.; Choudry, A.; Kallue, U.R.; Amin, N. Intraoperative superior hypogastric plexus block, to relieve postoperative pain in abdominal hysterectomies. Pak. Armed Forces Med. J. 2018, 68, 935–941. [Google Scholar]
- Subramanian, V.; Aggarwal, S.; Kale, S.; Parthasarathy, A.H.; Batra, A. Intraoperative superior hypogastric plexus block for postoperative pain following gynecological laparotomies. Anaesth. Pain Intensive Care 2019, 23, 157–161. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.G.S. (Ed.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Internet]. [Updated March 2011]. The Cochrane Collaboration and A.A.F. Available online: www.handbook.cochrane.org (accessed on 3 May 2022).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Dickersin, K.; Berlin, J.A. Meta-analysis: State-of-the-science. Epidemiol. Rev. 1992, 14, 154–176. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Phys. 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Myles, P.S.; Myles, D.B.; Galagher, W.; Boyd, D.; Chew, C.; MacDonald, N.; Dennis, A. Measuring acute postoperative pain using the visual analog scale: The minimal clinically important difference and patient acceptable symptom state. Br. J. Anaesth. 2017, 118, 424–429. [Google Scholar] [CrossRef]
- Khodaverdi, S.; Alebouyeh, M.R.; Sadegi, K.; Mehdizadehkashi, A.; Kaveh, M.; Entezari, S.R.; Mirzaei, H.; Khaledi, M.; Khodaverdi, M. Superior hypogastric plexus block as an effective treatment method for endometriosis-related chronic pelvic pain: An open-label pilot clinical trial. J. Obstet. Gynaecol. 2021, 41, 966–971. [Google Scholar] [CrossRef]
- Astepe, B.S.; Aytuluk, H.G.; Yavuz, A.; Türkay, Ü.; Terzi, H.; Kale, A. Intraoperative superior hypogastric plexus block during cesarean section: A new technique for pain relief. J. Matern. Fetal Neonatal Med. 2020, 33, 2657–2663. [Google Scholar] [CrossRef]
- Peker, H.; Atasayan, K.; Haliloglu Peker, B.; Kilicci, C. Intraoperative superior hypogastric plexus block for pain relief after a cesarean section: A case-control study. Croat. Med. J. 2021, 62, 472–479. [Google Scholar] [CrossRef]
- Malouhi, A.; Aschenbach, R.; Erbe, A.; Owsianowski, Z.; Rußwurm, S.; Runnebaum, I.B.; Teichgräber, U. Effectiveness of Superior Hypogastric Plexus Block for Pain Control Compared to Epidural Anesthesia in Women Requiring Uterine Artery Embolization for the Treatment of Uterine Fibroids—A Retrospective Evaluation. Rofo 2021, 193, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Aytuluk, H.G.; Kale, A.; Basol, G. Laparoscopic Superior Hypogastric Blocks for Postoperative Pain Management in Hysterectomies: A New Technique for Superior Hypogastric Blocks. J. Minim. Invasive Gynecol. 2019, 26, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.V.; Moore, K.; Maghsoudlou, P.; North, A.; Ajao, M.O.; Einarsson, J.I.; Louie, M.; Schiff, L.; Moawad, G.; Cohen, S.L.; et al. Superior Hypogastric Plexus Block to Reduce Pain After Laparoscopic Hysterectomy: A Randomized Controlled Trial. Obstet. Gynecol. 2021, 137, 648–656. [Google Scholar] [CrossRef] [PubMed]
- De Silva, P.; Daniels, S.; Bukhari, M.E.; Choi, S.; Liew, A.; Rosen, D.M.B.; Conrad, D.; Cario, G.M.; Chou, D. Superior Hypogastric Plexus Nerve Block in Minimally Invasive Gynecology: A Randomized Controlled Trial. J. Minim. Invasive Gynecol. 2022, 29, 94–102. [Google Scholar] [CrossRef]
- Baig, S.; Moon, J.Y.; Shankar, H. Review of Sympathetic Blocks: Anatomy, Sonoanatomy, Evidence, and Techniques. Reg. Anesth. Pain Med. 2017, 42, 377–391. [Google Scholar] [CrossRef]
- Hansen, T.G. Ropivacaine: A pharmacological review. Expert. Rev. Neurother. 2004, 4, 781–791. [Google Scholar] [CrossRef]
- Kuthiala, G.; Chaudhary, G. Ropivacaine: A review of its pharmacology and clinical use. Indian. J. Anaesth. 2011, 55, 104–110. [Google Scholar] [CrossRef]
