Vertical Levels of the Occipital Artery Origin
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheen, T.; Yen, K.L.; Ko, J.; Hsu, M. Usefulness of the C1 transverse process as a reference guide in the dissection of the upper lateral neck. Otolaryngol. Neck Surg. 2000, 122, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Koizumi, H.; Ishima, D.; Kuroda, H.; Shibahara, I.; Niki, J.; Miyasaka, K.; Watanabe, T.; Kondo, R.; Kumabe, T. Angiographic Characterization of the External Carotid Artery: Special Attention to Variations in Branching Patterns. Tohoku J. Exp. Med. 2019, 249, 185–192. [Google Scholar] [CrossRef]
- Power, J. Surgical Anatomy of the Arteries and Descriptive Anatomy of the Heart by the Late Valentine Flood, MD; Fannin and Co.: Dublin, Ireland, 1850. [Google Scholar]
- Bergman, R.A.; Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Nathan, H.; Levy, J. The course and relations of the hypoglossal nerve and the occipital artery. Am. J. Otolaryngol. 1982, 3, 128–132. [Google Scholar] [CrossRef]
- Moraru, L.; Rusu, M.C.; Popescu, Ş.A. True terminal pentafurcation of the external carotid artery and terminal trifurcation of the contralateral one, occipitoauricular trunk, retropharyngeal internal carotid artery. Surg. Radiol. Anat. 2021, 43, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Manta, M.D.; Jianu, A.M.; Rusu, M.C.; Popescu, Ş.A. Launay’s External Carotid Vein. Medicina 2021, 57, 985. [Google Scholar] [CrossRef] [PubMed]
- Horos Project 2018 DICOM Image Viewing and Measuring. Available online: http://horosproject.org/ (accessed on 1 January 2020).
- Gray, H.; Standring, S.; Anand, N.; Birch, R.; Collins, P.; Crossman, A.; Gleeson, M.; Jawaheer, G.; Smith, A.L.; Spratt, J.D.; et al. Gray’s Anatomy: The Anatomical Basis of Clinical Practice; Elsevier: London, UK, 2016. [Google Scholar]
- Rouviere, H.; Delmas, A. Anatomie Humaine. Tête et Cou; Masson: Paris, France, 1985. [Google Scholar]
- Paulsen, F.; Waschke, J. Head, Neck and Neuroanatomy; Urban & Fischer Verlag/Elsevier GmbH: Munich, Germany, 2013. [Google Scholar]
- Pernkopf, E. Atlas of Topographical and Applied Human Anatomy; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1980. [Google Scholar]
- Agur, A.M.; Dalley, A.F. Grant’s Atlas of Anatomy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Newton, T.H.; Young, D.A. Anomalous origin of the occipital artery from the internal carotid artery. Radiology 1968, 90, 550–552. [Google Scholar] [CrossRef]
