Diagnostic Test Accuracy of Artificial Intelligence in Detecting Periapical Periodontitis on Two-Dimensional Radiographs: A Retrospective Study and Literature Review
Abstract
1. Introduction
2. Literature Review
3. Materials and Methods
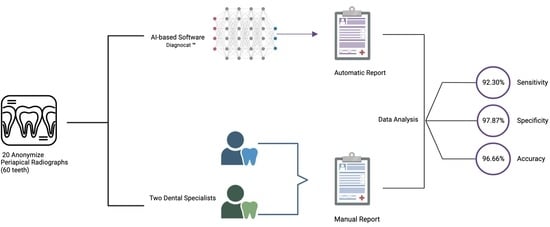
3.1. Image Data Set
3.2. Ground-Truth Evaluation
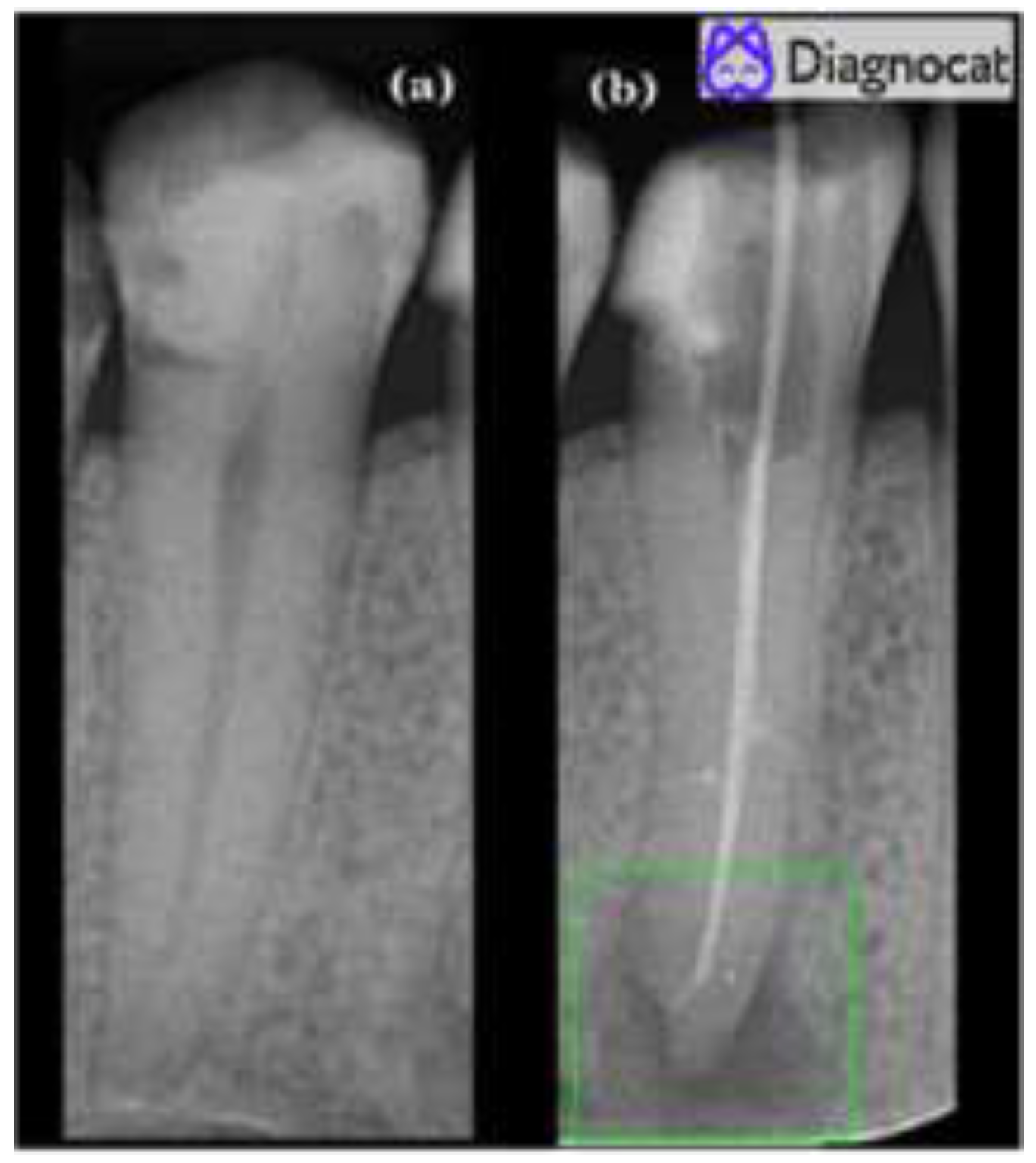
3.3. Automated Evaluation
4. Results
- Twelve true positives (TP) (teeth with apical periodontitis, unhealthy);
- One false positive (FP) (tooth with no signs of apical periodontitis was classified as unhealthy, over-diagnosed by the tool);
- Forty-six true negatives (TN) (healthy teeth, no signs of any apical periodontitis);
- One false negative (FN) (misdiagnosed by the tool; a tooth with apical periodontitis was classified as a healthy tooth).
