A Qualitative and Quantitative Study to Evaluate the Effectiveness and Safety of Magnetic Stimulation in Women with Urinary Incontinence Symptoms and Pelvic Floor Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Qualitative Evaluation with Validated Questionnaires
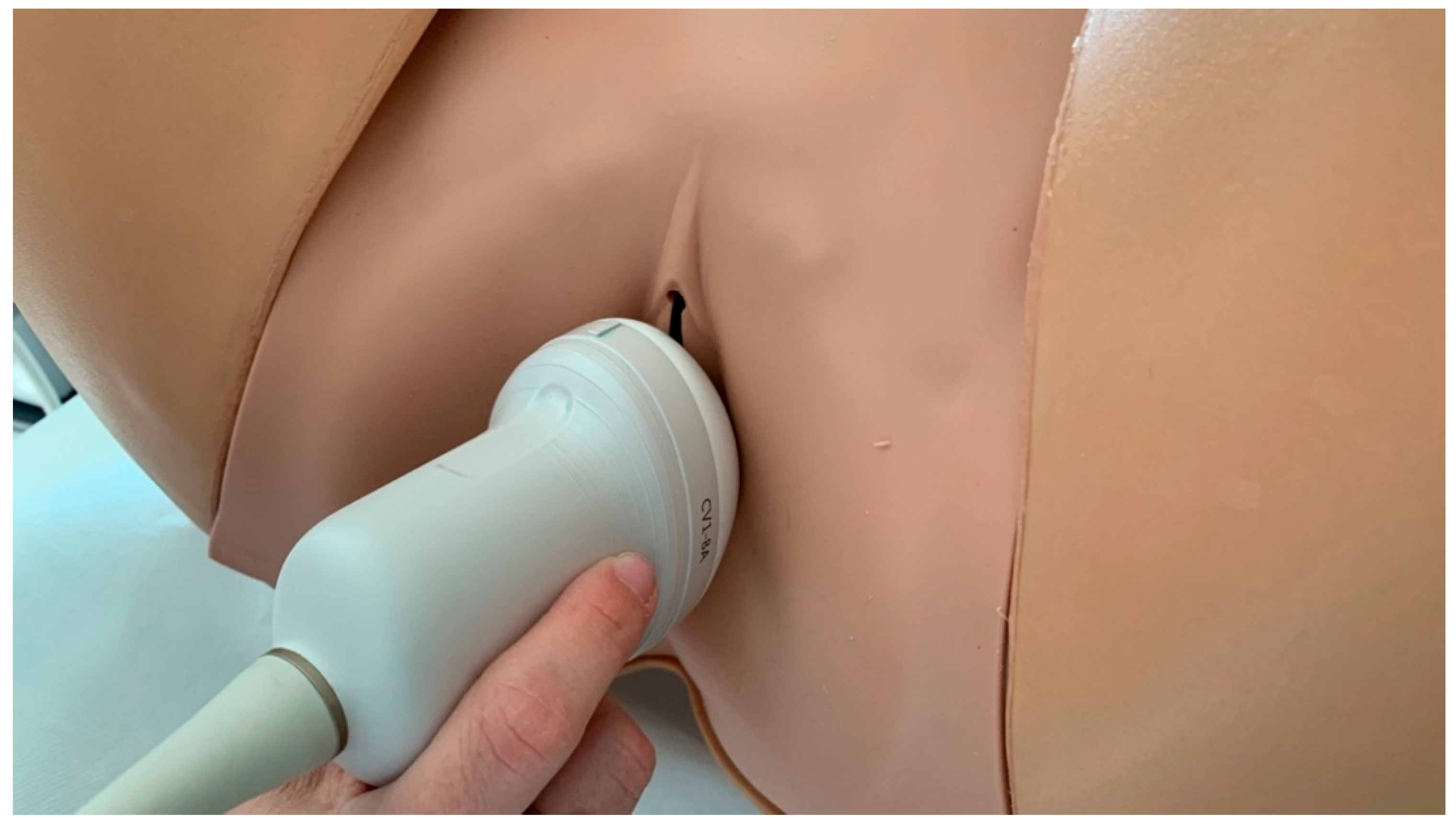
