A Randomized, Multicenter, Double-Blind, Parallel, Non-Inferiority Clinical Study to Compare the Efficacy and Safety of Unicenta and Melsmon for Menopausal Symptom Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Preparation of Medical Intervention
2.3. Statistical Analysis
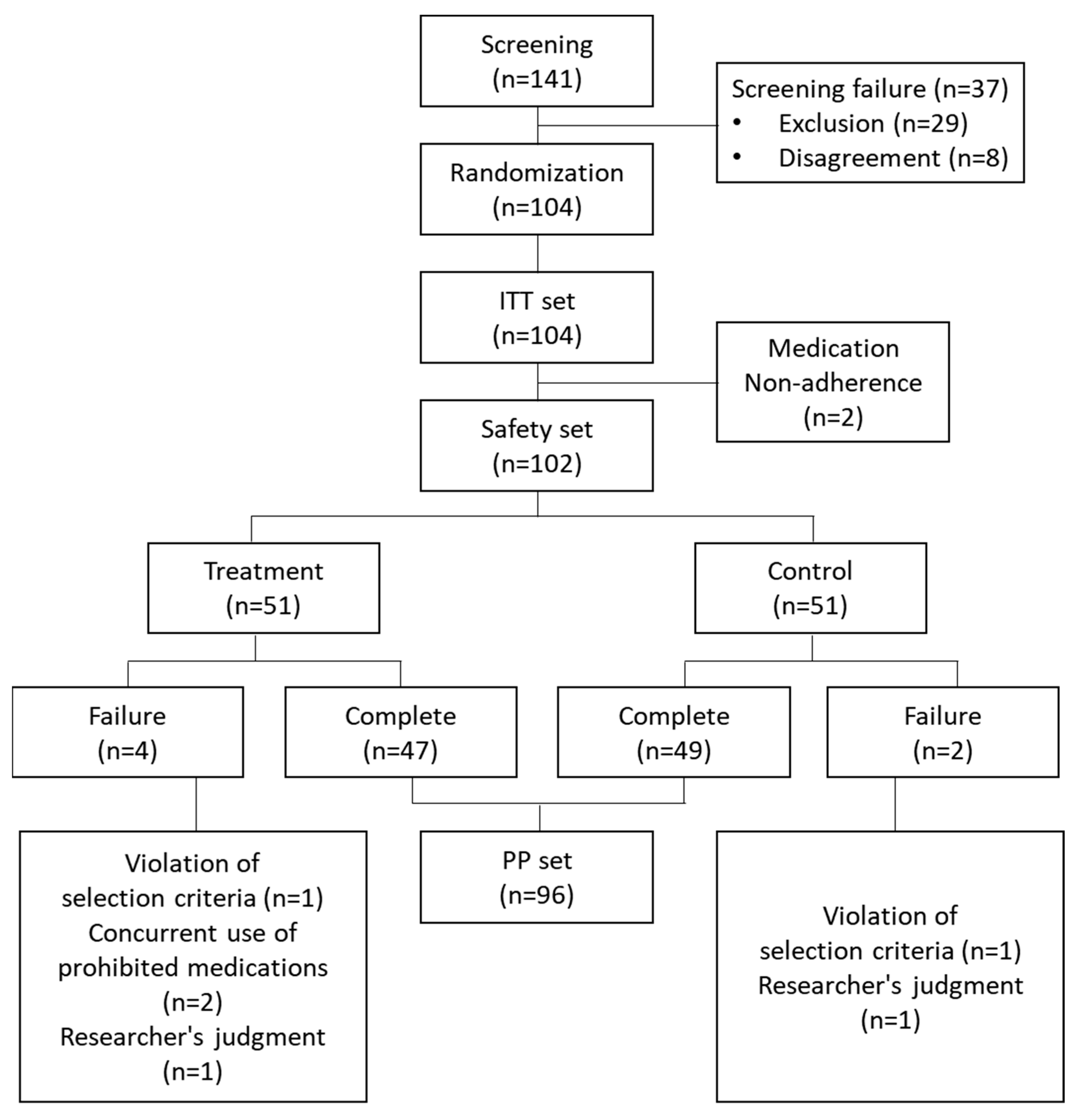
3. Results
3.1. Characteristics of Study Population
3.2. Primary Endpoint
3.3. Secondary Endpoints
3.4. Safety Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, B.-I.; Koh, S.-K.; Hwang, S.-O.; Park, J.-H.; Kim, C.-W. Comparative study on treatment of postmenopausal symptoms with black cohosh root extract and hormone replacement therapy. Korean J. Obstet. Gynecol. 2002, 45, 1330–1335. [Google Scholar]
- Karasawa, Y.; Iwasaki, Y.; Kagawa, S.; Saito, M.; Iwasaki, Y.; Kimura, Y.; Kobayashi, K.; Tsuyuguchi, M. Clinical treatment test of Melsmon on menopausal disorder. Med. Treat 1981, 9, 1–10. [Google Scholar]
- Park, K.M.; Cho, D.P.; Cho, T.H. Placenta Therapy: Its Biological Role of Anti-Inflammation and Regeneration. Placenta 2018, 113–134. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Prokopyuk, V.; Figueiredo, C.; Pogozhykh, D. Placenta and placental derivatives in regenerative therapies: Experimental studies, history, and prospects. Stem Cells Int. 2018, 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Costa, S.D.; Zenclussen, A.C. Endocrine factors modulating immune responses in pregnancy. Front Immunol 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Shukla, V.; Rasheed, M.; Kumar, M.; Gupta, S.; Pandey, S. A trial to determine the role of placental extract in the treatment of chronic non-healing wounds. J. Wound Care 2004, 13, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Park, C.L.; Kim, N.R.; Kim, H.Y.; Yoou, M.S.; Nam, S.Y.; Moon, P.D.; Jeong, H.J.; Kim, H.M. Protective effect of porcine placenta in a menopausal ovariectomized mouse. Reproduction 2016, 152, X1. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chung, H.H.; Kang, S.B. Efficacy and safety of human placenta extract in alleviating climacteric symptoms: Prospective, randomized, double-blind, placebo-controlled trial. J. Obs. Gynaecol Res. 2009, 35, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, H.T.; Lee, B.I.; Shin, J.H.; Park, H.M.; Kim, T. Comparison of the Efficacy and Safety of the Unicenta and Melsmon Injection for the Menopausal Symptoms. J. Korean Soc. Menopause 2013, 19, 36–44. [Google Scholar] [CrossRef]
