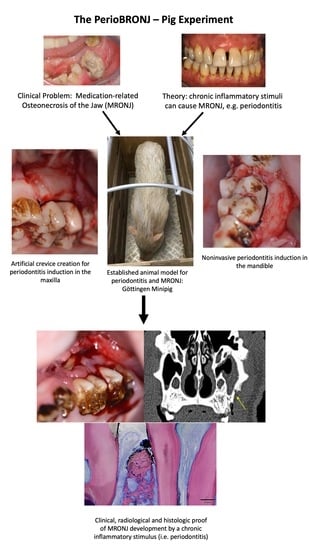
Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Selection and Experimental Group Design
2.2. Timeline of the PeriBRONJ Pig Experiment
2.3. MRONJ Induction Protocol
2.4. Definition of Gingivitis/Periodontitis
2.5. Periodontitis Induction Protocol
2.6. Reevaluation of Periodontitis Development and MRONJ Occurrence
2.7. Radiological Protocol
2.8. Further Periodontitis/MRONJ Development Observation Phase and Euthanasia
2.9. Post Mortem Analyses and Histological Protocol
2.10. Study Variables
2.11. Statistical Analysis
3. Results
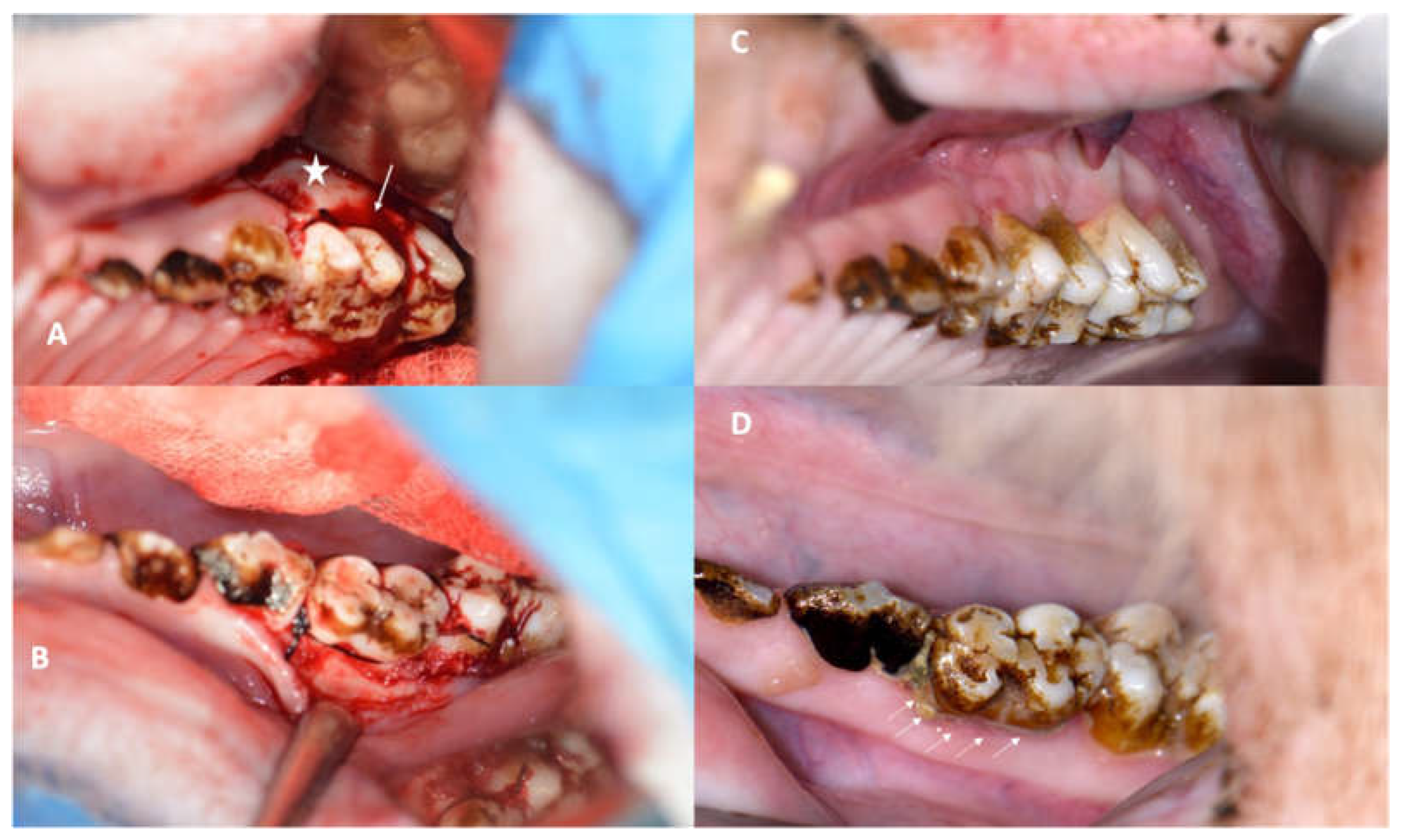
3.1. Success of the Periodontitis Induction Protocol
3.2. Osteonecrosis Induction
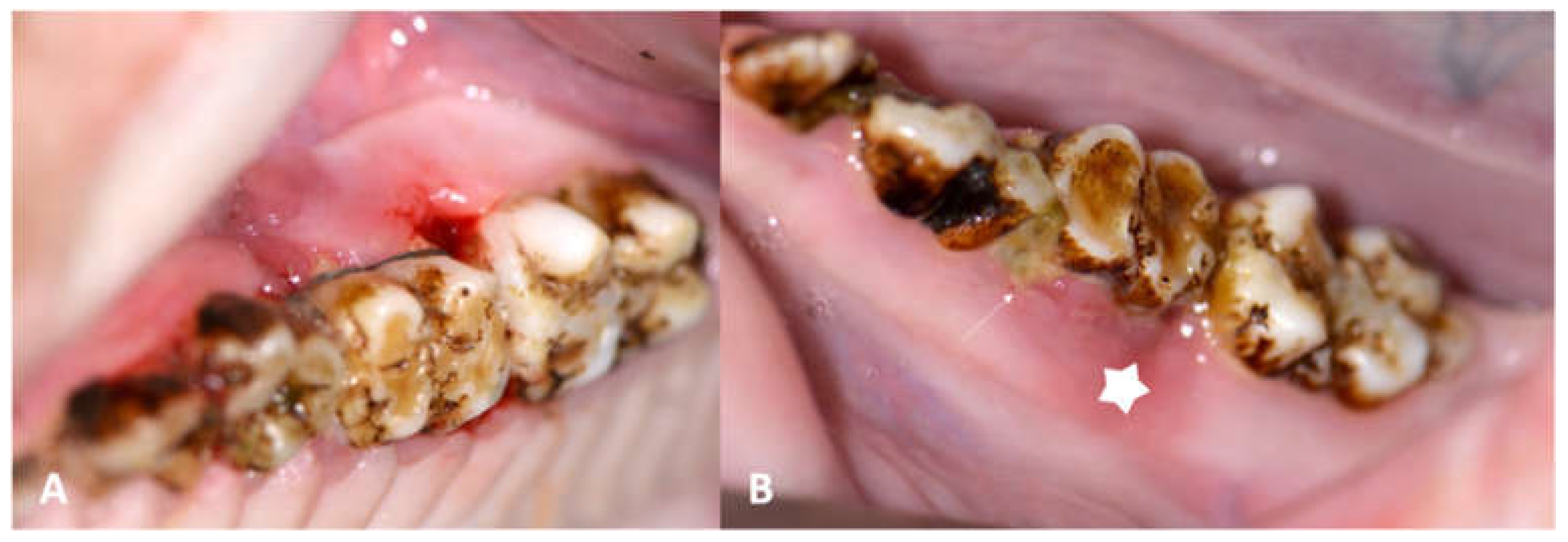
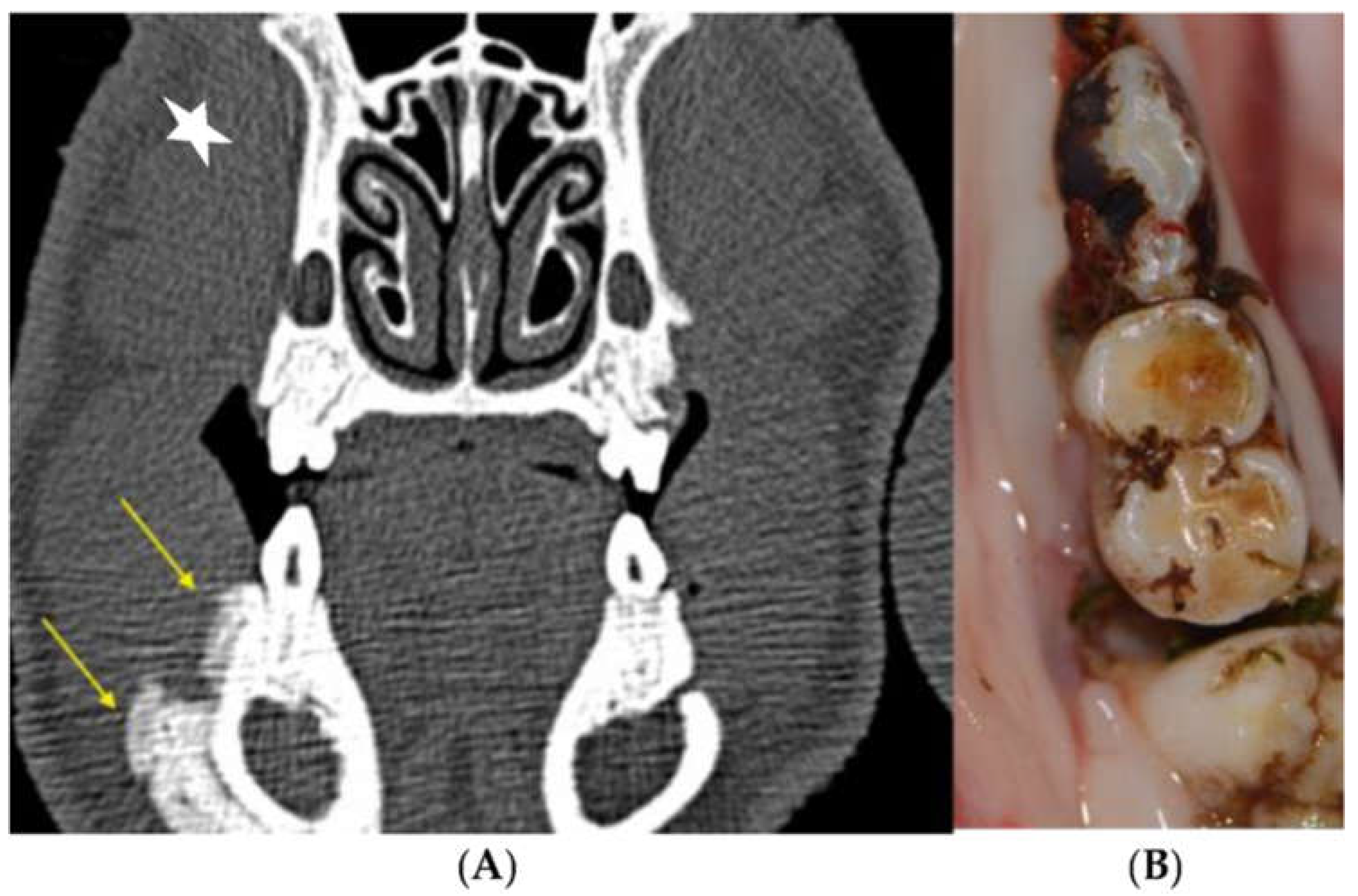
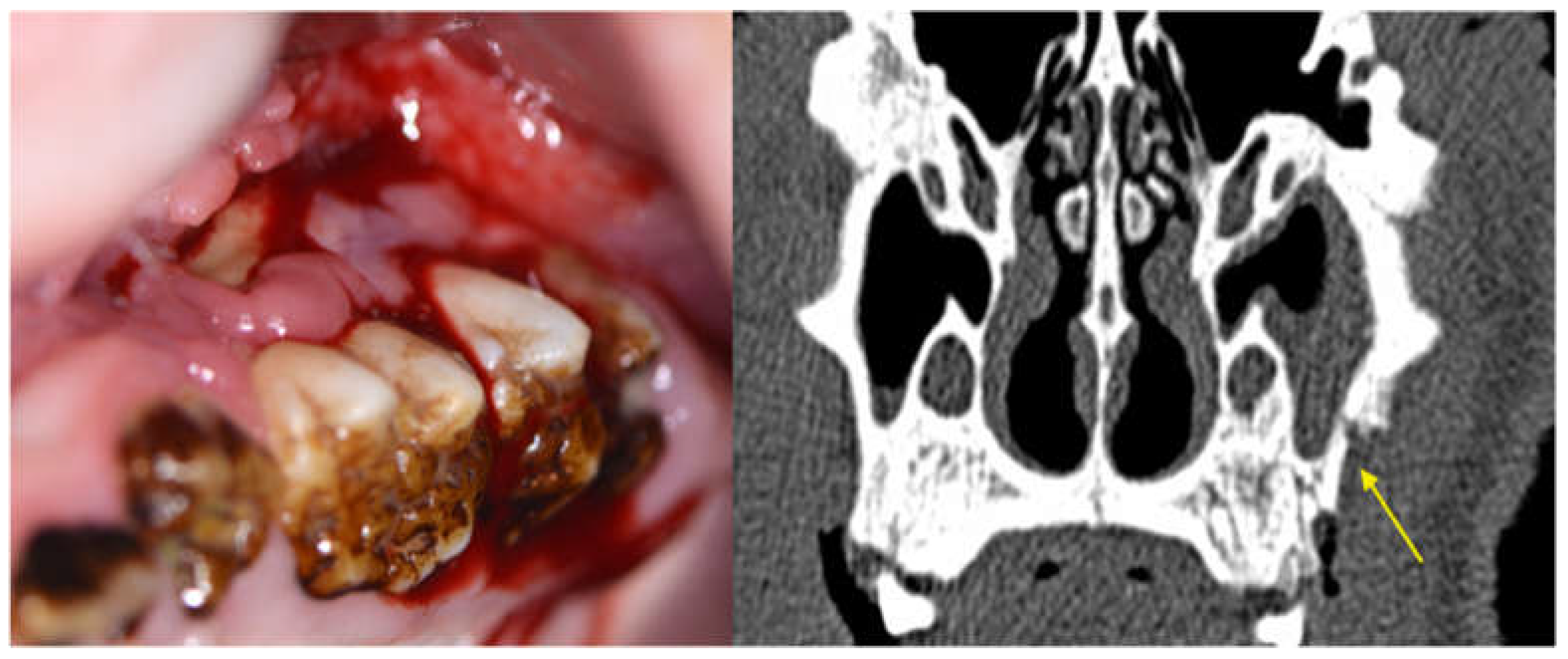
3.2.1. Clinical Evaluation
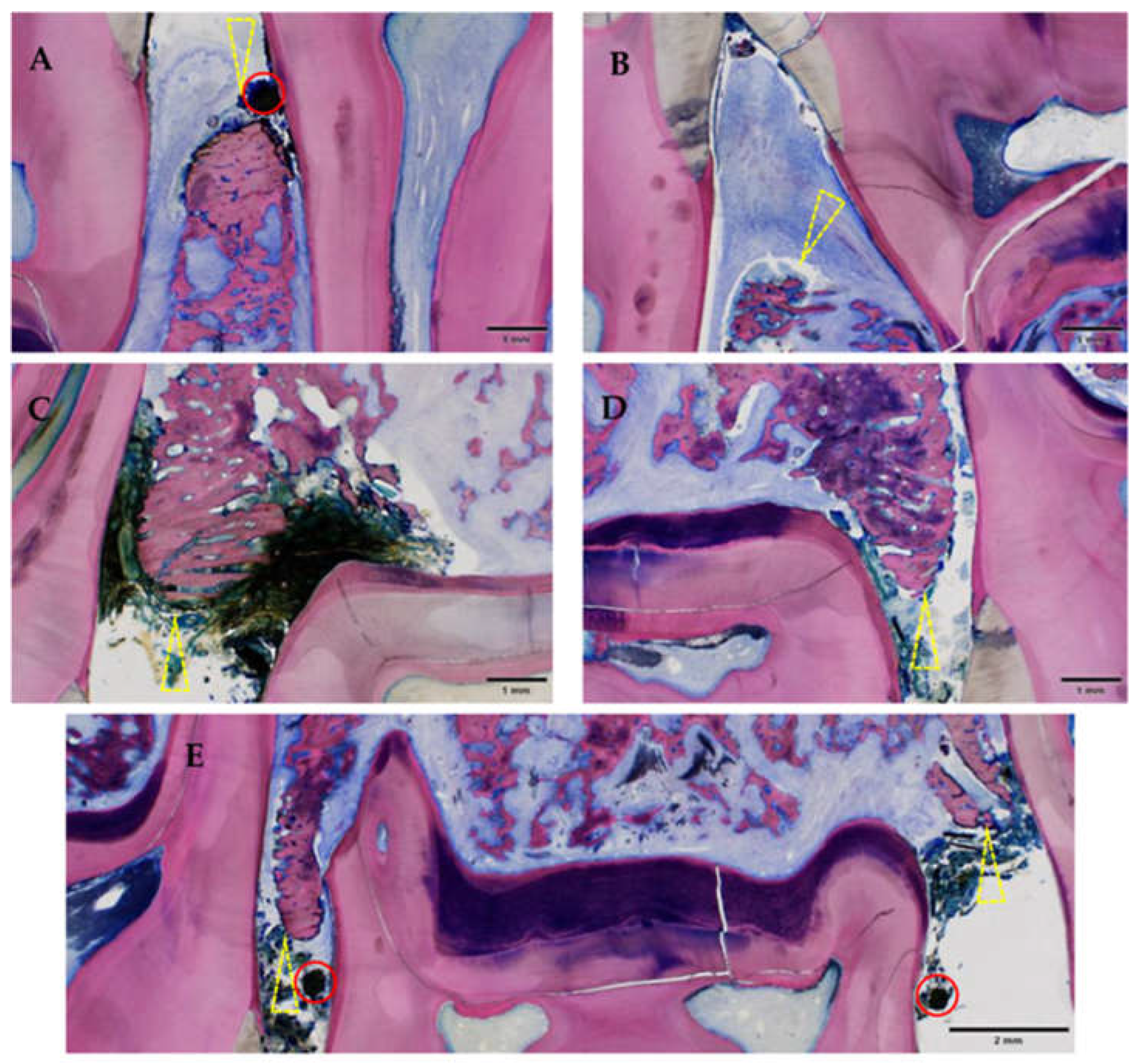
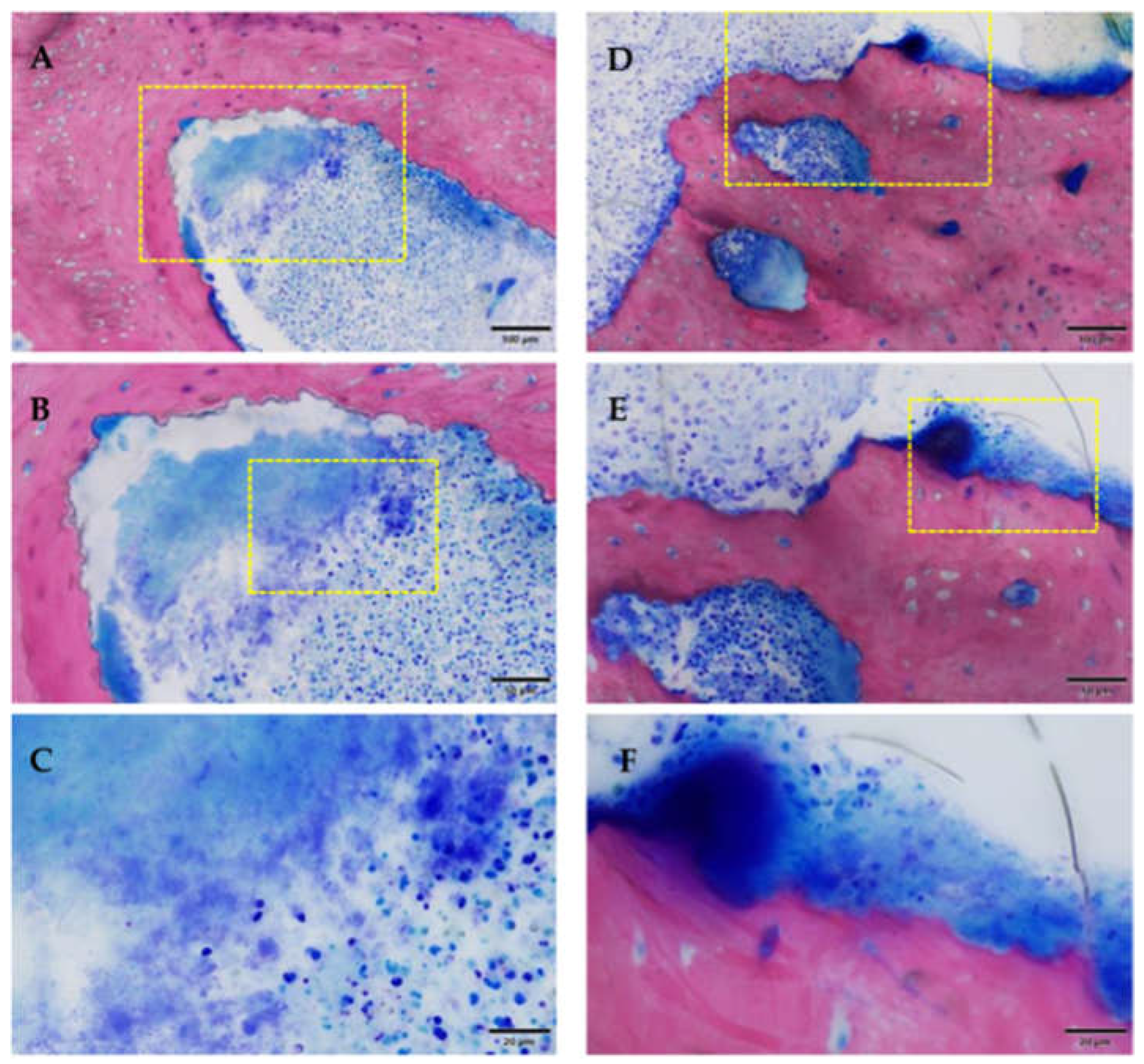
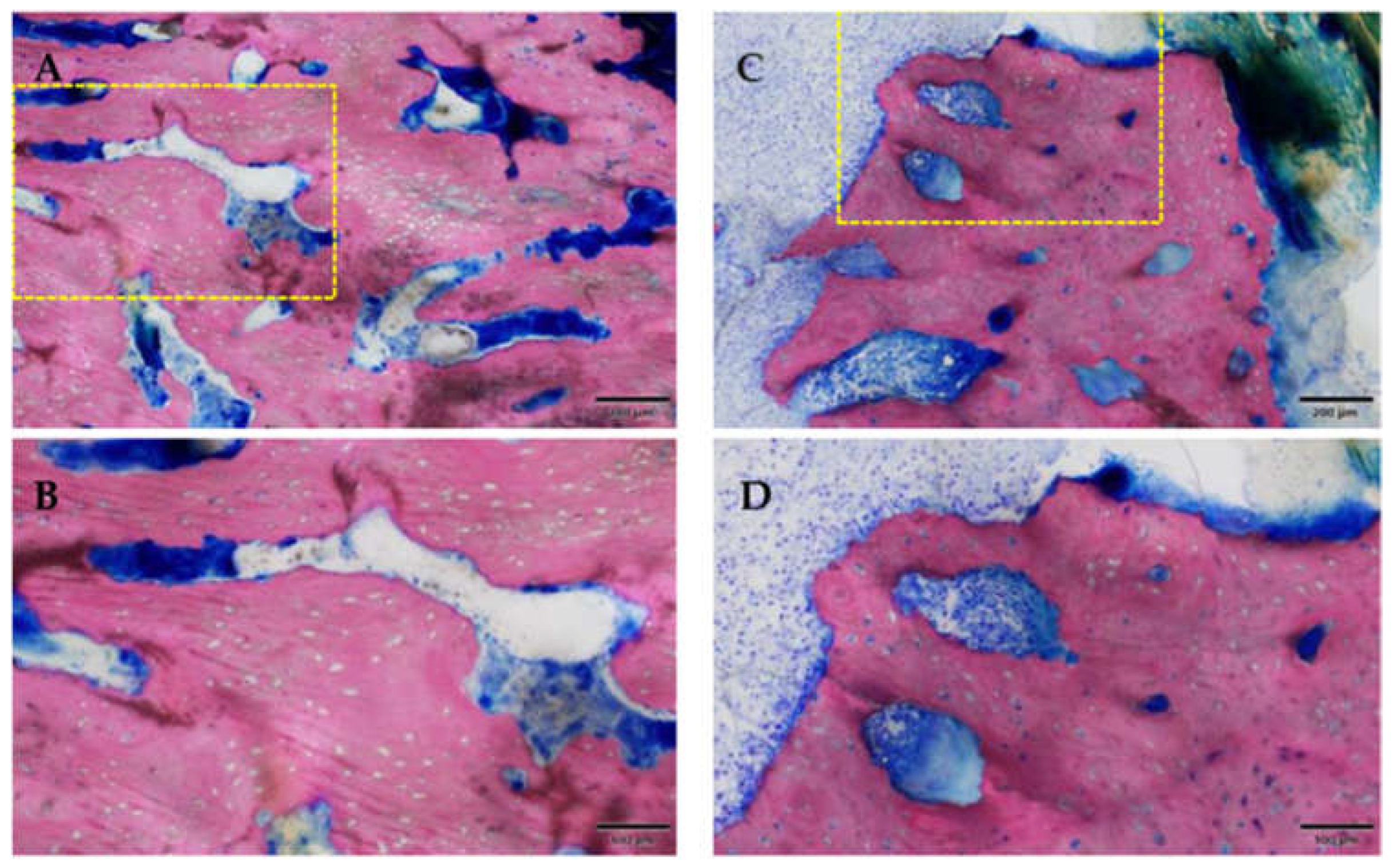
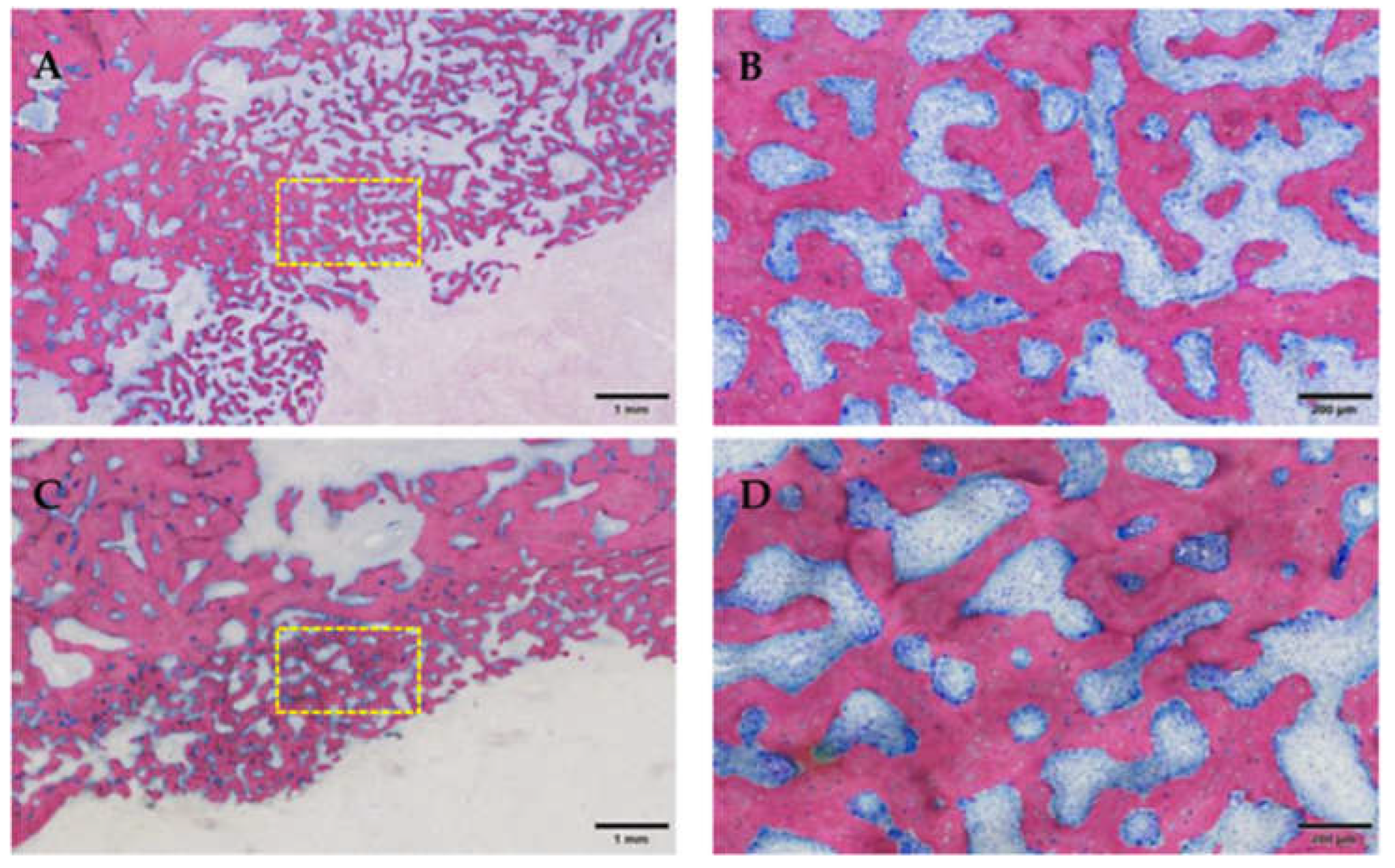
3.2.2. Histological Evaluation
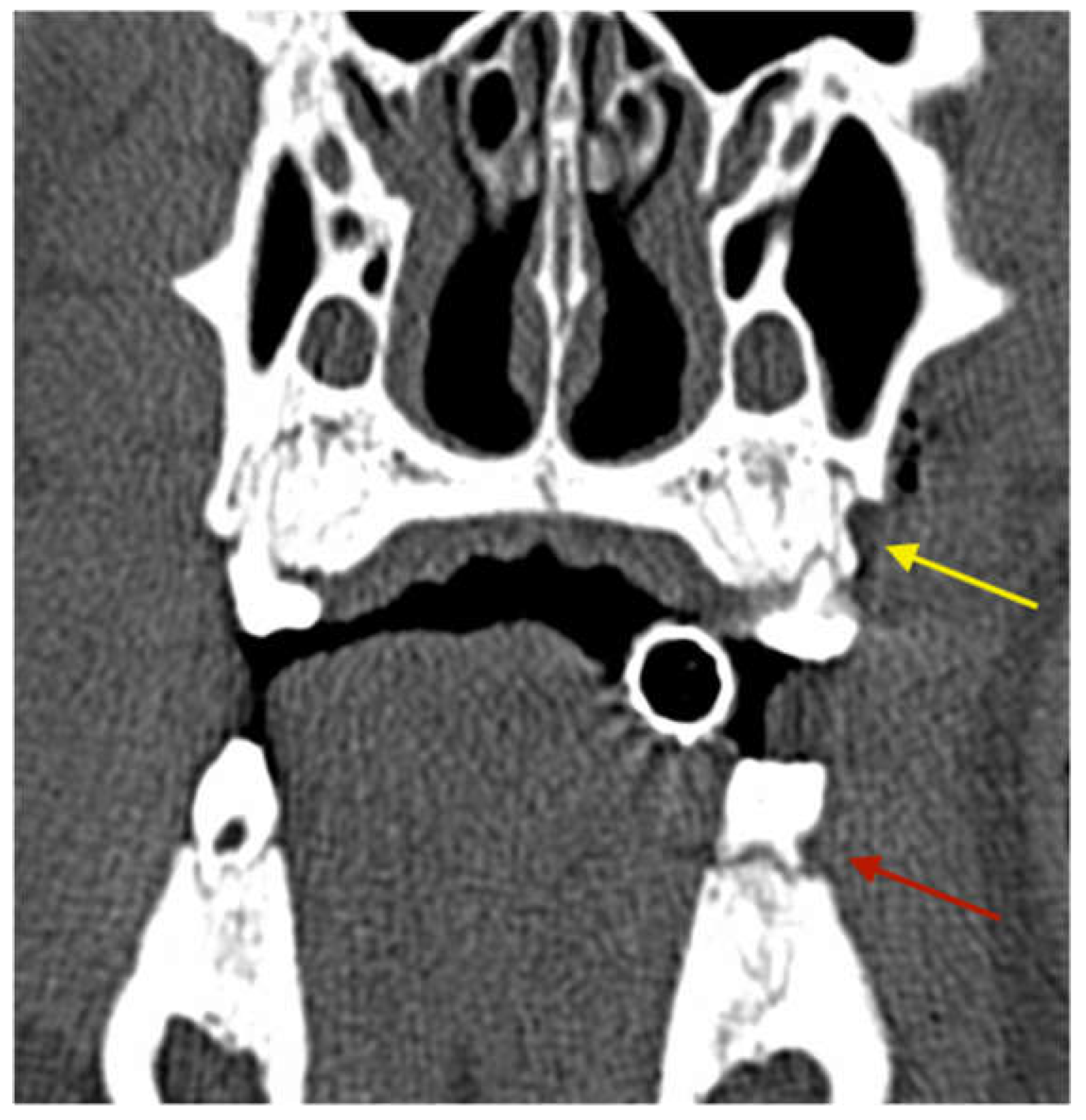
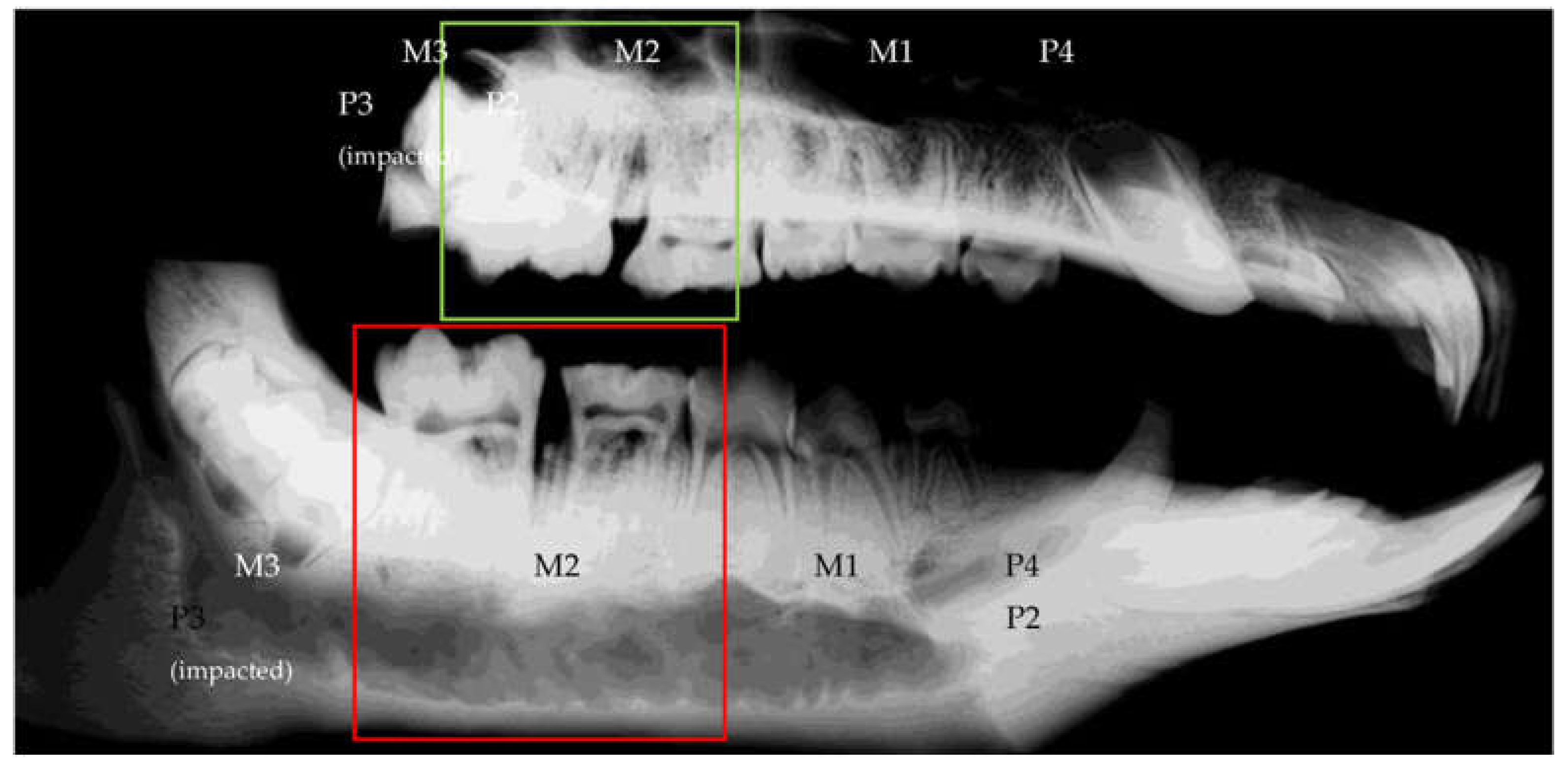
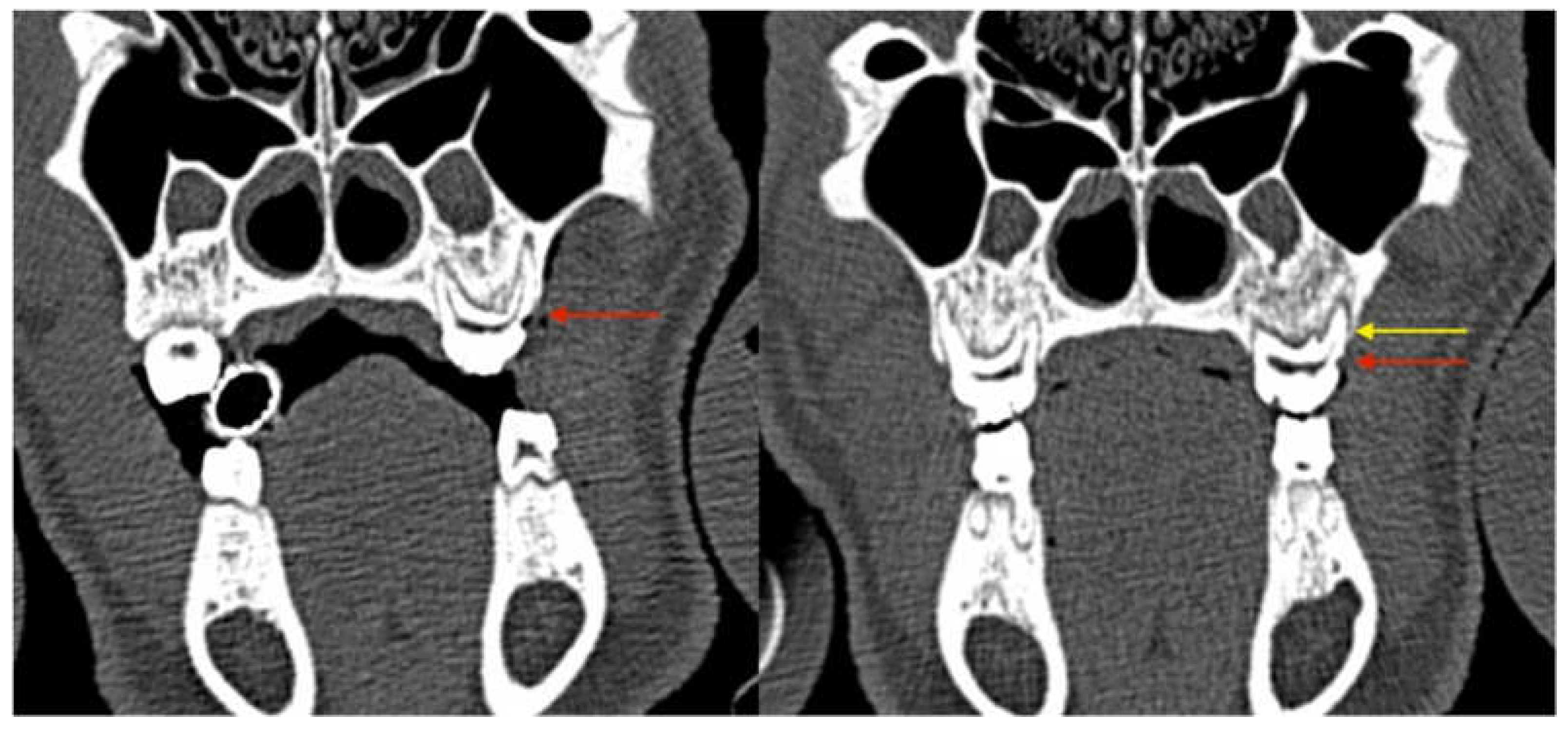
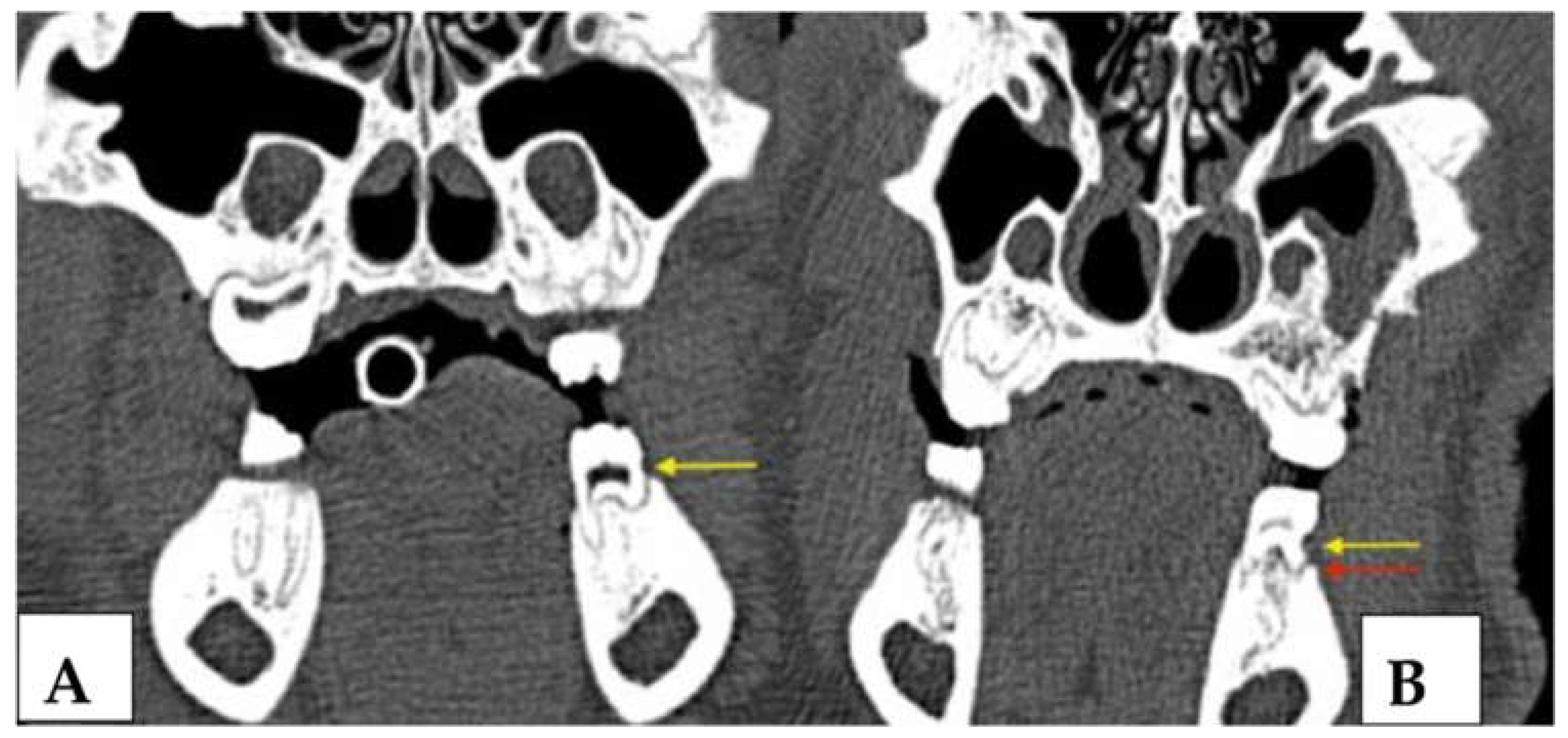
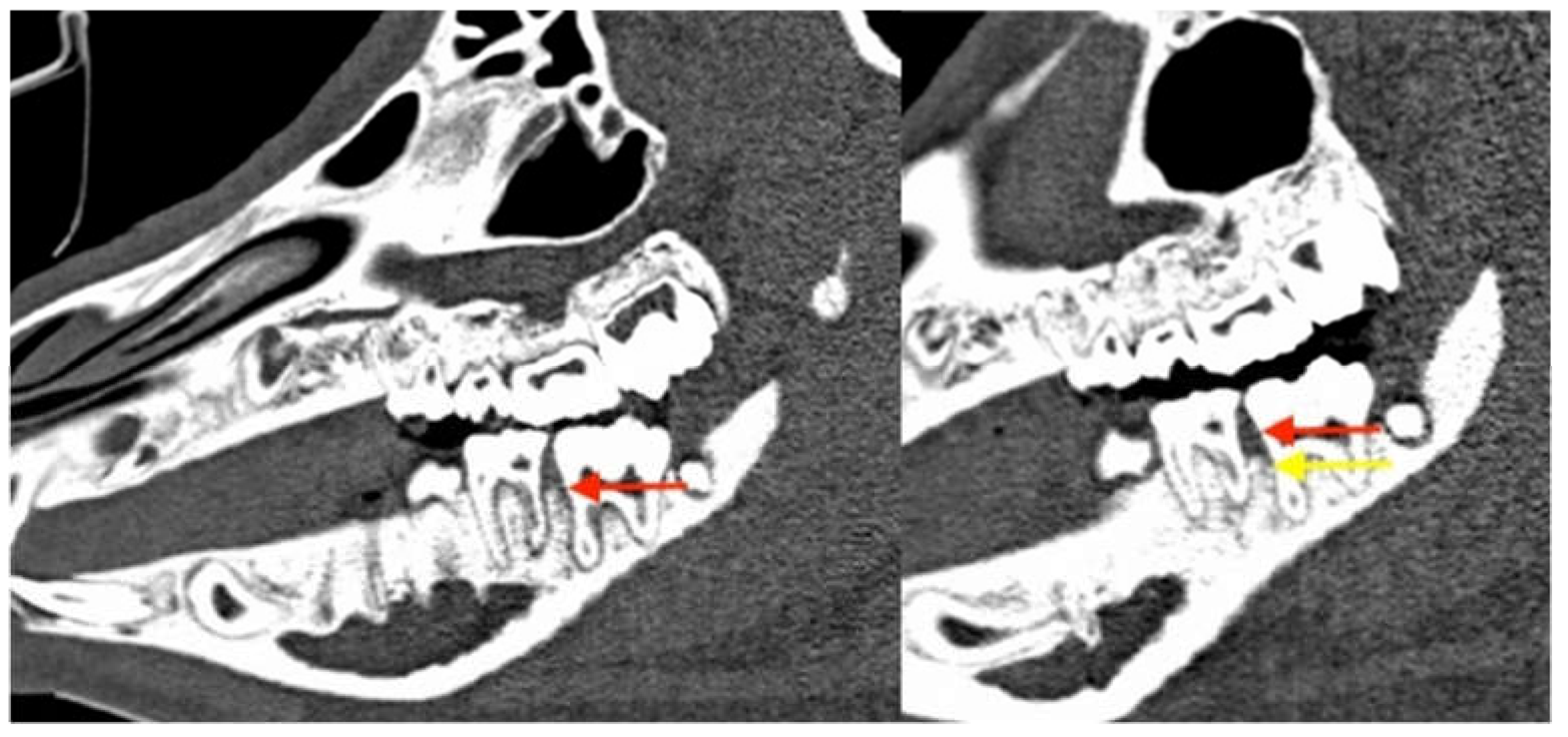
3.2.3. Radiological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral. Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Cillo, J.E., Jr.; Ulloa, J.J. Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral. Maxillofac. Surg. 2007, 65, 2397–2410. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral. Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral. Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral. Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Troeltzsch, M.; Woodlock, T.; Kriegelstein, S.; Steiner, T.; Messlinger, K.; Troeltzsch, M. Physiology and pharmacology of nonbisphosphonate drugs implicated in osteonecrosis of the jaw. J. Can. Dent. Assoc. 2012, 78, c85. [Google Scholar] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damiao, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Schiødt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Van den Wyngaert, T. Medication-related osteonecrosis of the jaw: Definition and best practice for prevention, diagnosis, and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Pautke, C.; Kreutzer, K.; Weitz, J.; Knodler, M.; Munzel, D.; Wexel, G.; Otto, S.; Hapfelmeier, A.; Sturzenbaum, S.; Tischer, T. Bisphosphonate related osteonecrosis of the jaw: A minipig large animal model. Bone 2012, 51, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Hafner, S.; Mast, G.; Tischer, T.; Volkmer, E.; Schieker, M.; Sturzenbaum, S.R.; von Tresckow, E.; Kolk, A.; Ehrenfeld, M.; et al. Bisphosphonate-related osteonecrosis of the jaw: Is pH the missing part in the pathogenesis puzzle? J. Oral. Maxillofac. Surg. 2010, 68, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, R.; Cozin, M.; Cremers, S.; Woo, V.; Kousteni, S.; Sinha, S.; Garrett-Sinha, L.; Raghavan, S. Inhibition of oral mucosal cell wound healing by bisphosphonates. J. Oral. Maxillofac. Surg. 2008, 66, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Pautke, C.; Martin Jurado, O.; Nehrbass, D.; Stoddart, M.J.; Ehrenfeld, M.; Zeiter, S. Further development of the MRONJ minipig large animal model. J. Craniomaxillofac. Surg. 2017, 45, 1503–1514. [Google Scholar] [CrossRef]