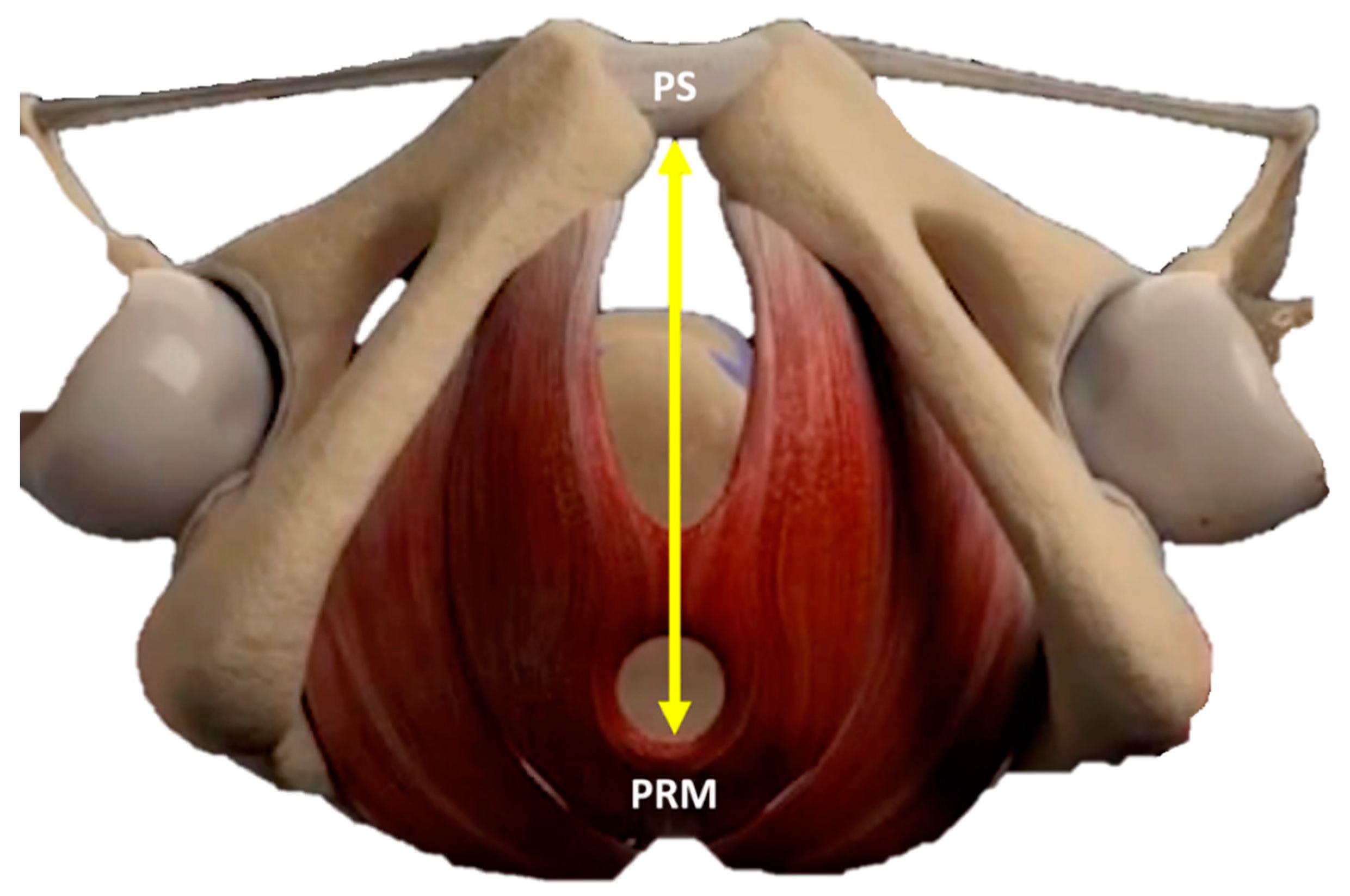
2.3. Quantitative Evaluation with Ultrasounds
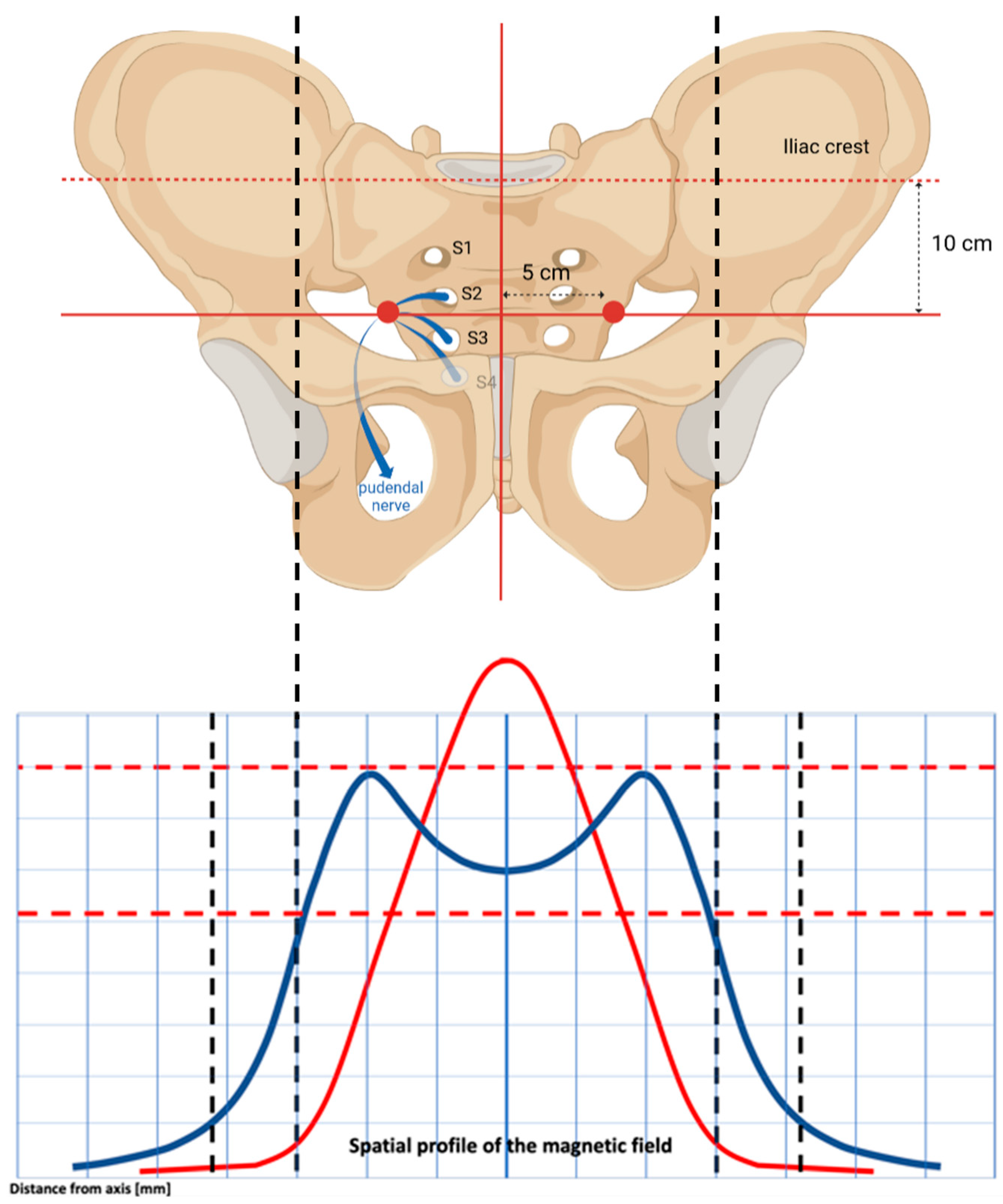
2.4. Study Device
2.5. Dr. ARNOLD Protocols and Assessments
2.6. Statistical Analysis
3. Results
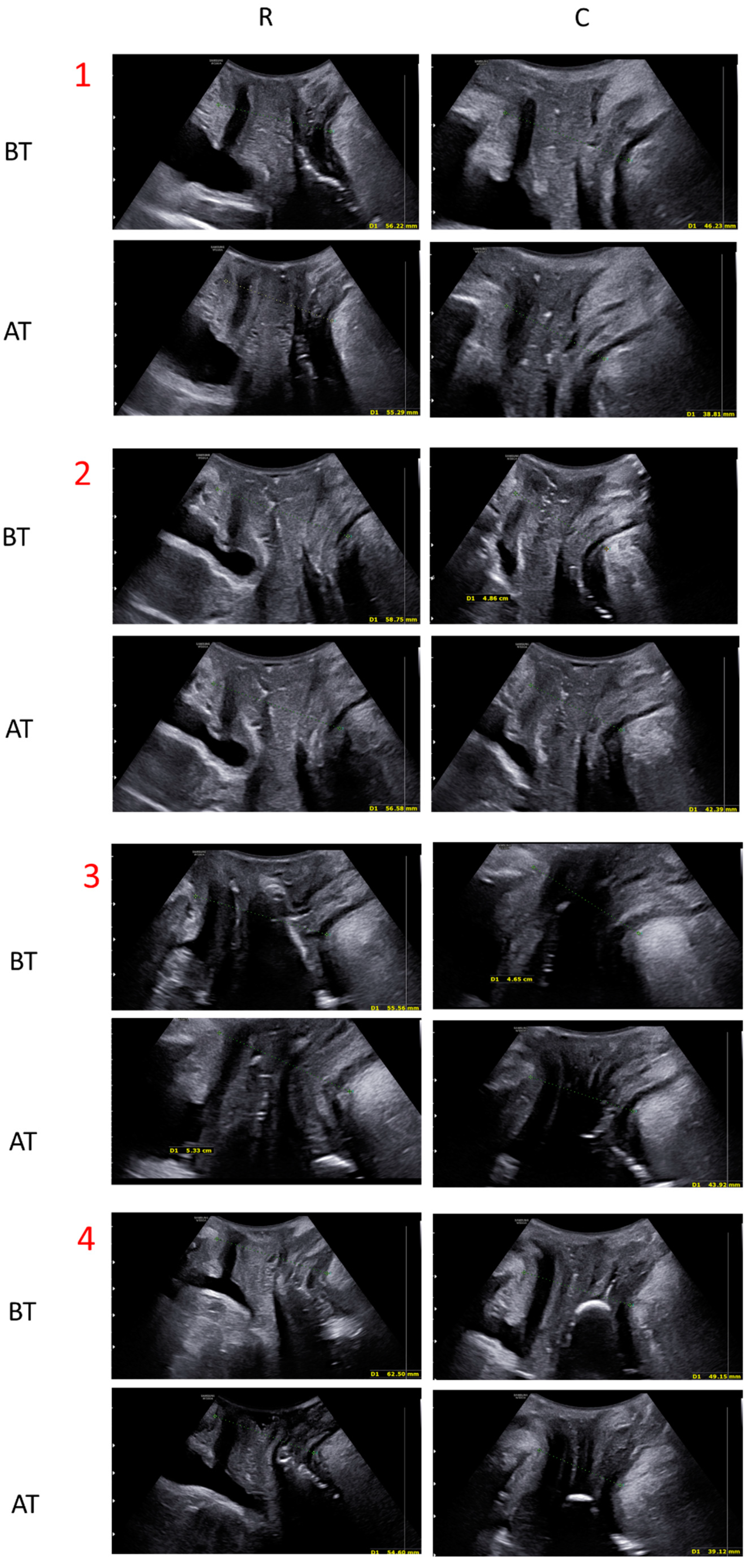
3.1. Quantitative Evaluation: Ultrasound Measurements
3.2. Qualitative Evaluation: Questionnaire Findings
SUI
3.3. Pelvic Organ Prolapse
3.4. Overactive Bladder Urge/UUI
3.5. Quality of Life
4. Discussion
Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.S.; Lue, T.F. Stem cell therapy for stress urinary incontinence: A critical review. Stem Cells Dev. 2012, 21, 834–843. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Dmochowski, R. Urinary incontinence in women: A comprehensive review of the pathophysiology, diagnosis and treatment. Minerva. Ginecol. 2014, 66, 469–478. Available online: https://europepmc.org/article/med/25078140 (accessed on 25 January 2023).