- Özgür, Ö.; Sindel, M.; Hizay, A.; Öztürk, S.; Aytaç, G.; Sindel, T. Occipital artery arising from the internal carotid artery: A case report. Surg. Radiol. Anat. 2016, 39, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Barral, J.-P.; Croibier, A. Visceral Vascular Manipulations; Elsevier Health Sciences: Paris, France, 2011. [Google Scholar]
- Di, G.; Fang, X.; Hu, Q.; Zhou, W.; Jiang, X. A Microanatomical Study of the Far Lateral Approach. World Neurosurg. 2019, 127, e932–e942. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Komune, N.; Akiyama, O.; Amano, T.; Nakamizo, A. Surgical Anatomy of the Donor Arteries for Extracranial-Intracranial Bypass Surgery: An Anatomic and Radiologic Study. World Neurosurg. 2020, 136, e447–e459. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, P.; Bonczar, M.; Plutecki, D.; Kwiecińska, M.; Rams, D.; Dziedzic, M.; Piątek-Koziej, K.; Przybycien, W.; Sporek, M.; Walocha, J.; et al. The occipital artery: A meta-analysis of its anatomy with clinical correlations. Anat. Sci. Int. 2022, 98, 12–21. [Google Scholar] [CrossRef]
- Seker, A.; Martins, C.; Rhoton, J.A.L. Meningeal Anatomy. In Meningiomas. A Comprehensive Text.; Pamir, M.N., Black, P.M., Fahlbusch, R., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2010; pp. 11–51. [Google Scholar]
- Uchino, A.; Saito, N. Occipital artery arising from the cervical internal carotid artery at the level of the C2 vertebral body: Three cases detected utilizing magnetic resonance angiography. Surg. Radiol. Anat. 2020, 42, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Alvernia, J.E.; Fraser, K.; Lanzino, G. The occipital artery: A microanatomical study. Neurosurgery 2006, 58, ONS114-122, discussion ONS114-122. [Google Scholar] [PubMed]
- Kawashima, M.; Rhoton, A.L., Jr.; Tanriover, N.; Ulm, A.J.; Yasuda, A.; Fujii, K. Microsurgical anatomy of cerebral revascularization. Part I: Anterior circulation. J. Neurosurg. 2005, 102, 116–131. [Google Scholar] [CrossRef]
- Acar, M.; Salbacak, A.; Sakarya, M.E.; Zararsiz, I.; Ulusoy, M. Análisis Morfométrico de la Arteria Carótida Externa y sus Ramas Mediante la Técnica de Angiografía por Tomografía Computarizada Multidetector. Int. J. Morphol. 2013, 31, 1407–1414. [Google Scholar] [CrossRef]