- Ristow, O.; Nehrbass, D.; Zeiter, S.; Arens, D.; Moratin, J.; Pautke, C.; Hoffmann, J.; Freudlsperger, C.; Otto, S. Differences between auto-fluorescence and tetracycline-fluorescence in medication-related osteonecrosis of the jaw-a preclinical proof of concept study in the mini-pig. Clin. Oral Investig. 2020, 24, 4625–4637. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Arens, D.; Poxleitner, P.; Eberli, U.; Nehrbass, D.; Zeiter, S.; Stoddart, M.J. A Drug Holiday Reduces the Frequency and Severity of Medication-Related Osteonecrosis of the Jaw in a Minipig Model. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 2179–2192. [Google Scholar] [CrossRef]
- Amarasena, N.; Ekanayaka, A.N.; Herath, L.; Miyazaki, H. Tobacco use and oral hygiene as risk indicators for periodontitis. Community Dent. Oral. Epidemiol. 2002, 30, 115–123. [Google Scholar] [CrossRef]
- American Academy of Periodontology. Parameter on comprehensive periodontal examination. J. Periodontol. 2000, 71, 847–848. [Google Scholar] [CrossRef]
- American Academy of Periodontology. Parameter on periodontal maintenance. J. Periodontol. 2000, 71, 849–850. [Google Scholar] [CrossRef]
- Thumbigere-Math, V.; Michalowicz, B.S.; Hodges, J.S.; Tsai, M.L.; Swenson, K.K.; Rockwell, L.; Gopalakrishnan, R. Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J. Periodontol. 2014, 85, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; Chamorro-Petronacci, C.; Gándara-Vila, P.; López-Jornet, P.; Carballo, J.; García-García, A. Association between periodontitis and medication-related osteonecrosis of the jaw: A systematic review and