- Kong, M.-H.; Lee, E.-J.; Lee, S.-Y.; Cho, S.-J.; Hong, Y.-S.; Park, S.-B. Effect of human placental extract on menopausal symptoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women. Menopause 2008, 15, 296–303. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, C.; Yoon, S.H.; Choi, H. Effect of porcine placental extract on menopausal symptoms in postmenopausal women: A prospective, randomized, double-blind, placebo-controlled trial. Taiwan J Obs. Gynecol 2020, 59, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, D.I.; Yoon, S.H.; Choi, C.M.; Yoo, J.E. Randomized, single-blind, placebo-controlled trial on Hominis placenta extract pharmacopuncture for hot flashes in peri- and post-menopausal women. Integr. Med. Res. 2022, 11, 100891. [Google Scholar] [CrossRef] [PubMed]
- Kupperman, H.S.; Blatt, M.H.; Wiesbader, H.; Filler, W. Comparative clinical evaluation of estrogenic preparations by the menopausal and amenorrheal indices. J. Clin. Endocrinol. Metab. 1953, 13, 688–703. [Google Scholar] [CrossRef]
- Alder, E. The Blatt-Kupperman menopausal index: A critique. Maturitas 1998, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]

| Unicenta (n = 51) | Melsmon (n = 51) | p Value | |
|---|---|---|---|
| Age | |||
| Mean, SD | 55.10, 4.41 | 55.60, 5.66 | 0.617 |
| Median (Min–Max) | 54 (46–67) | 56 (43–66) | |
| Weight (kg) | |||
| Mean, SD | 52.27, 10.00 | 58.31, 5.68 | 0.514 |
| Median (Min–Max) | 55.35 (44.0–96.8) | 58.95 (46.5–71.0) | |
| Height (cm) | |||
| Mean, SD | 156.48, 4.42 | 155.6, 5.02 | 0.342 |
| Median (Min–Max) | 156.0 (143.0–166.0) | 155.5 (142.0–166.0) | |
| History of illness (n, %) | 27 (51.92%) | 25 (48.08%) | 0.695 |
| Concurrent illness (n, %) | 21 (40.38%) | 29 (55.77%) | 0.116 |
| History of any drug (n, %) | 21 (40.38%) | 43 (65.38%) | 0.011 |
| Concurrent drug (n, %) | 15 (28.85%) | 22 (42.31%) | 0.152 |
| Visit | Unicenta | Melsmon | p Value * |
|---|---|---|---|
| PP | (n = 47) | (n = 49) | |
| Baseline (mean, SD) | 30.64, 6.79 | 31.31, 6.26 | |
| Visit 8 (mean, SD) | 13.38, 6.57 | 14.39, 5.33 | |