- Cobiella, R.; Quinones, S.; Aragones, P.; León, X.; Abramovic, A.; Vazquez, T.; Sanudo, J.R.; Maranillo, E.; Olewnik, L.; de Blas, C.S.; et al. Anatomic mapping of the collateral branches of the external carotid artery with regard to daily clinical practice. Ann. Anat.-Anat. Anz. 2021, 238, 151789. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.T.; Demirtas, I.; Topcu, F.S.; Karasu, S.; Ayyıldiz, B. A rare case report: Bilateral occipital artery arising from the vertebral artery. Surg. Radiol. Anat. 2021, 43, 1901–1904. [Google Scholar] [CrossRef]
- Öner, Z.; Öner, S.; Kahraman, A.S. The right vertebral artery originating from the right occipital artery and the absence of the transverse foramen: A rare anatomical variation. Surg. Radiol. Anat. 2017, 39, 1397–1400. [Google Scholar] [CrossRef]
- Touré, G.; Méningaud, J.P.; Vacher, C. Arterial vascularization of occipital scalp: Mapping of vascular cutaneous territories and surgical applications. Surg. Radiol. Anat. 2010, 32, 739–743. [Google Scholar] [CrossRef]
- Wolf, J.; Mattila, K.; Hietanen, J.; Kozeltsev, A.L. A stereoangiographic study of the arterial variations in the external carotid system. Dentomaxillofac. Radiol. 1985, 14, 45–51. [Google Scholar] [CrossRef]
- Hayashi, N.; Hori, E.; Ohtani, Y.; Ohtani, O.; Kuwayama, N.; Endo, S. Surgical anatomy of the cervical carotid artery for carotid endarterectomy. Neurol. Med.-Chir. 2005, 45, 25–30. [Google Scholar] [CrossRef]
- Lemaire, V.; Jacquemin, G.; Nelissen, X.; Heymans, O. Tip of the greater horn of the hyoid bone: A landmark for cervical surgery. Surg. Radiol. Anat. 2004, 27, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Adachi, B. Das Arteriensystem der Japaner; Kenkyusha Press: Kyoto, Japan, 1928; Volume 2, pp. 18–71. [Google Scholar]
- Sundick, S.A.; Weaver, M.; Faries, P.L.; Marin, M. Aberrant origin of occipital artery proximal to internal carotid artery stenosis. J. Vasc. Surg. 2014, 59, 244. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.T.; Hamer, J.D. Anomalous origin of the occipital artery from the cervical internal carotid artery. J. Vasc. Surg. 1988, 8, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Hyrtle, J. Einige in chirurgischer Hinsicht wichtige Gefassvarietaten. Med. Jahrbosterr Staats 1841, 33, 421. [Google Scholar]
- Quain, R. The Anatomy of the Arteries of the Human Body; Taylor and Walton: London, UK, 1844. [Google Scholar]