- Tennstedt, S.L.; Link, C.L.; Steers, W.D.; McKinlay, J.B. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: The Boston Area Community Health (BACH) Survey. Am. J. Epidemiol. 2008, 167, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Anger, J.T.; Saigal, C.S.; Litwin, M.S. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J. Urol. 2006, 175, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Minassian, V.A.; Yan, X.; Lichtenfeld, M.J.; Sun, H.; Stewart, W.F. The iceberg of health care utilization in women with urinary incontinence. Int. Urogynecol. J. 2012, 23, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Lopopolo, G.; Salsi, B.; Banfi, A.; Isaza, P.G.; Fusco, I. Is It Possible to Improve Urinary Incontinence and Quality of Life in Female Patients? A Clinical Evaluation of the Efficacy of Top Flat Magnetic Stimulation Technology. Bioengineering 2022, 9, 140. [Google Scholar] [CrossRef]
- Isaza, P.G.; Borrego, R.S.; Fusco, I. A case of stress urinary incontinence after radical prostatectomy successfully treated with an innovative device based on top flat magnetic stimulation. World J. Urol. 2022, 40, 1887–1889. [Google Scholar] [CrossRef]
- Faiena, I.; Patel, N.; Parihar, J.S.; Calabrese, M.; Tunuguntla, H.; Robert, R. Conservative Management of Urinary Incontinence in Women. Rev. Urol. 2015, 17, 129. [Google Scholar] [CrossRef]
- Mezzana, P.; Garibay, I.; Fusco, I. Vaginal Bipolar Radiofrequency Treatment of Mild SUI: A Pilot Retrospective Study. Medicina 2022, 58, 181. [Google Scholar] [CrossRef]
- Labrie, J.; Berghmans, B.L.; Fischer, K.; Milani, A.L.; van der Wijk, I.; Smalbraak, D.J.; Vollebregt, A.; Schellart, R.P.; Graziosi, G.C.; van der Ploeg, J.M.; et al. Surgery versus physiotherapy for stress urinary incontinence. N. Engl. J. Med. 2013, 369, 1124–1133. [Google Scholar] [CrossRef]
- Greer, J.A.; Arya, L.A.; Smith, A.L. Urinary Incontinence: Diagnosis and Treatment in the Elderly. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 66–75. [Google Scholar] [CrossRef]
- Yount, S.M.; Fay, R.A.; Kissler, K.J. Prenatal and Postpartum Experience, Knowledge and Engagement with Kegels: A Longitudinal, Prospective, Multisite Study. J. Womens Health 2021, 30, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, O.T.; Hassa, H.; Ozalp, S.; Yildirim, A.; Sener, T. Results of the anti-incontinence operations and Kegel exercises in patients with Type II anatomic stress incontinence. Acta Obstet. Gynecol. Scand. 1998, 77, 341–346. [Google Scholar] [CrossRef]
- Biondo, A.; Isaza, P.G.; Fusco, I. Efficacy of Top Flat Magnetic Stimulation Technology for Female Stress and Urge Urinary Incontinence: A Clinical Evaluation. World J. Nephrol. Urol. 2022, 11, 18–23. [Google Scholar] [CrossRef]
- Christensen, L.L.; Djurhuus, J.C.; Constantinou, C.E. Imaging of pelvic floor contractions using MRI. Neurourol. Urodyn. 1995, 14, 209–216. [Google Scholar] [CrossRef]
- Chi, T.W.C.; Chen, S.H. Dynamic magnetic resonance imaging used in evaluation of female pelvic prolapse: Experience from nine cases. Kaohsiung J. Med. Sci. 2007, 23, 302–308. [Google Scholar] [CrossRef]