- Bosscher, H. Blockade of the superior hypogastric plexus block for visceral pelvic pain. Pain Pract. 2001, 1, 162–170. [Google Scholar] [CrossRef] [PubMed]
- de Leon-Casasola, O.A.; Kent, E.; Lema, M.J. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Pain 1993, 54, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Alomar, O.; Abuzaid, M.; Abu-Zaid, A.; Al-Badawi, I.A.; Salem, H. Superior hypogastric plexus (SHP) block during minimally invasive hysterectomy: A systematic review. Turk. J. Obstet. Gynecol. 2022, 19, 170–177. [Google Scholar] [CrossRef]
- Shama, A.A.A.; Elgarhy, A.M.M.M.; Ewieda, T.M.A.; Ibrahim, W.M.E.; Elsayed, M.M.; Arafa, M.H.; Yahia, O.S.; Elsayed, A.H.I.; Almonayery, D.M.; Abdelhakim, A.M.; et al. Superior Hypogastric Plexus Block for Pain Management Post-Hysterectomy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Pain Palliat. Care Pharmacother. 2022, 36, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Efthimiou, O.; Mavridis, D.; Debray, T.P.; Samara, M.; Belger, M.; Siontis, G.C.; Leucht, S.; Salanti, G. Combining randomized and non-randomized evidence in network meta-analysis. Stat. Med. 2017, 36, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Sarri, G.; Patorno, E.; Yuan, H.; Guo, J.J.; Bennett, D.; Wen, X.; Zullo, A.R.; Largent, J.; Panaccio, M.; Gokhale, M.; et al. Framework for the synthesis of non-randomised studies and randomised controlled trials: A guidance on conducting a systematic review and meta-analysis for healthcare decision making. BMJ Evid. Based Med. 2022, 27, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Scheib, S.A.; Thomassee, M.; Kenner, J.L. Enhanced Recovery after Surgery in Gynecology: A Review of the Literature. J. Minim. Invasive Gynecol. 2019, 26, 327–343. [Google Scholar] [CrossRef]
- Giannini, A.; D’Oria, O.; Bogani, G.; Di Donato, V.; Vizza, E.; Chiantera, V.; Laganà, A.S.; Muzii, L.; Salerno, M.G.; Caserta, D.; et al. Hysterectomy: Let’s Step Up the Ladder of Evidence to Look Over the Horizon. J. Clin. Med. 2022, 11, 6940. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, H.; Bukhari, I.A.; Al Baalharith, M.; AlTahtam, N.; Alabdrabalamir, S.; Jamjoom, M.Z.; Baradwan, S.; Badghish, E.; Abuzaid, M.; AbuAlsaud, F.S.; et al. Analgesic Efficacy of Intraoperative Superior Hypogastric Plexus (SHP) Block during Abdominal Hysterectomy: A Systematic Review and Meta-Analysis of Controlled Trials. Medicina 2023, 59, 893. https://doi.org/10.3390/medicina59050893
Salem H, Bukhari IA, Al Baalharith M, AlTahtam N, Alabdrabalamir S, Jamjoom MZ, Baradwan S, Badghish E, Abuzaid M, AbuAlsaud FS, et al. Analgesic Efficacy of Intraoperative Superior Hypogastric Plexus (SHP) Block during Abdominal Hysterectomy: A Systematic Review and Meta-Analysis of Controlled Trials. Medicina. 2023; 59(5):893. https://doi.org/10.3390/medicina59050893
Chicago/Turabian StyleSalem, Hany, Ibtihal Abdulaziz Bukhari, Maha Al Baalharith, Nasser AlTahtam, Safa Alabdrabalamir, Mohammed Ziad Jamjoom, Saeed Baradwan, Ehab Badghish, Mohammed Abuzaid, Fatimah Shakir AbuAlsaud, and et al. 2023. "Analgesic Efficacy of Intraoperative Superior Hypogastric Plexus (SHP) Block during Abdominal Hysterectomy: A Systematic Review and Meta-Analysis of Controlled Trials" Medicina 59, no. 5: 893. https://doi.org/10.3390/medicina59050893
APA StyleSalem, H., Bukhari, I. A., Al Baalharith, M., AlTahtam, N., Alabdrabalamir, S., Jamjoom, M. Z., Baradwan, S., Badghish, E., Abuzaid, M., AbuAlsaud, F. S., Alomar, O., Alyousef, A., Abu-Zaid, A., & Al-Badawi, I. A. (2023). Analgesic Efficacy of Intraoperative Superior Hypogastric Plexus (SHP) Block during Abdominal Hysterectomy: A Systematic Review and Meta-Analysis of Controlled Trials. Medicina, 59(5), 893. https://doi.org/10.3390/medicina59050893