meta-analysis. J. Oral Pathol. Med. 2020, 49, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Bankosegger, P.; Otto, S.; Hohlweg-Majert, B.; Steiner, T.; Probst, F.; Ristow, O.; Pautke, C. Risk factors associated with onset of medication-related osteonecrosis of the jaw in patients treated with denosumab. Clin. Oral. Investig. 2022, 26, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Lin, K.C.; Liu, S.P.; Chang, C.T.; Muo, C.H.; Chang, P.J.; Tsai, C.H.; Wu, C.Z. The association between the severity of periodontitis and osteonecrosis of the jaw in patients with different cancer locations: A nationwide population-based study. Clin. Oral Investig. 2022, 26, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Kang, B.; Sung, E.C.; Shoff, M.; Ronconi, M.; Gotcher, J.E.; Bezouglaia, O.; Dry, S.M.; Tetradis, S. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 1871–1882. [Google Scholar] [CrossRef]
- Castillo, E.; Messer, J.; Abraham, A.; Jiron, J.; Alekseyenko, A.; Israel, R.; Thomas, S.; Gonzalez-Perez, G.; Croft, S.; Gohel, A. Preventing or controlling periodontitis reduces the occurrence of osteonecrosis of the jaw (ONJ) in rice rats (Oryzomys palustris). Bone 2021, 145, 115866. [Google Scholar] [CrossRef]
- Aguirre, J.; Castillo, E.; Kimmel, D. Preclinical models of medication-related osteonecrosis of the jaw (MRONJ). Bone 2021, 153, 116184. [Google Scholar] [CrossRef]
- Calciolari, E.; Donos, N.; Mardas, N. Osteoporotic Animal Models of Bone Healing: Advantages and Pitfalls. J. Investig. Surg. Off. J. Acad. Surg. Res. 2017, 30, 342–350. [Google Scholar] [CrossRef]