- Hoyte, L.; Schierlitz, L.; Zou, K.; Flesh, G.; Fielding, J.R. Two- and 3-dimensional MRI comparison of levator ani structure, volume, and integrity in women with stress incontinence and prolapse. Am. J. Obstet. Gynecol. 2001, 185, 11–19. [Google Scholar] [CrossRef]
- Dietz, H.P. Ultrasound imaging of the pelvic floor. Part II: Three-dimensional or volume imaging. Ultrasound Obstet. Gynecol. 2004, 23, 615–625. [Google Scholar] [CrossRef]
- Dietz, H.P.; Shek, C.; Clarke, B. Biometry of the pubovisceral muscle and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet. Gynecol. 2005, 25, 580–585. [Google Scholar] [CrossRef]
- Dietz, H.P.; Steensma, A.B.; Hastings, R. Three-dimensional ultrasound imaging of the pelvic floor: The effect of parturition on paravaginal support structures. Ultrasound Obstet. Gynecol. 2003, 21, 589–595. [Google Scholar] [CrossRef]
- Haylen, B.T.; De Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int. Urogynecol. J. 2010, 21, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Klovning, A.; Avery, K.; Sandvik, H.; Hunskaar, S. Comparison of two questionnaires for assessing the severity of urinary incontinence: The ICIQ-UI SF versus the incontinence severity index. Neurourol. Urodyn. 2009, 28, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Uebersax, J.S.; Wyman, J.F.; Shumaker, S.A.; McClish, D.K.; Fantl, J.A. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol. Urodyn. 1995, 14, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.P.H.; Lam, C.L.K.; Chin, W.Y. The Incontinence Impact Questionnaire-7 (IIQ-7) Can Be Applicable to Chinese Males and Females with Lower Urinary Tract Symptoms. Patient 2014, 7, 403–411. [Google Scholar] [CrossRef]
- Jackson, S.; Donovan, J.; Brookes, S.; Eckford, S.; Swithinbank, L.; Abrams, P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: Development and psychometric testing. Br. J. Urol. 1996, 77, 805–812. [Google Scholar] [CrossRef]
- Barber, M.D.; Walters, M.D.; Bump, R.C. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am. J. Obstet. Gynecol. 2005, 193, 103–113. [Google Scholar] [CrossRef]
- Utomo, E.; Blok, B.F.; Steensma, A.B.; Korfage, I.J. Validation of the Pelvic Floor Distress Inventory (PFDI-20) and Pelvic Floor Impact Questionnaire (PFIQ-7) in a Dutch population. Int. Urogynecol. J. 2014, 25, 531–544. [Google Scholar] [CrossRef]
- Digesu, G.A.; Khullar, V.; Cardozo, L.; Robinson, D.; Salvatore, S. P-QOL: A validated questionnaire to assess the symptoms and quality of life of women with urogenital prolapse. Int. Urogynecol. J. 2005, 16, 176–181. [Google Scholar] [CrossRef]
- Dietz, H.P.; Haylen, B.T.; Broome, J. Ultrasound in the quantification of female pelvic organ prolapse. Ultrasound Obstet. Gynecol. 2001, 18, 511–514. [Google Scholar] [CrossRef]
- Dominguez, A.P.; Isaza, P.G.; Pantoja, S.N.; Fusco, I. Role of top flat magnetic stimulation for urinary incontinence as a debilitating condition of pelvic floor dysfunction: An observational evaluation of Latin American population. World J. Urol. 2023, 41, 173–177. [Google Scholar] [CrossRef]