- Seidel, K. Arteriographic observation of a rare carotis anomaly. Fortschr. Geb. Rontgenstr. Nuklearmed. 1965, 103, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, G.; Kawashima, M.; Tsutsumi, K. Carotid endarterectomy for treatment of tandem carotid stenosis in the presence of the anomalous origin of the occipital artery arising from the cervical internal carotid artery: A case report. J. Med. Case Rep. 2013, 7, 254. [Google Scholar] [CrossRef]
- Iwai, T.; Izumi, T.; Inoue, T.; Maegawa, J.; Fuwa, N.; Mitsudo, K.; Tohnai, I. Occipital artery arising from the anterior aspect of the internal carotid artery identified by three-dimensional computed tomography angiography. Iran J. Radiol. 2012, 9, 103–105. [Google Scholar] [CrossRef]
- Uchino, A.; Saito, N.; Okano, N.; Kakehi, Y. Aberrant internal carotid artery associated with occipital artery arising from the internal carotid artery. Surg. Radiol. Anat. 2015, 37, 1137–1140. [Google Scholar] [CrossRef]
- Hachem, K.; Slaba, S.; Nassar, J.; Kanso, H.; Ashoush, R.; Ghossain, M. Imaging of an aberrant occipital artery arising from the extracranial segment of the internal carotid artery. J. Mal. Vasc. 2004, 29, 205–209. [Google Scholar] [CrossRef]
- Lippert, H.; Pabst, R. Arterial Variations in Man: Classification and Frequency; J.P. Bergmann Verlag: München, Germany, 1985. [Google Scholar]
- Uchino, A.; Saito, N.; Mizukoshi, W.; Okada, Y. Anomalous origin of the occipital artery diagnosed by magnetic resonance angiography. Neuroradiology 2010, 53, 853–857. [Google Scholar] [CrossRef]
- Iwai, T.; Izumi, T.; Inoue, T.; Maegawa, J.; Mitsudo, K.; Tohnai, I. Incidence of the occipital artery arising from the internal carotid artery identified by three-dimensional computed tomographic angiography. Br. J. Oral Maxillofac. Surg. 2012, 50, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Small, J.E.; Harrington, J.; Watkins, E. Prevalence of arterial branches arising from the extracranial internal carotid artery on CT angiography. Surg. Radiol. Anat. 2013, 36, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Evins, A.I.; Burrell, J.C.; Stieg, P.E.; Bernardo, A. A safe and effective technique for harvesting the occipital artery for posterior fossa bypass surgery: A cadaveric study. World Neurosurg. 2014, 82, e459–e465. [Google Scholar] [CrossRef] [PubMed]