- Holtmann, H.; Lommen, J.; Kübler, N.R.; Sproll, C.; Rana, M.; Karschuck, P.; Depprich, R. Pathogenesis of medication-related osteonecrosis of the jaw: A comparative study of in vivo and in vitro trials. J. Int. Med. Res. 2018, 46, 4277–4296. [Google Scholar] [CrossRef]
- Oz, H.S.; Puleo, D.A. Animal models for periodontal disease. J. Biomed. Biotechnol. 2011, 2011, 754857. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem. Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Hoare, A.; Hong, B.Y.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000 2021, 86, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.; Powell, R.; Aitken, J. Experimental gingivitis in humans: A clinical and histologic investigation. J. Periodontol. 1983, 54, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A. Is the chemical prevention of gingivitis necessary to prevent severe periodontitis? Periodontol. 2000 1997, 15, 15–24. [Google Scholar] [CrossRef]
- Ooya, K.; Yamamoto, H. A scanning electron microscopic study of the destruction of human alveolar crest in periodontal disease. J. Periodontal Res. 1978, 13, 498–503. [Google Scholar] [CrossRef]
- Carranza, F.; Takei, H. Clinical Diagnosis. In Clinical Periodontology; Newman, M., Takei, H., Klokkevold, P., Carranza, F., Eds.; Saunders: St. Louis, MO, USA, 2006; Volume 9, p. 1286. [Google Scholar]
- Armitage, G.C. Periodontal diagnoses and classification of periodontal diseases. Periodontol. 2000 2004, 34, 9–21. [Google Scholar] [CrossRef]
- Yang, J.R.; Hsu, C.W.; Liao, S.C.; Lin, Y.T.; Chen, L.R.; Yuan, K. Transplantation of embryonic stem cells improves the regeneration of periodontal furcation defects in a porcine model. J. Clin. Periodontol. 2013, 40, 364–371. [Google Scholar] [CrossRef]