- Frigerio, M.; Barba, M.; Cola, A.; Marino, G.; Volontè, S.; Melocchi, T.; De Vicari, D.; Maruccia, S. Flat Magnetic Stimulation for Stress Urinary Incontinence: A Prospective Comparison Study. Bioengineering 2023, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, M.; Palmieri, B.; Malagoli, A.; Laurino, C. High-power Magnetotherapy: A New Weapon in Urinary Incontinence? Low. Urin. Tract Symptoms 2018, 10, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.S.; Tseng, L.H.; Lin, Y.H.; Liang, C.C.; Lu, C.Y.; Pue, L.B. Effect of extracorporeal magnetic energy stimulation on bothersome lower urinary tract symptoms and quality of life in female patients with stress urinary incontinence and overactive bladder. J. Obstet. Gynaecol. Res. 2013, 39, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Chandi, D.D.; Groenendijk, P.M.; Venema, P.L. Functional extracorporeal magnetic stimulation as a treatment for female urinary incontinence: ‘the chair’. BJU Int. 2004, 93, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Vodušek, D.B. Anatomy and neurocontrol of the pelvic floor. Digestion 2004, 69, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, T.; Kaga, K.; Fuse, M.; Shibata, C.; Uchiyama, T. Neuromodulation for the Treatment of Lower Urinary Tract Symptoms. Low. Urin. Tract Symptoms 2015, 7, 121–132. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Khoury, S.; Wein, A. Chapter 15—Adult conservative management. In Incontinence Management; UK, 2005; Volume 2, pp. 855–965. Available online: www.consort-statement.org (accessed on 22 February 2023).
- Yamanishi, T.; Kamai, T.; Yoshida, K.I. Neuromodulation for the treatment of urinary incontinence. Int. J. Urol. 2008, 15, 665–672. [Google Scholar] [CrossRef]
- Okada, N.; Igawa, Y.; Ogawa, A. Transcutaneous electrical stimulation of thigh muscles in the treatment of detrusor overactivity. Br. J. Urol. 1998, 81, 560–564. [Google Scholar] [CrossRef]
- Yamanishi, T.; Yasuda, K.; Sakakibara, R.; Hattori, T.; Ito, H.; Murakami, S. Pelvic floor electrical stimulation in the treatment of stress incontinence: An investigational study and a placebo controlled double-blind trial. J. Urol. 1997, 158, 2127–2131. [Google Scholar] [CrossRef]
- Yamanishi, T.; Yasuda, K. Electrical stimulation for stress incontinence. Int. Urogynecol. J. 1998, 9, 281–290. [Google Scholar] [CrossRef]
- Der Zalm, P.J.V.-V.; Pelger, R.C.M.; Stiggelbout, A.M.; Elzevier, H.W.; Nijeholt, G.A.B.L.À. Effects of magnetic stimulation in the treatment of pelvic floor dysfunction. BJU Int. 2006, 97, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Rosen, N.O.; Dawson, S.J.; Brooks, M.; Kellogg-Spadt, S. Treatment of Vulvodynia: Pharmacological and Non-Pharmacological Approaches. DSrugs 2019, 79, 483–493. [Google Scholar] [CrossRef] [PubMed]


| Number of patients | 62 | |
| Average age (mean ± SD) | 55.1 ± 14.5 | |
| Menopausal patients (%) | 60% | |
| UI type (%) | SUI | UUI |
| 80% | 48% | |
| Prolapse (%) | 42.8% | |
| GRADE (% PATIENTS) | ||
| I (53%) II (47%) | ||
| Indication | Questionnaire Name | Score Range | Aim | Administration Time |
|---|---|---|---|---|
| SUI | ICIQ-UI-SF | 0–21 | Evaluation of clinical manifestations of urinary incontinence, everity of urinary loss, and impact on quality of life | T0, T4, T6, T8 |