- Sensitivity = TP/FP + TP
- Specificity = TN/FN + TN
- Accuracy = TP + TN/TP + TN + FP + TP
- F1 score = 2 ∗ TP / (2 ∗ TP + FP + FN)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulabivala, K.; Ng, Y.L. Biological and clinical rationale for root-canal treatment and management of its failure. Endodontics 2014, 4, 43–92. [Google Scholar] [CrossRef]
- García, C.C.; Sempere, F.V.; Diago, M.P.; Bowen, E.M. The post-endodontic periapical lesion: Histologic and etiopathogenic aspects. Med. Oral Patol. Oral Cir. Bucal 2007, 12, E585–E590. [Google Scholar] [PubMed]
- Gomes, B.P.; Pinheiro, E.T.; Gadê-Neto, C.R.; Sousa, E.L.; Ferraz, C.C.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Ferrocino, I.; Gavrilova, N.; Genova, T.; Dell’Acqua, A.; Cocolin, L.; Carossa, S. Apical periodontitis: Preliminary assessment of microbiota by 16S rRNA high throughput amplicon target sequencing. BMC Oral Health 2018, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, J.V.; Abraham, C.S.; Karthik, A.K.; Jayaprakash, N. Successful Nonsurgical Management of Periapical Lesions of Endodontic Origin: A Conservative Orthograde Approach. J. Pharm. Bioallied Sci. 2017, 9, S246–S251. [Google Scholar] [CrossRef] [PubMed]
- Gaddipati, R. Fascial Space Infections. In Oral and Maxillofacial Surgery for the Clinician; Springer: Singapore, 2021; pp. 441–459. [Google Scholar] [CrossRef]
- Luo, X.; Wan, Q.; Cheng, L.; Xu, R. Mechanisms of bone remodeling and therapeutic strategies in chronic apical periodontitis. Front. Cell. Infect. Microbiol. 2022, 12, 908859. [Google Scholar] [CrossRef]
- Mortazavi, H.; Baharvand, M. Review of common conditions associated with periodontal ligament widening. Imaging Sci. Dent. 2016, 46, 229–237. [Google Scholar] [CrossRef]
- Yu, V.S.; Messer, H.H.; Shen, L.; Yee, R.; Hsu, C.Y. Lesion progression in post-treatment persistent endodontic lesions. J. Endod. 2012, 38, 1316–1321. [Google Scholar] [CrossRef]
- Arslan, Z.B.; Demir, H.; Berker Yıldız, D.; Yaşar, F. Diagnostic accuracy of panoramic radiography and ultrasonography in detecting periapical lesions using periapical radiography as a gold standard. Dentomaxillofac. Radiol. 2020, 49, 20190290. [Google Scholar] [CrossRef]
- European Society of Endodontology. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.; Eaton, K.A. Selection Criteria for Dental Radiography; Faculty of General Dental Practice: London, UK, 2018; p. 3. Available online: https://cgdent.uk/wp-content/uploads/2021/08/FGDP-SCDR-ALL-Web.pdf (accessed on 5 October 2022).