- Pautke, C.; Bauer, F.; Otto, S.; Tischer, T.; Steiner, T.; Weitz, J.; Kreutzer, K.; Hohlweg-Majert, B.; Wolff, K.D.; Hafner, S.; et al. Fluorescence-guided bone resection in bisphosphonate-related osteonecrosis of the jaws: First clinical results of a prospective pilot study. J. Oral. Maxillofac. Surg. 2011, 69, 84–91. [Google Scholar] [CrossRef]
- American Academy of Periodontology. Parameter on chronic periodontitis with advanced loss of periodontal support. J. Periodontol. 2000, 71, 856–858. [Google Scholar] [CrossRef]
- American Academy of Periodontology. Parameter on chronic periodontitis with slight to moderate loss of periodontal support. J. Periodontol. 2000, 71, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.E.; Polson, A.M. Experimental marginal periodontitis in squirrel monkeys. J. Periodontol. 1973, 44, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, Y.; Tang, H.; Huang, L.; Tong, Z.; Hu, C.; Chen, X.; Tan, J. A Simplified and Effective Method for Generation of Experimental Murine Periodontitis Model. Front. Bioeng. Biotechnol. 2020, 8, 444. [Google Scholar] [CrossRef]
- Gao, J.; Cai, S.; Wang, Z.; Li, D.; Ou, M.; Zhang, X.; Tian, Z. The optimization of ligature/bone defect-induced periodontitis model in rats. Odontology 2022, 110, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Troltzsch, M.; Jambrovic, V.; Panya, S.; Probst, F.; Ristow, O.; Ehrenfeld, M.; Pautke, C. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: A trigger for BRONJ development? J. Craniomaxillofac. Surg. 2015, 43, 847–854. [Google Scholar] [CrossRef]