| [Baseline—Visit 8] (mean, SD) | 17.26, 7.64 | 16.92, 7.33 | 0.826 |
| p value † | <0.001 | <0.001 | |
| ITT | (n = 51) | (n = 51) | |
| Baseline (mean, SD) | 30.83, 6.51 | 31.63, 6.30 | |
| Visit 8 (mean, SD) | 14.14, 7.29 | 14.59, 5.79 | |
| [Baseline—Visit 8] (mean, SD) | 16.63, 8.08 | 17.04, 7.42 | 0.789 |
| p value † | <0.001 | <0.001 |
| Unicenta | Melsmon | p Value | |
|---|---|---|---|
| E2 change (Mean, SD) | −1.77, 8.60 | −4.11, 26.46 | 0.587 |
| FSH change (Mean, SD) | 4.18, 14.59 | 2.45, 13.93 | 0.561 |
| Hot flash number change during daytime (Mean, SD) | 2.81, 2.88 | 2.77, 2.91 | 0.946 |
| Hot flash number change during night (Mean, SD) | 1.77, 3.05 | 1.54, 1.74 | 0.637 |
| Hot flash index change during daytime (Mean, SD) | 5.81, 6.00 | 5.71, 6.36 | 0.937 |
| Hot flash index change during night (Mean, SD) | 3.63, 6.55 | 2.92, 3.59 | 0.494 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, S.; Ahn, K.-H.; Park, H.-T.; Song, J.-Y.; Hong, S.-C.; Kim, T. A Randomized, Multicenter, Double-Blind, Parallel, Non-Inferiority Clinical Study to Compare the Efficacy and Safety of Unicenta and Melsmon for Menopausal Symptom Improvement. Medicina 2023, 59, 1382. https://doi.org/10.3390/medicina59081382
Kim S, Lee S, Ahn K-H, Park H-T, Song J-Y, Hong S-C, Kim T. A Randomized, Multicenter, Double-Blind, Parallel, Non-Inferiority Clinical Study to Compare the Efficacy and Safety of Unicenta and Melsmon for Menopausal Symptom Improvement. Medicina. 2023; 59(8):1382. https://doi.org/10.3390/medicina59081382
Chicago/Turabian StyleKim, Seongmin, Sanghoon Lee, Ki-Hoon Ahn, Hyun-Tae Park, Jae-Yun Song, Soon-Cheol Hong, and Tak Kim. 2023. "A Randomized, Multicenter, Double-Blind, Parallel, Non-Inferiority Clinical Study to Compare the Efficacy and Safety of Unicenta and Melsmon for Menopausal Symptom Improvement" Medicina 59, no. 8: 1382. https://doi.org/10.3390/medicina59081382
APA StyleKim, S., Lee, S., Ahn, K.-H., Park, H.-T., Song, J.-Y., Hong, S.-C., & Kim, T. (2023). A Randomized, Multicenter, Double-Blind, Parallel, Non-Inferiority Clinical Study to Compare the Efficacy and Safety of Unicenta and Melsmon for Menopausal Symptom Improvement. Medicina, 59(8), 1382. https://doi.org/10.3390/medicina59081382