- Updegrave, W.J. The paralleling extension-cone technique in intraoral dental radiography. Oral Surg. Oral Med. Oral Pathol. 1951, 4, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, J.; Halse, A. Radiographic simulation of a periapical lesion comparing the paralleling and the bisecting-angle techniques. Int. Endod. J. 1994, 27, 133–138. [Google Scholar] [CrossRef]
- Patel, S.; Dawood, A.; Whaites, E.; Pitt Ford, T. New dimensions in endodontic imaging: Part 1. Conventional and alternative radiographic systems. Int. Endod. J. 2009, 42, 447–462. [Google Scholar] [CrossRef]
- Schwendicke, F.; Samek, W.; Krois, J. Artificial Intelligence in Dentistry: Chances and Challenges. J. Dent. Res. 2020, 99, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Sarker, I.H. AI-Based Modeling: Techniques, Applications and Research Issues Towards Automation, Intelligent and Smart Systems. SN Comput. Sci. 2022, 3, 158. [Google Scholar] [CrossRef]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional neural networks: An overview and application in radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.; Wang, L.; Rao, J.; Lim, T. Deep Learning Applications in Medical Image Analysis. IEEE Access 2018, 6, 9375–9389. [Google Scholar] [CrossRef]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Rohban, M.H.; Krois, J.; Uribe, S.E.; Mahmoudinia, E.; Rokhshad, R.; Nadimi, M.; Schwendicke, F. Deep learning for caries detection: A systematic review. J. Dent. 2022, 122, 104115. [Google Scholar] [CrossRef]
- Al-Rawi, N.; Sultan, A.; Rajai, B.; Shuaeeb, H.; Alnajjar, M.; Alketbi, M.; Mohammad, Y.; Shetty, S.R.; Mashrah, M.A. The Effectiveness of Artificial Intelligence in Detection of Oral Cancer. Int. Dent. J. 2022, 72, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Khanagar, S.B.; Al-Ehaideb, A.; Maganur, P.C.; Vishwanathaiah, S.; Patil, S.; Baeshen, H.A.; Sarode, S.C.; Bhandi, S. Developments, application, and performance of artificial intelligence in dentistry—A systematic review. J. Dent. Sci. 2021, 16, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.; Olszewski, R.; Dyszkiewicz-Konwińska, M. The Effectiveness of Semi-Automated and Fully Automatic Segmentation for Inferior Alveolar Canal Localization on CBCT Scans: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 560. [Google Scholar] [CrossRef]
- Center for Devices and Radiological Health. Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. U.S. Food and Drug Administration. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (accessed on 5 October 2022).
- Hamdan, M.H.; Tuzova, L.; Mol, A.; Tawil, P.Z.; Tuzoff, D.; Tyndall, D.A. The effect of a deep-learning tool on dentists’ performances in detecting apical radiolucencies on periapical radiographs. Dentomaxillofac. Radiol. 2022, 51, 20220122. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhou, Z.; Zhou, Z.; Wu, X.; Li, Y.; Wang, S.; Liao, W.; Ying, S.; Zhao, Z. Artificial intelligence for caries and periapical periodontitis detection. J. Dent. 2022, 122, 104107. [Google Scholar] [CrossRef]
- Moidu, N.P.; Sharma, S.; Chawla, A.; Kumar, V.; Logani, A. Deep learning for categorization of endodontic lesion based on radiographic periapical index scoring system. Clin. Oral Investig. 2022, 26, 651–658. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Zhao, Y.; Zhao, J.; Wang, Y. Dental disease detection on periapical radiographs based on deep convolutional neural networks. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 649–661. [Google Scholar] [CrossRef]