- Kwoen, M.J.; Park, J.H.; Kim, K.S.; Lee, J.R.; Kim, J.W.; Lee, H.; Lee, H.J. Association between periodontal disease, tooth extraction, and medication-related osteonecrosis of the jaw in women receiving bisphosphonates: A national cohort-based study. J. Periodontol. 2023, 94, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Hadaya, D.; Soundia, A.; Gkouveris, I.; Dry, S.M.; Aghaloo, T.L.; Tetradis, S. Development of medication-related osteonecrosis of the jaw after extraction of teeth with experimental periapical disease. J. Oral Maxillofac. Surg. 2019, 77, 71–86. [Google Scholar] [CrossRef]
- Williams, D.W.; Ho, K.; Lenon, A.; Kim, S.; Kim, T.; Gwack, Y.; Kim, R.H. Long-Term Ligature-Induced Periodontitis Exacerbates Development of Bisphosphonate-Related Osteonecrosis of the Jaw in Mice. J. Bone Miner. Res. 2022, 37, 1400–1410. [Google Scholar] [CrossRef]




| Group | Duration of Experiment | Number of Animals |
|---|---|---|
| Group 1: ZOL | 24 weeks | 8 |
| Group 2: NON-ZOL | 24 weeks | 8 |
| Total | 16 |
| Time | Action | All Minipigs | |
|---|---|---|---|
| 4 weeks before experiment inception (-4 weeks) | Arrival of Animals | Quarantine | |
| Inception of experiment (0 weeks) | Assignment to groups | Group 1 (n = 8) Zoledronate group | Group 2 (n = 8) Control group (no zoledronate) |
| Pretreatment phase (12 weeks) | Induction protocol of BRONJ-Pig Experiment [11] | 0.05 mg/KG body weight zoledronate iv weekly | No i.v. treatment |
| Baseline | Periodontitis induction | Maxillary defect + suture; Mandibular suture | Maxillary defect + suture; Mandibular suture |
| Progression time (6 weeks) | Assessment exam | Intraoral exam, CT | Intraoral exam, CT |