- Ateş, Ö.; Ahmed, A.S.; Niemann, D.; Başkaya, M.K. The occipital artery for posterior circulation bypass: Microsurgical anatomy. Neurosurg. Focus 2008, 24, E9. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, H.; Chen, X.; Yu, J. Clinical importance of the occipital artery in vascular lesions: A review of the literature. Neuroradiol. J. 2019, 32, 366–375. [Google Scholar] [CrossRef]
- Manders, E.K. Regional Pedicle Flaps. In Operative Otolaryngology: Head and Neck Surgery; Myers, E.N., Carrau, R.L., Eibling, D.E., Ferguson, B.J., Ferris, R.L., Gillman, G.S., Golla, S., Grandis, J.R., Hirsch, B.E., Johnson, J.T., et al., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2008; pp. 753–763. [Google Scholar]
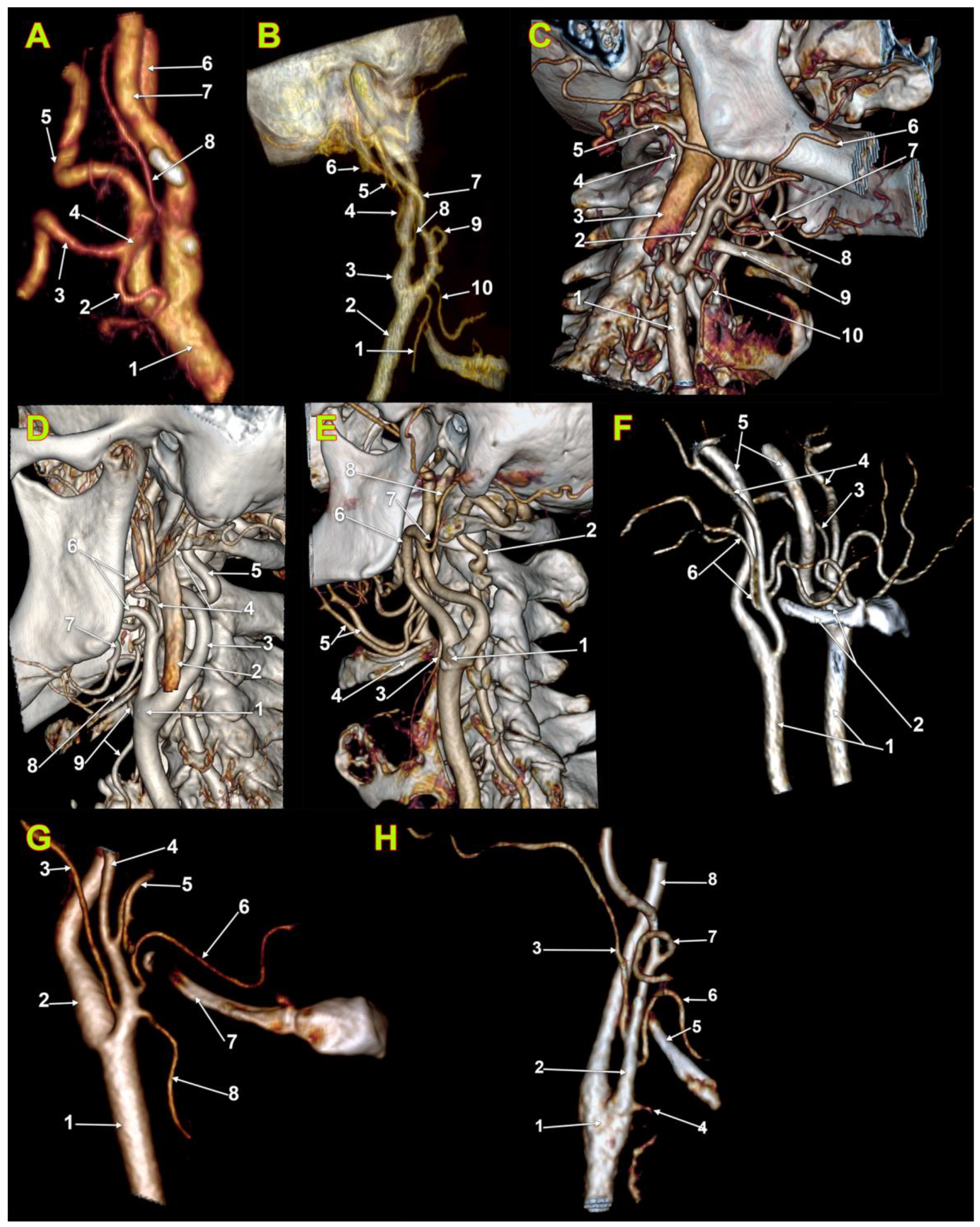
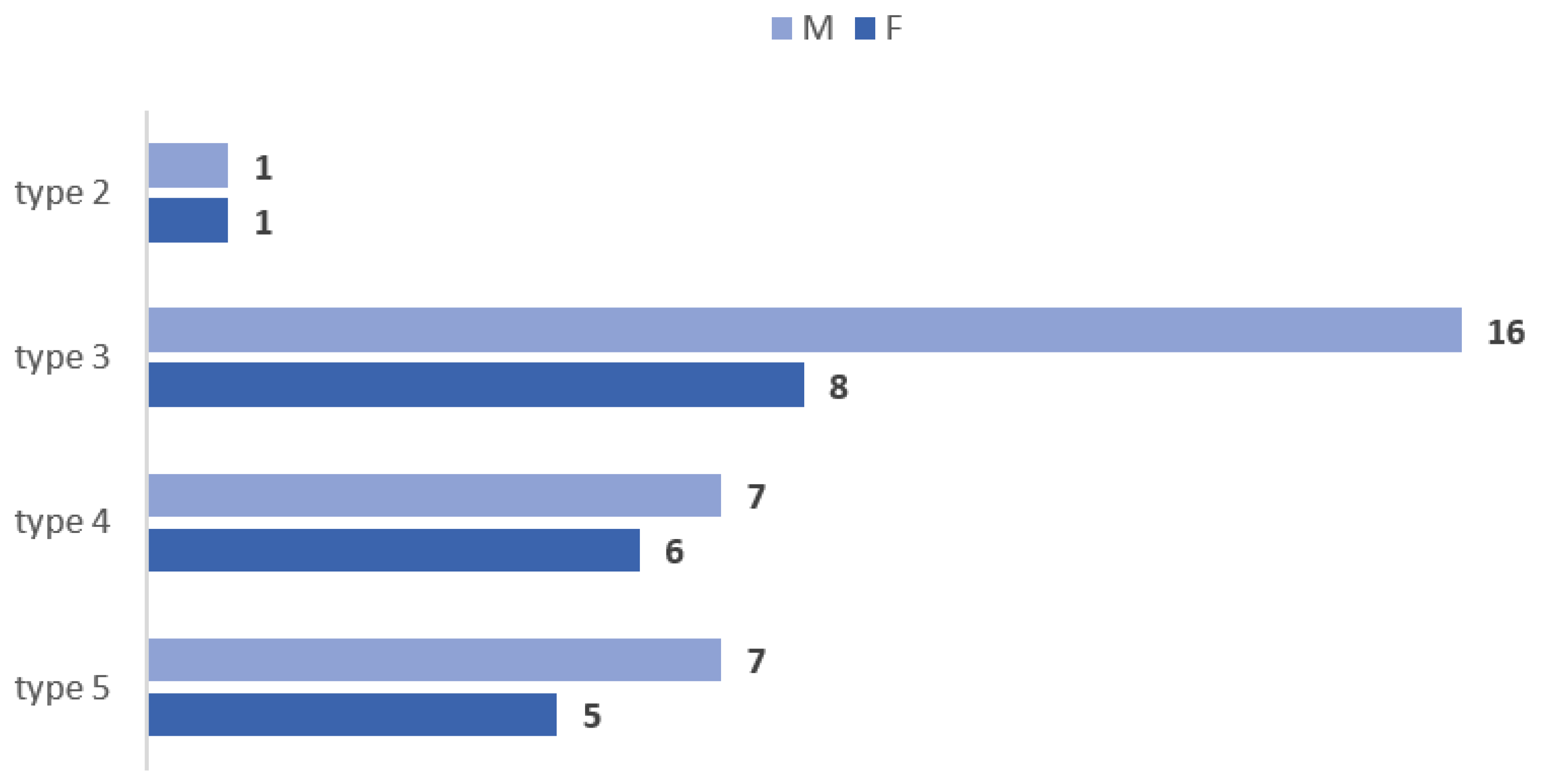
| Vertical Levels of Origin of the OA (N = 180) | Count, % |
|---|---|
| type 1—infrahyoid level of origin | 2 (1.11%) |
| type 2—hyoid level of origin | 10 (5.56%) |
| type 3—suprahyoid level of origin | 73 (40.56%) |
| type 4—gonial level of origin | 51 (28.33%) |
| type 5—supragonial level of origin | 42 (23.33%) |
| type 6—ICA origin | 2 (1.11%) |
| Type | General Lot (90 Cases) | Males (53 Cases) | Females (37 Cases) | |||
|---|---|---|---|---|---|---|
| Right Side | Left Side | Right Side | Left Side | Right Side | Left Side | |
| 1 | 2.22% | 0 | 0 | 0 | 5.41% | 0 |
| 2 | 3.33% | 7.78% | 3.77% | 7.55% | 2.7% | 8.11% |
| 3 | 36.67% | 44.44% | 39.62% | 49.06% | 32.43% | 37.84% |
| 4 | 25.56% | 31.11% | 22.64% | 26.42% | 29.73% | 37.84% |
| 5 | 30% | 16.67% | 30.19% | 16.98% | 29.73% | 16.22% |
| 6 | 2.22% | 0 | 3.77% | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, C.C.; Hostiuc, S.; Vrapciu, A.D.; Rusu, M.C. Vertical Levels of the Occipital Artery Origin. Medicina 2023, 59, 317. https://doi.org/10.3390/medicina59020317
Dumitru CC, Hostiuc S, Vrapciu AD, Rusu MC. Vertical Levels of the Occipital Artery Origin. Medicina. 2023; 59(2):317. https://doi.org/10.3390/medicina59020317
Chicago/Turabian StyleDumitru, Cătălin Constantin, Sorin Hostiuc, Alexandra Diana Vrapciu, and Mugurel Constantin Rusu. 2023. "Vertical Levels of the Occipital Artery Origin" Medicina 59, no. 2: 317. https://doi.org/10.3390/medicina59020317
APA StyleDumitru, C. C., Hostiuc, S., Vrapciu, A. D., & Rusu, M. C. (2023). Vertical Levels of the Occipital Artery Origin. Medicina, 59(2), 317. https://doi.org/10.3390/medicina59020317