- Li, C.W.; Lin, S.Y.; Chou, H.S.; Chen, T.Y.; Chen, Y.A.; Liu, S.Y.; Liu, Y.L.; Chen, C.A.; Huang, Y.C.; Chen, S.L.; et al. Detection of Dental Apical Lesions Using CNNs on Periapical Radiograph. Sensors 2021, 21, 7049. [Google Scholar] [CrossRef]
- Li, S.; Fevens, T.; Krzyżak, A.; Jin, C.; Li, S. Semi-automatic computer aided lesion detection in dental X-rays using variational level set. Pattern Recognit. 2007, 40, 2861–2873. [Google Scholar] [CrossRef]
- Caputo, B.; Gigante, G.E. Analysis of periapical lesion using statistical textural features. Stud. Health Technol. Inform. 2000, 77, 1231–1234. [Google Scholar]
- Orstavik, D.; Kerekes, K.; Eriksen, H.M. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Endod. Dent. Traumatol. 1986, 2, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Setzer, F.C.; Shi, K.J.; Zhang, Z.; Yan, H.; Yoon, H.; Mupparapu, M.; Li, J. Artificial Intelligence for the Computer-aided Detection of Periapical Lesions in Cone-beam Computed Tomographic Images. J. Endod. 2020, 46, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Rysavy, S.; Enciso, R.; Okada, K. Non-invasive differential diagnosis of dental periapical lesions in cone-beam CT. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, 28 June–1 July 2009; pp. 566–569. [Google Scholar] [CrossRef]
- Ezhov, M.; Gusarev, M.; Golitsyna, M.; Yates, J.M.; Kushnerev, E.; Tamimi, D.; Aksoy, S.; Shumilov, E.; Sanders, A.; Orhan, K. Clinically applicable artificial intelligence system for dental diagnosis with CBCT. Sci. Rep. 2021, 11, 15006. [Google Scholar] [CrossRef]
- Orhan, K.; Bayrakdar, I.S.; Ezhov, M.; Kravtsov, A.; Özyürek, T. Evaluation of artificial intelligence for detecting periapical pathosis on cone-beam computed tomography scans. Int. Endod. J. 2020, 53, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, A.E.; Gültekin, S.; Simsar, E.; Özdemir, Ş.D.; Gündoğar, M.; Tokgöz, S.B.; Hamamcı, İ.E. Dental enumeration and multiple treatment detection on panoramic X-rays using deep learning. Int. Endod. J. 2020, 53, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.G.; Hillen, F.; Salloumis, M.; Sedaghat, A.R.; Niehues, S.M.; Quatela, O.; Hanken, H.; Smeets, R.; Beck-Broichsitter, B.; Rendenbach, C.; et al. Development of a Deep Learning Algorithm for Periapical Disease Detection in Dental Radiographs. Diagnostics 2020, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Ekert, T.; Krois, J.; Meinhold, L.; Elhennawy, K.; Emara, R.; Golla, T.; Schwendicke, F. Deep Learning for the Radiographic Detection of Apical Lesions. J. Endod. 2019, 45, 917–922.e5. [Google Scholar] [CrossRef]
- Bayrakdar, I.S.; Orhan, K.; Çelik, Ö.; Bilgir, E.; Sağlam, H.; Kaplan, F.A.; Görür, S.A.; Odabaş, A.; Aslan, A.F.; Różyło-Kalinowska, I. A U-Net Approach to Apical Lesion Segmentation on Panoramic Radiographs. Biomed. Res. Int. 2022, 2022, 7035367. [Google Scholar] [CrossRef]
- Zadrożny, Ł.; Regulski, P.; Brus-Sawczuk, K.; Czajkowska, M.; Parkanyi, L.; Ganz, S.; Mijiritsky, E. Artificial Intelligence Application in Assessment of Panoramic Radiographs. Diagnostics 2022, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Antony, D.P.; Thomas, T.; Nivedhitha, M.S. Two-dimensional Periapical, Panoramic Radiography Versus Three-dimensional Cone-beam Computed Tomography in the Detection of Periapical Lesion After Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7736. [Google Scholar] [CrossRef] [PubMed]