| Progression/observation time (6 weeks) | Assessment exam | Intraoral exam, CT, euthanasia | Intraoral exam, CT, euthanasia |
| Group 1 (ZOL) | Group 2 (NON-ZOL) | Group 1 (ZOL) | Group 2 (NON-ZOL) | |
|---|---|---|---|---|
| Parameter | Baseline (December 2017) | Baseline (December 2017) | Follow-up (February 2018) | Follow-up (February 2018) |
| PPD | 7 mm | 4 mm | 13 mm | 9 mm |
| BOP | 42% | 35% | 100% | 75% |
| SBI | 53% | 45% | 100% | 100% |
| MRONJ Stage | Maxilla | Mandible |
|---|---|---|
| I | 1 | 3 |
| II | 3 | 4 |
| III | 4 | 0 |
| Total | 8 | 7 |
| MRONJ Stage | Maxilla | Mandible |
|---|---|---|
| I | 0 | 1 |
| II | 2 | 5 |
| III | 6 | 2 |
| Total | 8 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troeltzsch, M.; Zeiter, S.; Arens, D.; Nehrbass, D.; Probst, F.A.; Liokatis, P.; Ehrenfeld, M.; Otto, S. Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model). Medicina 2023, 59, 1000. https://doi.org/10.3390/medicina59051000
Troeltzsch M, Zeiter S, Arens D, Nehrbass D, Probst FA, Liokatis P, Ehrenfeld M, Otto S. Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model). Medicina. 2023; 59(5):1000. https://doi.org/10.3390/medicina59051000
Chicago/Turabian StyleTroeltzsch, Matthias, Stephan Zeiter, Daniel Arens, Dirk Nehrbass, Florian A. Probst, Paris Liokatis, Michael Ehrenfeld, and Sven Otto. 2023. "Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model)" Medicina 59, no. 5: 1000. https://doi.org/10.3390/medicina59051000
APA StyleTroeltzsch, M., Zeiter, S., Arens, D., Nehrbass, D., Probst, F. A., Liokatis, P., Ehrenfeld, M., & Otto, S. (2023). Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model). Medicina, 59(5), 1000. https://doi.org/10.3390/medicina59051000