| UDI-6 | 0–100 | Urogenital Distress Inventory in daily life | ||
| IIQ-7 (SUI) | 0–100 | Evaluation of the impact of urinary incontinence on activities, relationships and emotional states | ||
| Pelvic Organ Prolapse | PFDI-SF20 | 0–300 | Evaluation of the intensity of distress caused by the presence of PFD symptoms | |
| POPDI-6 | 0–100 | |||
| CRADI 8 | 0–100 | |||
| Overactive Bladder Urge/UUI | ICIQ-OAB | 0–16 | For overactive bladder, evaluation of urgency, frequency, nocturia and urgency leakage | |
| IIQ-7 (OAB) | 0–100 | Evaluation of the impact of urinary incontinence on activities, relationships and emotional states | ||
| Quality of Life | IQoL | 0–110 | Evaluation of the impact of urinary/pelvic floor disorder on the everyday quality of life, including socializing, sexuality and emotional states | T0, T8 |
| Rest (mm) | Contraction (mm) | |
|---|---|---|
| Before treatment | 59.74 ± 7.05 | 53.31 ± 8.47 |
| After treatment | 56.37 ± 8.14 | 49.44 ± 8.98 |
| Time Points | ||||
|---|---|---|---|---|
| Questionnaires | T0 | T4 | T6 | T8 |
| ICIQ-UI-SF | 12.44 ± 5.30 | 9.57 ± 6.05 | 7.93 ± 6.11 | 6.75 ± 6.22 |
| IIQ-7 (SUI) | 46.91 ± 26.57 | 37.39 ± 28.13 | 28.80 ± 27.80 | 27.88 ± 27.69 |
| UDI-6 | 49.26 ± 20.84 | 32.84 ± 19.31 | 25.73 ± 22.64 | 20.83 ± 24.60 |
| PFDI-SF20 | 115.62 ± 23.28 | 104.77 ± 23.15 | 99.56 ± 19.43 | 88.45 ± 12.18 |
| POPDI-6 | 25.69 ± 13.51 | 18.40 ± 13.58 | 11.11 ± 10.85 | 11.45 ± 13.66 |
| CRADI 8 | 24.55 ± 11.03 | 18.75 ± 11.41 | 15.17 ± 10.58 | 13.39 ± 8.59 |
| ICIQ-OAB | 7.2 ± 3.73 | 6.24 ± 2.96 | 5.48 ± 3.02 | 4.76 ± 3.12 |
| IIQ-7 (OAB) | 50.16 ± 31.79 | 45.40 ± 31.72 | 38.92 ± 35.96 | 38.92 ± 33.89 |
| IQoL | 72.05 ± 21.03 | - | - | 89.21 ± 20.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, M.; Biordi, N.; Curcio, A.; Comito, A.; Pennati, B.M.; Farinelli, M. A Qualitative and Quantitative Study to Evaluate the Effectiveness and Safety of Magnetic Stimulation in Women with Urinary Incontinence Symptoms and Pelvic Floor Disorders. Medicina 2023, 59, 879. https://doi.org/10.3390/medicina59050879
Filippini M, Biordi N, Curcio A, Comito A, Pennati BM, Farinelli M. A Qualitative and Quantitative Study to Evaluate the Effectiveness and Safety of Magnetic Stimulation in Women with Urinary Incontinence Symptoms and Pelvic Floor Disorders. Medicina. 2023; 59(5):879. https://doi.org/10.3390/medicina59050879
Chicago/Turabian StyleFilippini, Maurizio, Nicoletta Biordi, Antonella Curcio, Alessandra Comito, Beatrice Marina Pennati, and Miriam Farinelli. 2023. "A Qualitative and Quantitative Study to Evaluate the Effectiveness and Safety of Magnetic Stimulation in Women with Urinary Incontinence Symptoms and Pelvic Floor Disorders" Medicina 59, no. 5: 879. https://doi.org/10.3390/medicina59050879
APA StyleFilippini, M., Biordi, N., Curcio, A., Comito, A., Pennati, B. M., & Farinelli, M. (2023). A Qualitative and Quantitative Study to Evaluate the Effectiveness and Safety of Magnetic Stimulation in Women with Urinary Incontinence Symptoms and Pelvic Floor Disorders. Medicina, 59(5), 879. https://doi.org/10.3390/medicina59050879