- Rohlin, M.; Kullendorff, B.; Ahlqwist, M.; Henrikson, C.O.; Hollender, L.; Stenström, B. Comparison between panoramic and periapical radiography in the diagnosis of periapical bone lesions. Dentomaxillofac. Radiol. 1989, 18, 151–155. [Google Scholar] [CrossRef] [PubMed]

| Author, Study Location, and Year of Publication | Sample Size (Periapical Radiographs) | X-ray Technology | AI Model | Sensitivity | Specificity | Accuracy | F1 Score |
|---|---|---|---|---|---|---|---|
| Hamdan et al., United States, 2022 [28] | 68 | Photostimulable phosphor (PSP) plates scanned using ScanX (Air Techniques, Hicksville, NY, USA), Soredex Digora Optime (Kavo Dental, Charlotte, NC, USA) Sirona Schick33 Direct Digital Sensor (Dentsply Sirona, Charlotte, NC, USA) XDR Anatomic Sensor (Cyber Medical Imaging, Los Angeles, CA, USA). | CNN (Denti.AI) | 93.1% (by case) 88.8% (by lesion) | 73.3% (by case) | N/A | N/A |
| Li et al., China, 2022 [29] | 4129 | N/A | ResNet-18 | 82% | 84% | N/A | 0.82 |
| Moidu et al., India, 2022 [30] | 1950 | Size-2 CMOS RVG sensor (Kodak RVG 5100, Eastman Kodak Company, France) | YOLO (You Only Look Once) version 3 | 92.1% | 76% | 86.3% | 0.89 |
| Chen et al., China, 2021 [31] | 2900 | Digital | Fast-R-CNN (Fast Region-based convolutional neural network) | N/A | N/A | N/A | N/A |
| Li et al., Taiwan, 2021 [32] | 476 | N/A | CNN | 94.87% | 90% | 92.75% | N/A |
| Li et al., Canada, 2007 [33] | 60 | N/A | SVM (support vector machine) | N/A | N/A | N/A | N/A |
| Caputo et al., Italy, 2000. [34] | 54 | Digital RadioVisioGraphy (RVG) | Neural network | N/A | N/A | N/A | N/A |
| Healthy | Unhealthy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AI-based method | False Negative | False Positive | ||||||||
| 46 | 47 | 1 | 12 | 13 | 1 | |||||
| True Negative | True Positive | |||||||||
| Ground truth method | 47 | 13 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, J.; Jaber, M.; Rifai, I.; Mozdziak, P.; Kempisty, B.; Dyszkiewicz-Konwińska, M. Diagnostic Test Accuracy of Artificial Intelligence in Detecting Periapical Periodontitis on Two-Dimensional Radiographs: A Retrospective Study and Literature Review. Medicina 2023, 59, 768. https://doi.org/10.3390/medicina59040768
Issa J, Jaber M, Rifai I, Mozdziak P, Kempisty B, Dyszkiewicz-Konwińska M. Diagnostic Test Accuracy of Artificial Intelligence in Detecting Periapical Periodontitis on Two-Dimensional Radiographs: A Retrospective Study and Literature Review. Medicina. 2023; 59(4):768. https://doi.org/10.3390/medicina59040768
Chicago/Turabian StyleIssa, Julien, Mouna Jaber, Ismail Rifai, Paul Mozdziak, Bartosz Kempisty, and Marta Dyszkiewicz-Konwińska. 2023. "Diagnostic Test Accuracy of Artificial Intelligence in Detecting Periapical Periodontitis on Two-Dimensional Radiographs: A Retrospective Study and Literature Review" Medicina 59, no. 4: 768. https://doi.org/10.3390/medicina59040768
APA StyleIssa, J., Jaber, M., Rifai, I., Mozdziak, P., Kempisty, B., & Dyszkiewicz-Konwińska, M. (2023). Diagnostic Test Accuracy of Artificial Intelligence in Detecting Periapical Periodontitis on Two-Dimensional Radiographs: A Retrospective Study and Literature Review. Medicina, 59(4), 768. https://doi.org/10.3390/medicina59040768